Abstract
This prospective observational study evaluated the safety and feasibility of a low threshold testing process in a Triage and Test Center (TTC) during the early course of the coronavirus disease 19 (COVID-19) pandemic. In addition, we aimed to identify clinical predictors for a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) swab result. Patients underwent informal triage, standardized history taking, and physician evaluation, only where indicated. Patients were observed for 30 days. Safety was the primary outcome and was defined as a COVID-19-related 30 day re-presentation rate <5% and mortality rate <1% in patients presenting to the TTC. Feasibility was defined as an overruling of informal triage <5%. Among 4815 presentations, 572 (11.9%) were tested positive for SARS-CoV-2, and 4774 were discharged. Mortality at 30-days was 0.04% (2 patients, one of which related to COVID-19). Fever (OR 2.03 [95% CI 1.70;2.42]), myalgia (OR 1.94 [1.63;2.31]), chills (OR 1.77 [1.44;2.16]), headache (OR 1.61 [1.34;1.94]), cough (OR 1.50 [1.24;1.83]), weakness (OR 1.46 [1.21;1.76]), and confusion (OR 1.39 [1.06;1.80]) were associated with test positivity. Re-presentation rate was 8% overall and 1.4% in COVID-19 related re-presentation (69 of 4774). The overruling rate of informal triage was 1.5%. According to our study, a low-threshold testing process in a TTC appeared to be safe (low re-presentation and low mortality) and is feasible (low overruling of informal triage). A COVID-19 diagnosis based on clinical parameters only does not appear possible.
1. Introduction
Regular, widespread testing to identify and isolate individuals with coronavirus disease 19 (COVID-19) appears to be crucial to reduce transmission [1,2], but limitations on testing availability has been a challenge during the early phase of COVID-19 pandemic. To implement a low-threshold testing, we setup a Triage and Test Center (TTC) in the early phase of the pandemic. Expecting a high number of patients willing to be tested, we designed a quick and easy walk-in process. This process was based on previous safety data regarding informal triage and physiological reserve (e.g., mobility). In detail, unimpaired mobility, low physician disease severity ratings (PDSR), and normal vital signs are associated with a very favorable prognosis [3,4,5,6,7]. We hypothesized that these principles hold true for a coordinated large-scale testing process. Several similar approaches, such as a drive-thru process in COVID-19 testing have been described, but to date, safety data are lacking [8].
In the present study, we evaluated feasibility and safety of a Triage and Test Center for low-threshold testing, to determine patient outcomes, and to identify clinical predictors for a positive nasopharyngeal SARS-CoV-2 swab result.
2. Methods
The emergency department (ED) of the University Hospital Basel (UHBS), a tertiary care center, has a yearly census of 54,000 patients. UHBS serves as a major provider for a population of about 176,000 inhabitants and is the designated COVID-19 hospital for the Canton (State). The TTC was located in a church in close vicinity (entrance 30 m from the ED front door). A signpost diverted patients with flu-like symptoms from the ED and other front doors to the TTC. To ensure social distancing while waiting for testing, a waiting area supervised by security staff was marked outside of the TTC. Opening hours were from 8 am to 11 pm. The TTC was designed to provide a maximal test capacity of 500 tests daily. A simulation was performed prior to the launch of the TTC to ensure patient-flow and safety. We briefed team members before each shift in adherence to personal protective equipment guidelines, the use of informal triage, and test administration. Health authorities endorsed testing in symptomatic patients and advertised the location through media (newspaper, internet, radio, television, and social media). After evaluation in the TTC, patients deemed in need of further work-up were transferred to the ED. We established a notification pathway for providing test results through text messaging to patients. Local health authorities were informed automatically about all tested cases. Patients were instructed to present to the ED if their symptoms worsened. We performed patient follow-ups for COVID-19 positive cases.
This study was approved by the local ethics committee (EKNZ-identifier: 2020-00790 and 2020-00769). The study is reported according to Strobe guidelines.
2.1. Study Population
The eligible population were consecutive patients who presented and met testing criteria for COVID-19, including those with respiratory symptoms (such as shortness of breath), other flu-like symptoms (such as fever, sore throat, or cough) of any degree, and/or self-reported exposure to COVID-19.
2.2. Methods of Measurement
At the TTC front door, a senior physician screened patients for symptoms and acuity. Informal triage using physician disease severity rating (PDSR) was used to identify patients in need of a clinical evaluation. PDSR was based on a very brief subjective first impression, using a numeric rating scale from 0 to 10 points by asking “how ill does this patient look” (0 = not appearing ill at all, 10 = appearing very seriously ill). PDSR was previously shown to be non-inferior to formal triage regarding safety outcomes, such as mortality [3]. In case of a PDSR higher than 2/10, complete vital sign assessment was to be performed and the patient was evaluated by a physician. Patients filled out a questionnaire regarding symptoms and comorbidities on a machine-readable case-report form. Previously trained medical students reviewed the questionnaire, and performed swabs [9].
In order to implement a low-threshold testing strategy in the virology lab, we provided cross-validated laboratory-developed and commercial SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) assays detecting the viral S-gene, E-gene, and ORF8-gene sequences including the Roche Cobas 6800 assay (Roche Diagnostics, Rotkreuz, Switzerland) [10,11]. Two swabs from the naso- and oropharyngeal sites (NOPS), respectively, were taken and combined into one universal transport medium (UTM) tube (Copan, Brescia, Italy). Total nucleic acids (TNAs) were extracted from the UTM using the MagNA Pure 96 system (Roche Diagnostics, Rotkreuz, Switzerland). We tested for SARS-CoV-2 RNA using RT-PCR assays targeting specific viral sequences (S-gene, Basel-SCoV2-S-112bp, in-house methods, not commercial kits) and simultaneously ran a commercial assay (E-gene; Roche Diagnostics, Rotkreuz, Switzerland) head-to-head. Concordant positive or negative results were signed out directly, while discordant results were retested using the ORF8-gene assay (Basel-SCoV2-ORF8-97bp) and signed out accordingly. Validation of the Roche Cobas 6800 demonstrated concordant results with the Basel-SCoV2-S-112bp and E-gene assays of 97.0% of 1344 tests, again using BaselSCoV2-ORF8-97bp testing for discordant results [11].
