Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. SMH Displacement or Regression Rate
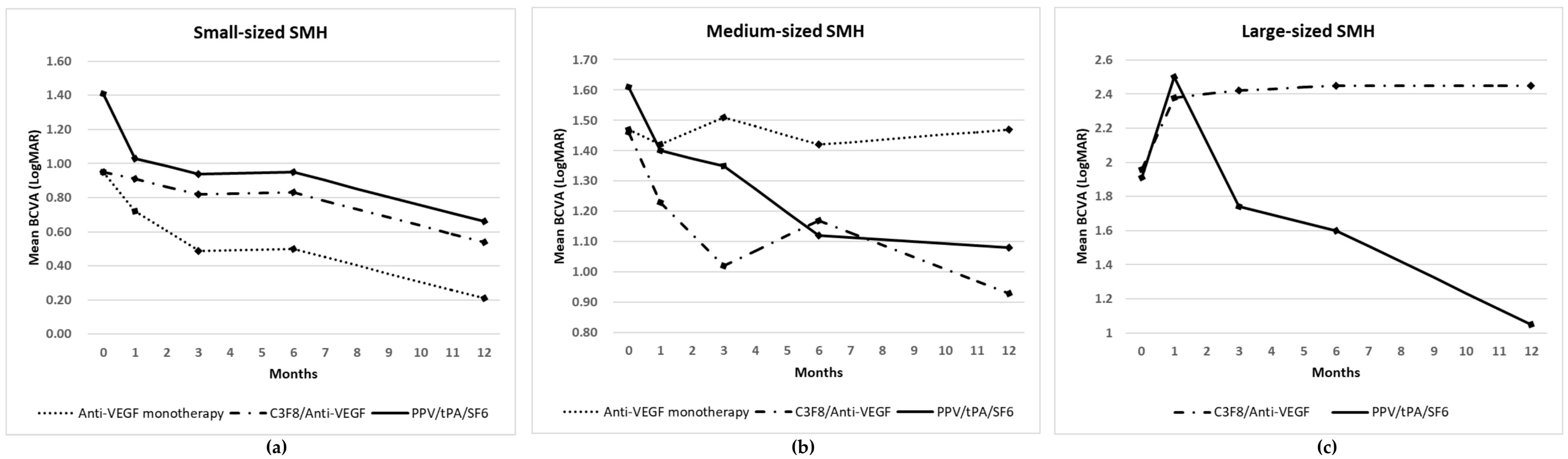
3.3. Visual Outcomes
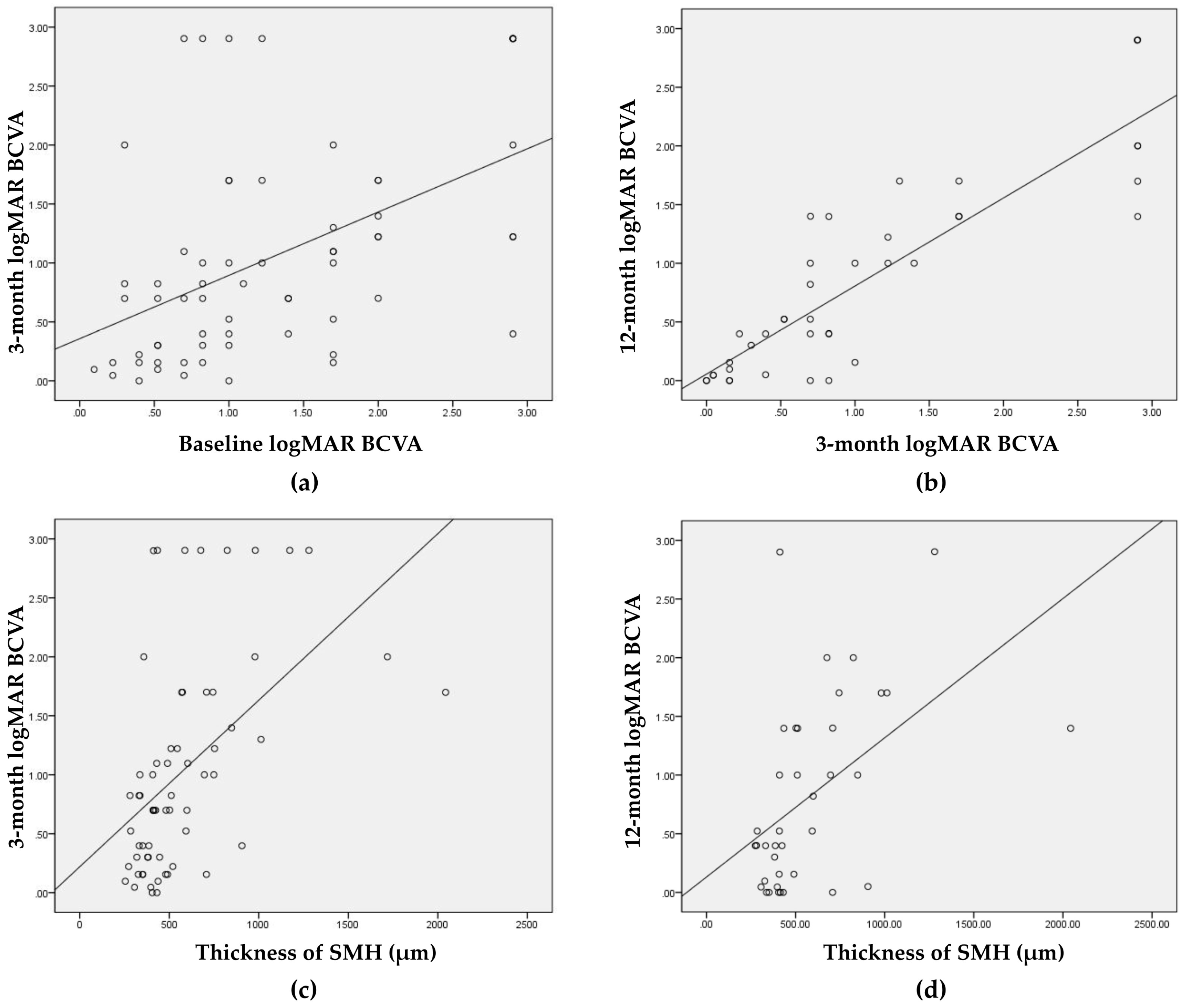
3.4. Factors Associated with Visual Outcomes
3.5. Frequency of Additional Anti-VEGF Injections
3.6. Complications
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Glatt, H.; Machemer, R. Experimental subretinal hemorrhage in rabbits. Am. J. Ophthalmol. 1982, 94, 762–773. [Google Scholar] [PubMed]
- Avery, R.L.; Fekrat, S.; Hawkins, B.S.; Bressler, N.M. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996, 16, 183–189. [Google Scholar] [PubMed]
- Rijken, D.C. Plasminogen activators and plasminogen activator inhibitors: Biochemical aspects. Bailliere Clin. Haematol. 1995, 8, 291–312. [Google Scholar]
- Soliman, W.; Lund-Andersen, H.; Larsen, M. Resolution of subretinal haemorrhage and fluid after intravitreal bevacizumab in aggressive peripapillary subretinal neovascularization. Acta Ophthalmol. Scand. 2006, 84, 707–708. [Google Scholar]
- Stanescu-Segall, D.; Balta, F.; Jackson, T.L. Submacular hemorrhage in neovascular age-related macular degeneration: A synthesis of the literature. Surv. Ophthalmol. 2016, 61, 18–32. [Google Scholar]
- Chang, M.A.; Do, D.V.; Bressler, S.B.; Cassard, S.D.; Gower, E.W.; Bressler, N.M. Prospective one-year study of ranibizumab for predominantly hemorrhagic choroidal neovascular lesions in age-related macular degeneration. Retina 2010, 30, 1171–1176. [Google Scholar]
- Cho, H.J.; Koh, K.M.; Kim, H.S.; Lee, T.G.; Kim, C.G.; Kim, J.W. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2013, 156, 524–531. [Google Scholar]
- Spaide, R.F.; Fisher, Y.L. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006, 26, 275–278. [Google Scholar]
- Shienbaum, G.; Garcia Filho, C.A.; Flynn, H.W., Jr.; Nunes, R.P.; Smiddy, W.E.; Rosenfeld, P.J. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am. J. Ophthalmol. 2013, 155, 1009–1013. [Google Scholar]
- Irvine, W.D.; Johnson, M.W.; Hernandez, E.; Olsen, K.R. Retinal toxicity of human tissue plasminogen activator in vitrectomized rabbit eyes. Arch. Ophthalmol. 1991, 109, 718–722. [Google Scholar]