2.3. Outcomes
The primary outcome was the safety of the TTC process (performing informal triage in all patients and physician evaluation including vital sign assessment only in patients deemed to be ill, i.e., PDSR >2) [3]. Safety was defined as a re-presentation rate <5% after discharge from the TTC and a mortality rate <1% during the follow-up of 30 days. Re-presentation was defined as any non-elective re-presentation to UHBS with the exception of re-presentation to the TTC for repeated testing. Re-presentations were stratified into related and unrelated re-presentations. Related re-presentation was defined as a COVID-19 case with re-presentation due to symptoms of COVID-19 (like progressive dyspnea, increasing cough or persistent fever) and a patient with newly diagnosed COVID-19 after initial negative testing in the TTC.
Secondary outcomes were feasibility, the description of population and outcomes, and the identification of clinical predictors for a positive nasopharyngeal SARS-CoV-2 swab result. Feasibility was defined as the successful implementation of the process based on informal triage and the inclusion of a student-staffed swab-team [9], an overruling of discharge in patients with PDSR ≤2 below 5%, and the ability to provide the throughput as planned.
Other outcomes were the characterization of the population and the rate of positive swabs for SARS-CoV-2 over time. We chose potential clinical predictors a priori based on available evidence at that time (8th March 2020) [12,13,14,15].
2.4. Statistical Analysis
Descriptive statistics were presented as counts and frequencies for categorical data and medians (Min, Max) for metric or ordinal variables. p-values corresponded to multivariable logistic regressions. A p-value < 0.05 was considered significant. Odds ratios (OR) were calculated with 95% confidence intervals. For metric and ordinal predictors, ORs were presented as the ratios of the odds increasing the predictor by one unit. In forest plots, ORs were expressed increasing the predictor by one IQR (interquartile range). Adjusted ORs were calculated using multivariable logistic regression including age and gender as covariates. We performed all evaluations with the R software language and environment for statistical computing, R foundation for statistical computing, Vienna, Austria, 2020 (R version 4.0.0).
3. Results
3.1. Characteristics of Study Subjects
Between 19th March and 29th April 2020, 5063 presentations occurred to the TTC. We excluded 248 presentations (4.9%) for the following reasons: 172 patients did not provide informed consent, 23 did not receive a nasopharyngeal swab, and 53 had missing data. Therefore, 4815 presentations were included. 3563 patient presentations (74%) had PDSR ≤2, and 1252 (26%) PDSR >2 (Figure 1).
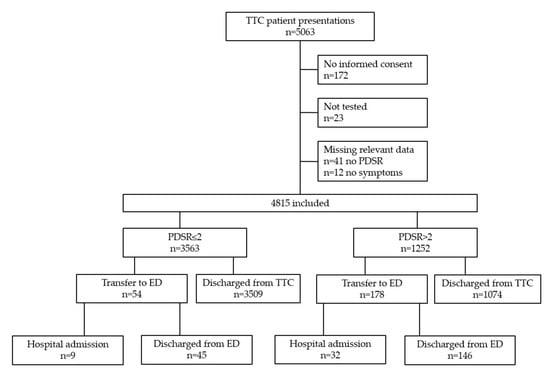
Figure 1.
Flowchart of inclusion. TTC = Triage and Test Center; PDSR = Physician Disease Severity Rating; ED = Emergency Department.
3.2. Safety Outcomes
Among all 4774 patient presentations who were discharged (4583 from the TTC (3509 + 1074), 191 from the ED (45 + 146), Figure 1), 384 (8%) patients represented to the hospital during 30-day follow up (Figure 2). Of these, 174 patients (3.6%) represented the ED, 92 patients (1.9%) represented the TTC, and 118 patients (2.5%) represented elective consultation or hospitalization. Most re-presentations were unrelated to COVID-19 (315/384 patients, 82%). Moreover, 69 patients out of 4774 (1.4%) discharged presentations were related to COVID-19. Among these, 9 patients had newly diagnosed COVID-19 (7/92 patients were diagnosed in the TTC, 2/174 patients in the ED). Median time for COVID-19 related and COVID-19 unrelated re-presentations was 6 (min 1, max 29) and 12 (min 1, max 30) days, respectively. Among all included patient presentations, 2 (0.04%) died during 30-day follow-up. Both patients were discharged from the TTC with PDSR 3 (1 patient tested positive and 1 patient tested negative). The first patient, a 58 year-old male, who was tested positive, represented the ED on day 5. He died in the intensive care unit (ICU) of multi-organ failure. The second patient, a 77-year-old female, who tested negative, sustained a ground level fall 29 days after presentation to the TTC. She died postoperatively after a complicated hip fracture. Repeated testing for COVID-19 was negative. Moreover, 13 patients who tested positive for COVID-19 were lost to follow-up. Nine patients lived in another country and 4 patients provided incorrect personal data.
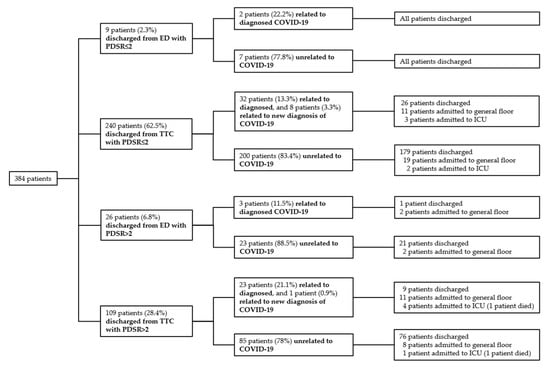
Figure 2.
Re-presentations of discharged patients during 30-day follow-up; ED = Emergency Department; TTC = Triage and Test Center; PDSR = Physician Disease Severity Rating; ICU = Intensive Care Unit.
3.3. Feasibility of the Triage and Test Center (TTC)
In 54 out of 3563 (1.5%) patient presentations with PDSR ≤2, the standard process of discharge without vital signs and physician examination was overruled by referring patients to the ED (for details, see Table A1). Among these, 9 patients were hospitalized (2 related to COVID-19) and 45 discharged from the ED. Next, 178/1252 (14.2%) presentations with PDSR >2 were transferred to the ED for further evaluation, of which 32 were hospitalized (12 related to COVID-19) and 146 discharged. The majority of all presentations (4583, 95.2%) were discharged from the TTC after a nasopharyngeal swab was performed (Figure 1). During opening hours, 93 patients (min 25, max 358) per day were tested. Most tests were performed on March 19th (first day of the study).
3.4. Clinical Predictors for Positive Nasopharyngeal SARS-CoV-2 Swab Result
Among all presentations, 572 (11.9%) had a positive swab for SARS-CoV-2, 4243 (88.1%) were negative (Table 1). Most positive testing occurred in the first 3 weeks of the study and started to decline after inclusion of about 2500 presentations (Figure A1 and Figure A2).

Table 1.
Baseline demographic and clinical characteristics of all included presentations.
The most common reported symptom was cough (3132 presentations, 65%), followed by sore throat (2694, 56%), headache (2693, 55.9%), and coryza (2575, 53.5%). In positive cases, the most common symptom was cough (415, 72.6%), followed by headache (366, 64%), measured/reported fever (323, 56.5%), and coryza (314, 54.9%). Among the 572 positive cases, 192 (33.6%) reported symptoms for seven or more days. Patients with positive swab results were older (OR 1.31 [95% CI 1.15;1.50]). The symptoms most strongly associated with positive tests were measured/reported fever (OR 2.03 [95% CI 1.70;2.42]), myalgia (OR 1.94 [95% CI 1.63;2.31]), chills (OR 1.77 [95% CI 1.44;2.16]), headache (OR 1.61 [1.34;1.94]), and cough (OR 1.50 [95% CI 1.24;1.83]). Wheezing (OR 0.44 [95% CI 0.32;0.61]), lymphadenopathy (OR 0.54 [95% CI 0.37;0.76]), sore throat (OR 0.59 [95% CI 0.49;0.70]), and dyspnea (OR 0.65 [95% CI 0.52;0.82]) were inversely associated with positive tests. Regarding comorbidities, diabetes (OR 1.64 [95% CI 1.07;2.450]) was associated with a positive test, whereas pulmonary disorders (OR 0.27 [95% CI 0.13;0.50]) were inversely associated with a positive test (Figure 3).
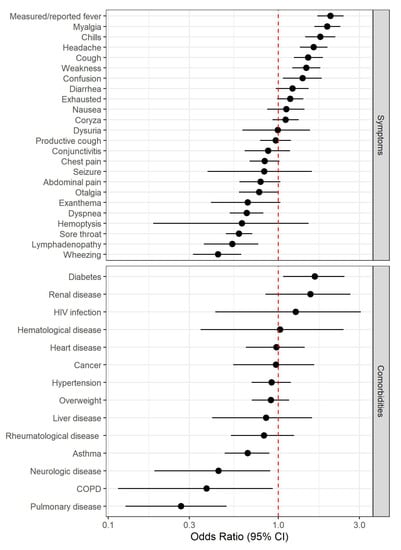
Figure 3.
Forest plot for symptoms and comorbidities. COPD = Chronic Obstructive Pulmonary Disease.
Regarding vital signs, elevated temperature (OR 1.52 [95% CI 1.30;1.78]) and respiratory rate (OR 1.42 [1.17;1.71]) were associated with a positive test. Finally, the decision to admit to hospital was associated with a positive test for SARS-CoV-2 (OR 2.78 [95% CI 1.32;5.43])
For all hospitalized patients (n = 41, related and unrelated to COVID-19) the most common reported symptoms were cough and exhaustion (26 patients, 63.4%), followed by measured/reported fever (23 patients, 56.1%) and headache (22 patients, 53.7%). Higher PDSR (OR 10.2 [95% CI 5.05;23.00]), seizure (OR 6.39 [95% CI 1.84;16.50]), nausea (OR 4.97 [95% CI 2.62;9.27]), confusion (OR 2.79 [95% CI 1.28;5.55]), dyspnea (OR 2.76 [95% CI 1.47;5.14]), and weakness (OR 2.73 [95% CI 1.44;5.49]) were most strongly associated with hospitalization. Regarding comorbidities, renal disease (OR 13.3 [95% CI 5.20; 29.40] and diabetes (OR 10.6 [4.83; 21.40]) were associated with hospitalization (Table A2).