- Hassan, A.S.; Johnson, M.W.; Schneiderman, T.E.; Regillo, C.D.; Tornambe, P.E.; Poliner, L.S.; Blodi, B.A.; Elner, S.G. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology 1999, 106, 1900–1906. [Google Scholar] [PubMed]
- Johnson, M.W.; Olsen, K.R.; Hernandez, E. Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Retina 1991, 11, 250–258. [Google Scholar] [PubMed]
- Ohji, M.; Saito, Y.; Hayashi, A.; Lewis, J.M.; Tano, Y. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch. Ophthalmol. 1998, 116, 1326–1332. [Google Scholar] [PubMed]
- Stifter, E.; Michels, S.; Prager, F.; Georgopoulos, M.; Polak, K.; Hirn, C.; Schmidt-Erfurth, U. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am. J. Ophthalmol. 2007, 144, 886–892. [Google Scholar] [PubMed]
- Al-Hity, A.; Steel, D.H.; Yorston, D.; Gilmour, D.; Koshy, Z.; Young, D.; Hillenkamp, J.; McGowan, G. Incidence of submacular haemorrhage (SMH) in Scotland: A Scottish Ophthalmic Surveillance Unit (SOSU) study. Eye 2019, 33, 486–491. [Google Scholar] [PubMed]
- Chang, Y.S.; Kim, J.H.; Kim, J.W.; Kim, C.G.; Lee, D.W. Development of submacular hemorrhage in neovascular age-related macular degeneration: Influence on visual prognosis in a clinical setting. Korean J. Ophthalmol. 2018, 32, 361–368. [Google Scholar]
- Klettner, A.; Groteluschen, S.; Treumer, F.; Roider, J.; Hillenkamp, J. Compatibility of recombinant tissue plasminogen activator (rtPA) and aflibercept or ranibizumab coapplied for neovascular age-related macular degeneration with submacular haemorrhage. Br. J. Ophthalmol. 2015, 99, 864–869. [Google Scholar]
- Kim, J.H.; Chang, Y.S.; Kim, J.W.; Kim, C.G.; Yoo, S.J.; Cho, H.J. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology 2014, 121, 926–935. [Google Scholar]
- Sacu, S.; Stifter, E.; Vecsei-Marlovits, P.V.; Michels, S.; Schutze, C.; Prunte, C.; Schmidt-Erfurth, U. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye 2009, 23, 1404–1410. [Google Scholar]