Among all 572 SARS-CoV-2 positive tested presentations, 561 (98.1%) were discharged from the TTC (n = 538) and the ED (n = 23). All were immediately quarantined by the local health authorities. The most common diagnosis in patients discharged from the ED was presumed viral infection tested negative for SARS-CoV-2 (102 of 191 patients, 53.4%). Regarding hospitalization, COVID-19 was the leading cause (14 of 41 patients, 34.1%). Of note, in 11 out of 14 (positive) hospitalized patients, testing for SARS-CoV-2 was positive after the first swab. In 3 patients, testing was initially negative and repeated testing revealed a positive result during hospitalization (Table 2).

Table 2.
Diagnoses of Triage and Test Center (TTC) presentations.
4. Discussion
The main results of our study indicate safety, feasibility, and the excellent clinical outcomes in a low-threshold Triage and Test Center for SARS-CoV-2. Safety was shown by a low re-presentation rate and low mortality during follow-up. Outcomes were excellent, as documented by low hospitalization and ED transfer rates. Feasibility was shown by the successful implementation of the process based on informal triage, the ability to provide a high throughput employing medical students as “swab-team” [9], and a low overruling rate. Analysis of clinical predictors showed that it is virtually impossible to include or exclude COVID-19 on clinical grounds only. Even though certain markers defining influenza-like illness, such as fever, myalgia, chills, and cough, were somewhat predictive of positive swab results in our setting, nonspecific symptoms, such as weakness and confusion [16] were similarly predictive. Although we previously demonstrated that nonspecific symptoms such as weakness are associated with unfavorable prognosis [17], these symptoms do not generally increase the probability of infection in (older) adults in the ED [18,19]. These symptoms appear to be unable to distinguish COVID-19 from other (infectious) illness. Therefore, clinical prediction based on symptoms and comorbidities alone cannot replace molecular diagnostic testing, and repeated testing may be indicated in patients with symptoms, but initially negative PCR test results. Low-threshold testing appears to be useful in order to identify cases that might otherwise have gone undetected. Chameleons, i.e., presentations that do not appear to be positive initially, but later turn out to be COVID-19 are not infrequent [20]. Conversely, COVID-19-mimics, such as the examples provided in Table 2 (apart from patients with COVID-19), accounted for almost two-thirds of hospitalized patients. Data from a large prospective cohort study showed that patients hospitalized for COVID-19 usually presented with fever, cough, and shortness of breath, as well as met the world health organization (WHO) case definitions for COVID-19 [21], but a control group was lacking. Similarly, in a large meta-analysis based on retrospective studies, cough was present only in 58% patients, which is similar to our study [22]. Dyspnea and fever, however, occurred less frequently in our setting, which is mostly due to inclusion of a healthier outpatient population. Another multicenter study developed a clinical score at hospital admission for predicting critical illness. In that study, dyspnea, hemoptysis, and unconsciousness were clinical predictors of critical illness [23]. While several clinical predictors for severe COVID-19 (dyspnea [24], old age [25], type II diabetes [26], obesity [27], possibly hypertension [28], history of cancer [29], cardiovascular disease [30], or increased ratios of CD4/CD8 [31], neutrophils/lymphocytes [32], and CRP/lymphocytes [33]) have been identified, no study has assessed clinical predictors for SARS-CoV-2 positivity. This knowledge is important for the upcoming phase, when the ratio of patients affected to patients tested will remain low in most test centers. The advantage of our approach, waiving formal triage, vital sign assessment, and physician examination in most patients is the reduction in resources used, while safety and feasibility are given. We speculate that, when effectively implemented, a low-threshold approach using a TTC may contribute to subduing the COVID-19 pandemic. This, however, will only be among a wide range of interventions, such as social distancing, contact tracing, and self-isolation of individuals tested positive for SARS-CoV-2.
5. Limitations
This study had several limitations. First, this is a single center study with limited external validity. Second, patients presented with symptoms were considered to be attributable to COVID-19. A self-referral bias appears likely. Third, as our patients were walk-in patients at the TTC, it must be assumed that this is a rather healthy population, which could cause a possible spectrum bias [34]. Verification bias appears to be minimal in our study, as almost all patients presenting to the TTC received RT-PCR testing. Fourth, patients filled out a questionnaire regarding symptoms and comorbidities by themselves. In 86 cases, for example, seizure was reported as a symptom. Therefore, response bias is likely. Fifth, we did not systematically assess hyposmia/anosmia or hypogeusia/ageusia as a predictor of COVID-19 positivity [35], as these predictors were not generally accepted on March 8th, when the study and the test process was designed. Sixth, we did not formally assess reasons for overruling. It can be speculated that patients who were deemed low risk (PDSR ≤ 2) but reported symptoms prompting further evaluation (e.g., dyspnea, chest pain) had their vital signs taken and were evaluated by medical doctor in charge. Seventh, we relied upon RT-PCR as the criterion standard for the diagnosis of COVID-19, which is an imperfect gold standard. The possibility of false-negative or false-positive RT-PCR results needs to be considered, especially as the pre-test probability or estimated risk of disease before testing was not formally assessed in our study [36], but was rather low. Moreover, the false-negative rate appears to be dependent on symptom duration [37] and the pre-analytical quality of the swabs. Our procedure to routinely combine nasopharyngeal with oropharyngeal swabs into one transport medium (as NOPS) is likely to increase the preanalytical quality of sampling and to reduce the number of false negatives. This is also supported by our two-target approach showing 97.5% to 99% concordance reflecting the very high SARS-CoV-2 loads in the transport medium of 10 million copies/mL (median 7.2 log10 copies/mL, IQR 5.8–8.4). Eighth, 13 COVID-19 positive patients were lost to follow-up. In addition, we could have missed other patients being tested positive at a later time point. However, as we are the only general public hospital in our Canton (State), and nearly all patients with possible COVID-19 symptoms presented or were referred to our hospital, other relevant missing data is unlikely, but possible. Family physicians, at that time, were not equipped with sufficient personal protective gear and were therefore reluctant to see COVID-19 patients. Further, local health authorities had a tight control on all cases who were tested positive. Finally, national health authorities did not endorse the low-threshold testing; instead, testing was recommended for patients with severe symptoms only. However, the overall positivity rates were comparable to a national average of roughly 14% in the period of testing [38].
6. Conclusions
Our study shows that installing a low-threshold walk-in TTC near the ED of a tertiary care hospital appears to be safe and is feasible. A clinical diagnosis of COVID-19 appears virtually impossible, given the only moderate associations of clinical predictors with SARS-CoV-2 positive PCR test results.
Author Contributions
Conceptualization, C.H.N.; methodology, G.M., R.B. and C.H.N.; validation, G.M., R.B. and C.H.N.; formal analysis, G.M., R.B. and C.H.N.; writing—original draft preparation, G.M., R.B. and C.H.N.; visualization, G.M., R.B. and C.H.N.; supervision, G.M., R.B. and C.H.N.; project administration, G.M., R.B. and C.H.N.; investigation, G.M., M.R., A.F.W., S.T.-S., M.B., J.H., K.K.S., A.E., B.S., K.L., H.H.H., A.M., T.B., M.M., R.B. and C.H.N.; resources, G.M., M.R., A.F.W., S.T.-S., M.B., J.H., K.K.S., A.E., B.S., K.L., H.H.H., A.M., T.B., M.M., R.B. and C.H.N.; writing—review and editing, G.M., M.R., A.F.W., S.T.-S., M.B., J.H., K.K.S., A.E., B.S., K.L., H.H.H., A.M., T.B., M.M., R.B. and C.H.N.; data curation, G.M.; funding acquisition, R.B. and C.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by scientific funds from the UHBS.
Acknowledgments
The authors wish to thank medical students of the University of Basel and the physicians working in the Triage and Test Center; Simon Fuchs and Carla Schaubhut (Cantonal Health Office); Sebastian Kölliker, Otto Schmid, and Artur Stickel (Organization of the Triage and Test-Center); Marc Dangel, Klaus Baumgartl, and Andreas Schötzau for statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Overruling of patient presentations.
Table A1.
Overruling of patient presentations.
| PDSR | Total Presentations | Vitals Measured | Vitals not Neasured |
|---|---|---|---|
| PDSR 1 | 2041 | 223 1 | 1818 2 |
| PDSR 2 | 1522 | 346 3 | 1176 4 |
| PDSR ≤2 | 3563 | 569 (16%) | 2994 (84%) |
| PDSR 3 | 1055 | 1037 5 | 18 6 |
| PDSR 4 | 155 | 151 7 | 4 8 |
| PDSR 5 | 36 | 35 9 | 1 10 |
| PDSR 6 | 6 | 6 11 | 0 |
| PDSR >2 | 1252 | 1229 (98.2%) | 23 (1.8%) |
1 of which 9 were transferred to ED for further work-up, 9 outpatient treatment; 2 of which 5 were transferred to ED for further work-up, 3 outpatient treatment, 2 hospitalized; 3 of which 38 were transferred to ED for further work-up, 32 outpatient treatment, 6 hospitalized; 4 of which 2 were transferred to ED for further work-up, 1 outpatient treatment, 1 hospitalized; 5 of which 121 were transferred to ED for further work-up, 105 outpatient treatment, 16 hospitalized; 6 of which 3 were transferred to ED for further work-up, 1 outpatient treatment, 2 hospitalized; 7 of which 39 were transferred to ED for further work-up, 31 outpatient treatment, 8 hospitalized; 8 of which 3 were transferred to ED for further work-up, 1 outpatient treatment, 2 hospitalized; 9 of which 9 were transferred to ED for further work-up, 6 outpatient treatment, 3 hospitalized; 10 of which 1 were transferred to ED for further work-up, 1 outpatient treatment; 11 of which 2 were transferred to ED for further work-up, 1 outpatient treatment, 1 hospitalized; PDSR = Physician Disease Severity Rating; ED = Emergency Department.

Table A2.
Characteristics stratified by disposition status for all presentations (related and unrelated to COVID-19).
Table A2.
Characteristics stratified by disposition status for all presentations (related and unrelated to COVID-19).