- Cho, H.J.; Koh, K.M.; Kim, J.H.; Kim, H.S.; Han, J.I.; Lew, Y.J.; Lee, T.G.; Kim, J.W. Intravitreal ranibizumab injections with and without pneumatic displacement for treating submacular hemorrhage secondary to neovascular age-related macular degeneration. Retina 2015, 35, 205–212. [Google Scholar]
- Shin, J.Y.; Lee, J.M.; Byeon, S.H. Anti-vascular endothelial growth factor with or without pneumatic displacement for submacular hemorrhage. Am. J. Ophthalmol. 2015, 159, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, J.M.; Sherman, M.P.; Barr, C.C.; Schaal, S. Tissue plasminogen activator for subfoveal hemorrhage due to age-related macular degeneration: Comparison of 3 treatment modalities. Retina 2016, 36, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Treumer, F.; Wienand, S.; Purtskhvanidze, K.; Roider, J.; Hillenkamp, J. The role of pigment epithelial detachment in AMD with submacular hemorrhage treated with vitrectomy and subretinal co-application of rtPA and anti-VEGF. Graefe Arch. Clin. Exp. Ophthalmol. 2017, 255, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Klais, C.; Koch, F.H.; Gumbel, H.O. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology 2001, 108, 1485–1492. [Google Scholar] [CrossRef]
- Schulze, S.D.; Hesse, L. Tissue plasminogen activator plus gas injection in patients with subretinal hemorrhage caused by age-related macular degeneration: Predictive variables for visual outcome. Graefe Arch. Clin. Exp. Ophthalmol. 2002, 240, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Gerding, H.; Loukopoulos, V.; Riese, J.; Hefner, L.; Timmermann, M. Results of flexible ranibizumab treatment in age-related macular degeneration and search for parameters with impact on outcome. Graefe Arch. Clin. Exp. Ophthalmol. 2011, 249, 653–662. [Google Scholar] [CrossRef]
- Treumer, F.; Roider, J.; Hillenkamp, J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br. J. Ophthalmol. 2012, 96, 708–713. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, J.B.; Kim, J.E.; Thordsen, J.; Dayani, P.; Ober, M.; Mahmoud, T.H. Pneumatic displacement of submacular hemorrhage with subretinal air and tissue plasminogen activator: Initial United States experience. Ophthalmol. Retina 2018, 2, 180–186. [Google Scholar]