| All (n = 4815) | Discharged (n = 4774) | Hospitalized (n = 41) | OR [95% CI] | p-Value | |
|---|---|---|---|---|---|
| Age (years, median [min;max]) | 41.6 [13.4;93.3] | 41.4 [13.4;93.3] | 62.1 [21.2;90.7] | 1.08 [1.06;1.10] | <0.001 |
| Male | 2188 (45.4%) | 2162 (45.3%) | 26 (63.4%) | Ref. | Ref. |
| Female | 2627 (54.6%) | 2612 (54.7%) | 15 (36.6%) | 0.48 [0.25;0.90] | 0.022 |
| PDSR≤2 PDSR>2 | 3563 (74%) 1252 (26%) | 3554 (74.4%) 1220 (25.6%) | 9 (22.0%) 32 (78%) | Ref. 10.2; [5.05;23.00] | Ref. <0.001 |
| Symptoms | |||||
| Duration of symptoms (days, median [min;max]) 1 | 4 [1;119] | 4 [1;119] | 6.5 [1;27] | 1.01 [0.98;1.04] | 0.671 |
| Measured/reported fever | 2012 (41.8%) | 1989 (41.7%) | 23 (56.1%) | 1.79 [0.96;3.37] | 0.067 |
| Chills 2 | 1025 (23.8%) | 1008 (23.6%) | 17 (41.5%) | 2.30 [1.20;4.28] | 0.012 |
| Myalgia | 1859 (38.6%) | 1840 (38.5%) | 19 (46.3%) | 1.38 [0.73;2.56] | 0.313 |
| Lymphadenopathy | 491 (10.2%) | 484 (10.1%) | 7 (17.1%) | 1.86 [0.74;3.98] | 0.170 |
| Headache | 2693 (55.9%) | 2671 (55.9%) | 22 (53.7%) | 0.91 [0.49;1.71] | 0.768 |
| Seizure | 86 (1.79%) | 52 (1.7%) | 4 (9.8%) | 6.39 [1.84;16.5] | 0.007 |
| Confusion | 509 (10.6%) | 499 (10.5%) | 10 (24.4%) | 2.79 [1.28;5.55] | 0.011 |
| Nausea | 668 (13.9%) | 650 (13.6%) | 18 (43.9%) | 4.97 [2.62;9.27] | <0.001 |
| Conjunctivitis | 469 (9.7%) | 465 (9.7%) | 4 (9.8%) | 1.04 [0.30;2.61] | 0.946 |
| Exanthema | 244 (5.1%) | 239 (5%) | 5 (12.2%) | 2.71 [0.91;6.38] | 0.070 |
| Coryza | 2575 (53.5%) | 2557 (53.6%) | 18 (43.9%) | 0.68 [0.36;1.26] | 0.223 |
| Otalgia | 749 (15.6%) | 745 (15.6%) | 4 (9.8%) | 0.61 [0.18;1.52] | 0.313 |
| Sore throat | 2694 (56%) | 2679 (56.1%) | 15 (36.6%) | 0.45 [0.23;0.85] | 0.013 |
| Dyspnea | 1158 (24%) | 1139 (23.9%) | 19 (46.3%) | 2.76 [1.47;5.14] | 0.002 |
| Wheezing | 675 (14%) | 664 (13.9%) | 11 (26.8%) | 2.29 [1.09;4.48] | 0.031 |
| Cough | 3132 (65%) | 3106 (65.1%) | 26 (63.4%) | 0.93 [0.49;1.80] | 0.817 |
| Productive cough | 1119 (23.2%) | 1104 (23.1%) | 15 (36.6%) | 1.93 [0.99;3.62] | 0.054 |
| Hemoptysis | 53 (1.1%) | 52 (1.1%) | 1 (2.4%) | 2.58 [0.11;12.1] | 0.440 |
| Chest pain | 1335 (27.7%) | 1324 (27.7%) | 11 (26.8%) | 0.96 [0.46;1.88] | 0.919 |
| Abdominal pain | 664 (13.8%) | 653 (13.7%) | 11 (26.8%) | 2.33 [1.11;4.56] | 0.027 |
| Diarrhea | 842 (17.5%) | 833 (17.4%) | 9 (22%) | 1.35 [0.60;2.73] | 0.448 |
| Dysuria | 184 (3.8%) | 180 (3.8%) | 4 (9.8%) | 2.85 [0.83;7.24] | 0.089 |
| Exhausted | 2131 (44.3%) | 2105 (44.1%) | 26 (63.4%) | 2.19 [1.17;4.26] | 0.014 |
| Weakness 2 | 1901 (44.1%) | 1873 (43.9%) | 28 (68.3%) | 2.73 [1.44;5.49] | 0.002 |
| Comorbidities | |||||
| Regular medication | 1695 (35.2%) | 1673 (35%) | 22 (53.7%) | 2.14 [1.15;4.02] | 0.016 |
| Neurologic disease | 112 (2.3%) | 109 (2.3%) | 3 (7.3%) | 3.54 [0.82;10.0] | 0.084 |
| Hypertension | 648 (13.5%) | 638 (13.4%) | 10 (24.4%) | 2.11 [0.97;4.20] | 0.058 |
| Diabetes | 152 (3.2%) | 142 (3%) | 10 (24.4%) | 10.6 [4.83;21.4] | <0.001 |
| Overweight | 705 (14.6%) | 693 (14.5%) | 12 (29.3%) | 2.46 [1.19;4.73] | 0.016 |
| Heart disease | 232 (4.8%) | 228 (4.8%) | 4 (9.8%) | 2.23 [0.65;5.64] | 0.178 |
| Pulmonary disease | 221 (4.6%) | 218 (4.6%) | 3 (7.3%) | 1.73 [0.40;4.84] | 0.407 |
| Asthma | 607 (12.6%) | 604 (12.7%) | 3 (7.3%) | 0.57 [0.13;1.59] | 0.317 |
| Chronic obstructive pulmonary disease | 67 (1.4%) | 67 (1.4%) | 0 (0%) | ||
| Renal disease | 81 (1.7%) | 74 (1.6%) | 7 (17.1%) | 13.3 [5.20;29.4] | <0.001 |
| Liver disease | 86 (1.8%) | 84 (1.8%) | 2 (4.9%) | 3.07 [0.46;10.2] | 0.020 |
| Hematological disease | 39 (0.81%) | 37 (0.8%) | 2 (4.9%) | 7.01 [1.03;24.2] | 0.047 |
| HIV infection | 31 (0.6%) | 31 (0.6%) | 0 (0%) | ||
| Rheumatological disease | 231 (4.8%) | 229 (4.8%) | 2 (4.9%) | 1.09 [0.16;3.59] | 0.907 |
| Cancer | 118 (2.5%) | 113 (2.4%) | 5 (12.2%) | 5.88 [1.96;14.0] | 0.003 |
| Vital signs | |||||
| Systolic blood pressure (mmHg, median [min;max]) 3 | 141 [95;222] | 141 [95;222] | 137 [102;165] | 1.01 [0.99;1.03] | 0.151 |
| Diastolic blood pressure (mmHg, median [min;max])3 | 82 [43;130] | 82 [43;130] | 76.5 [47;106] | 1.04 [1.01;1.07] | 0.008 |
| Heart rate (beats per minute, median [min;max]) 4 | 83 [44;141] | 83 [44;141] | 93 [65;136] | 1.05 [1.02;1.07] | <0.001 |
| Respiratory rate (breath per minute, median [min;max]) 5 | 17 [6;33] | 17 [6;33] | 20 [12;26] | 1.23 [1.13;1.34] | <0.001 |
| Oxygen saturation (median [min;max]) 6 | 98 [90;100] | 98 [90;100] | 96 [92;99] | 1.89 [1.58;2.26] | <0.001 |
| Temperature (°C, median [min;max]) 6 | 36.8 [35;39.5] | 36.7 [35;39.5] | 37.2 [36;39.5] | 3.73 [2.44;5.71] | <0.001 |
1 data available for 4402 presentations, 2 data available for 4307 presentations, 3 data available for 1788 presentations, 4 data available for 1797 presentations, 5 data available for 1782 presentations, 6 data available for 1798 presentations; COVID-19 = coronavirus disease 19; OR = Odds Ratio; CI = Confidence Interval; Ref. = Reference; PDSR = Physician Disease Severity Rating.
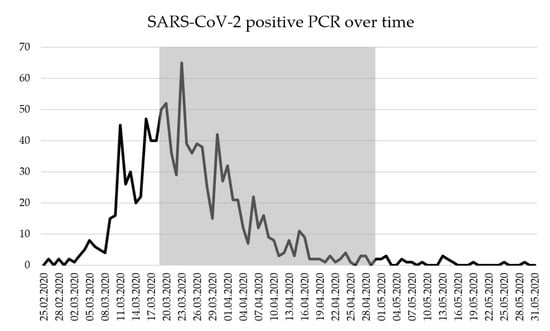
Figure A1.
SARS-CoV-2 positive PCR during study period from 19th March to 29th April 2020 (gray marking); SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; PCR = Polymerase Chain Reaction.
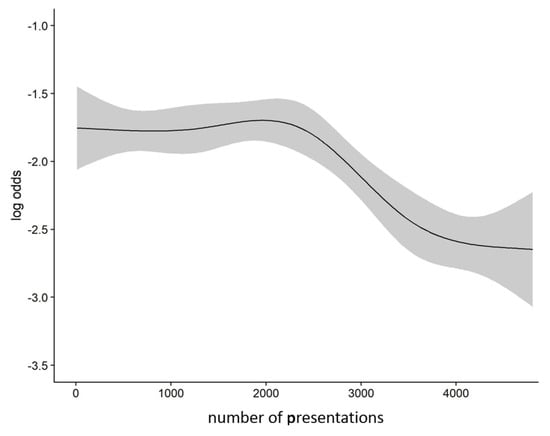
Figure A2.
Odds for positive SARS-CoV-2-testing during study period; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
References
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Salathé, M.; Althaus, C.L.; Neher, R.; Stringhini, S.; Hodcroft, E.; Fellay, J.; Zwahlen, M.; Senti, G.; Battegay, M.; Wilder-Smith, A.; et al. COVID-19 epidemic in Switzerland: On the importance of testing, contact tracing and isolation. Swiss Med. Wkly. 2020, 150, w20225. [Google Scholar] [CrossRef] [PubMed]
- Bingisser, R.; Baerlocher, S.M.; Kuster, T.; Ortega, R.N.; Nickel, C.H. Physicians’ Disease Severity Ratings are Non-Inferior to the Emergency Severity Index. J. Clin. Med. 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Nickel, C.H.; Kellett, J.; Ortega, R.N.; Lyngholm, L.; Hanson, S.; Cooksley, T.; Bingisser, R.; Brabrand, M. A simple prognostic score predicts one-year mortality of alert and calm emergency department patients: A prospective two-center observational study. Int. J. Clin. Pr. 2020, 74, e13481. [Google Scholar] [CrossRef] [PubMed]
- Kellett, J.; Nickel, C.H.; Skyttberg, N.; Brabrand, M. Is it possible to quickly identify acutely unwell patients who can be safely managed as outpatients? The need for a "Universal Safe to Discharge Score". Eur. J. Intern. Med. 2019, 67, e13–e15. [Google Scholar] [CrossRef]
- Nickel, C.H.; Kellett, J.; Ortega, R.N.; Lyngholm, L.; Wasingya-Kasereka, L.; Brabrand, M.; Lyngholm, H. Mobility Identifies Acutely Ill Patients at Low Risk of In-Hospital Mortality: A Prospective Multicenter Study. Chest 2019, 156, 316–322. [Google Scholar] [CrossRef]
- Brabrand, M.; Kellett, J.; Opio, M.; Cooksley, T.; Nickel, C.H. Should impaired mobility on presentation be a vital sign? Acta Anaesthesiol. Scand. 2018, 62, 945–952. [Google Scholar] [CrossRef]
- Shah, A.; Challener, D.; Tande, A.J.; Mahmood, M.; O’Horo, J.C.; Berbari, E.; Crane, S.J. Drive-Through Testing. Mayo Clin. Proc. 2020, 95, 1420–1425. [Google Scholar] [CrossRef]
- Klasen, J.M.; Meienberg, A.; Nickel, C.; Bingisser, R. SWAB team instead of SWAT team: Medical students as a frontline force during the COVID-19 pandemic. Med. Educ. 2020, 54, 860. [Google Scholar] [CrossRef]
- Leuzinger, K.; Roloff, T.; Gosert, R.; Sogaard, K.; Naegele, K.; Rentsch, K.; Bingisser, R.; Nickel, C.H.; Pargger, H.; Bassetti, S.; et al. Epidemiology of SARS-CoV-2 Emergence Amidst Community-Acquired Respiratory Viruses. J. Infect. Dis. 2020, 222, 1270–1279. [Google Scholar] [CrossRef]
- Leuzinger, K.; Gosert, R.; Sogaard, K.; Naegele, K.; Bielicki, J.; Roloff, T.; Bingisser, R.; Nickel, C.H.; Khanna, N.; Tschudin, S.; et al. Epidemiology and precision of SARS-CoV-2 detection following lockdown and relaxation measures. medRxiv. (under review).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. SSRN Electron. J. 2020, 395, 10223. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K.; Gupta, A. Encephalopathy in patients with COVID-19: A review. J. Med. Virol. 2020, 1–17. [Google Scholar] [CrossRef]
- Bingisser, R.; Dietrich, M.; Ortega, R.N.; Malinovska, A.; Bosia, T.; Nickel, C. Systematically assessed symptoms as outcome predictors in emergency patients. Eur. J. Intern. Med. 2017, 45, 8–12. [Google Scholar] [CrossRef]
- Caterino, J.M.; Kline, D.M.; Leininger, R.; Southerland, L.T.; Carpenter, C.R.; Baugh, C.W.; Pallin, D.J.; Hunold, K.M.; Stevenson, K.B. Nonspecific Symptoms Lack Diagnostic Accuracy for Infection in Older Patients in the Emergency Department. J. Am. Geriatr. Soc. 2018, 67, 484–492. [Google Scholar] [CrossRef]
- Karakoumis, J.; Nickel, C.H.; Kirsch, M.; Rohacek, M.; Geigy, N.; Müller, B.; Ackermann, S.; Bingisser, R. Emergency Presentations With Nonspecific Complaints—The Burden of Morbidity and the Spectrum of Underlying Disease. Medicine 2015, 94, e840. [Google Scholar] [CrossRef]
- Nickel, C.H.; Bingisser, R. Mimics and chameleons of COVID-19. Swiss Med. Wkly. 2020, 150, w20231. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, A.C.; Hardwick, E.H.; Pius, R.; Norman, L.; Holden, A.K.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Y.; Wang, Y.; Duan, G.; Yang, H. Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef]
- Pinto, L.C.; Bertoluci, M.C. Type 2 diabetes as a major risk factor for COVID-19 severity: A meta-analysis. Arch. Endocrinol. Metab. 2020, 64, 199–200. [Google Scholar] [CrossRef]
- Klang, E.; Kassim, G.; Soffer, S.; Freeman, R.; Levin, M.A.; Reich, D.L. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than. Obesity 2020, 28, 1595–1599. [Google Scholar] [CrossRef]
- Drager, L.F.; Pio-Abreu, A.; Lopes, R.D.; Bortolotto, L.A. Is Hypertension a Real Risk Factor for Poor Prognosis in the COVID-19 Pandemic? Curr. Hypertens. Rep. 2020, 22, 43. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, W.; Guo, E.; Liu, J.; Yang, B.; Wu, P.; Lin, S.; Peng, T.; Fu, Y.; Li, F.; et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: A propensity score-matched analysis. J. Hematol. Oncol. 2020, 13, 75. [Google Scholar] [CrossRef]
- Russo, V.; Bottino, R.; Carbone, A.; Rago, A.; Papa, A.A.; Golino, P.; Nigro, G. COVID-19 and Heart: From Clinical Features to Pharmacological Implications. J. Clin. Med. 2020, 9, 1944. [Google Scholar] [CrossRef]
- Pallotto, C.; Suardi, L.R.; Esperti, S.; Tarquini, R.; Grifoni, E.; Simone, M.; Valoriani, A.; di Martino, S.; Cei, F.; Sisti, E.; et al. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): A retrospective multicentre study. Infect Dis. 2020, 52, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Ahn, H.; Ryu, H.S.; Kim, B.-J.; Jang, J.; Jung, M.; Kim, J.; Jeong, S.H. Clinical Characteristics and Disease Progression in Early-Stage COVID-19 Patients in South Korea: The Retrospective Cohort Study. SSRN Electron. J. 2020, 9, 1959. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Mudd, P.; West, C.P.; Wilber, E.; Wilber, S.T. Diagnosing COVID-19 in the Emergency Department: A Scoping Review of Clinical Exam, Labs, Imaging Accuracy and Biases. Acad. Emerg. Med. 2020, 27, 653–670. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.-M.; et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Watson, J.; Whiting, P.F.; E Brush, J. Interpreting a covid-19 test result. BMJ 2020, 369, m1808. [Google Scholar]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]
- Bundesamt für Gesundheit BAG, D.Ö.G. 7.5.2020, Zwischenbilanz zum neuen Coronavirus in der Schweiz und im Fürstentum Liechtenstein. 2020 07.05.2020 Coronavirus Krankheit 2019 (COVID-19). Available online: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/k-und-i/aktuelle-ausbrueche-pandemien/2019-nCoV/covid-19-zwischenbilanz-mai-2020.pdf.download.pdf/BAG_Epidemiologische_Zwischenbilanz_zum_neuen_Coronavirus.pdf (accessed on 5 July 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).