- Hillenkamp, J.; Surguch, V.; Framme, C.; Gabel, V.P.; Sachs, H.G. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefe Arch. Clin. Exp. Ophthalmol. 2010, 248, 5–11. [Google Scholar] [CrossRef]
| Small-Sized SMH | Medium-Sized SMH | Large-Size dSMH | p-Value | Total | |
|---|---|---|---|---|---|
| No. of patients | 45 | 21 | 11 | 77 | |
| Mean age, years | 71.5 ± 10.9 | 74.8 ± 11.1 | 72.3 ± 9.0 | 0.164 a | 73.2 ± 10.8 |
| Sex (%) | 0.755 b | ||||
| Male | 33 (73.3) | 14 (66.7) | 7 (63.6) | 54 (70.1) | |
| Female | 12 (26.7) | 7 (33.3) | 4 (36.4) | 23 (29.9) | |
| Systemic anticoagulants (%) | 9 (20.0) | 10 (47.6) | 3 (27.4) | 0.068 b | 22 (100.0) |
| Duration of SMH, days | 16.6 ± 30.6 | 10.6 ± 19.7 | 12.4 ± 11.9 | 0.653 a | 14.3 ± 25.8 |
| Mean size of SMH, ODD | 2.45 ± 0.90 | 5.09 ± 1.27 | 7.55 ± 2.27 | <0.001 a | 3.90 ± 2.26 |
| Mean thickness of SMH, µm | 474.6 ± 149.6 | 791.9 ± 482.0 | 955.4 ± 375.9 | <0.001 a | 625.5 ± 357.7 |
| Classification of lesions (%) | 0.028 b | ||||
| CNV | 15 (33.3) | 12 (57.1) | 8 (72.7) | 35 (45.5) | |
| PCV | 30 (66.7) | 9 (42.9) | 3 (27.3) | 42 (54.5) | |
| Preoperative anti-VEGF treatment | 2 (4.4) | 3 (14.3) | 5 (45.5) | 0.005 b | 10 (13.0) |
| Lens status (%) | 0.119 b | ||||
| Phakic | 40 (88.9) | 14 (66.7) | 8 (72.7) | 62 (80.5) | |
| Pseudophakic | 5 (11.1) | 7 (33.3) | 3 (27.3) | 15 (19.5) | |
| Treatment modalities (%) b | 0.011 b | ||||
| Intravitreal anti-VEGF monotherapy | 23 (51.1) | 6 (28.6) | 0 (0.0) | 29 (37.7) | |
| Intravitreal anti-VEGF with C3F8 gas | 12 (26.7) | 9 (42.8) | 4 (36.4) | 25 (32.5) | |
| PPV with subretinal tPA and SF6 gas | 10 (22.2) | 6 (28.6) | 7 (63.6) | 23 (29.8) |
| Small-Sized SMH | Medium-Sized SMH | Large-Sized SMH | p-Value | Total | |
|---|---|---|---|---|---|
| LogMAR BCVA | |||||
| Baseline (n = 77) | 1.06 ± 0.70 | 1.5 ± 0.89 | 1.93 ± 0.84 | 0.015 a | 1.30 ± 0.83 |
| 1 month (n = 77) | 0.83 ± 0.55 | 1.33 ± 0.83 | 2.45 ± 0.94 | 0.002 a | 1.19 ± 0.87 |
| 3 months (n = 64) | 0.69 ± 0.65 | 1.26 ± 0.83 | 2.04 ± 1.05 | 0.018 a | 1.03 ± 0.95 |
| 6 months (n = 51) | 0.59 ± 0.56 | 1.24 ± 0.90 | 1.84 ± 1.11 | 0.145 a | 1.00 ± 0.93 |
| 12 months (n = 41) | 0.41 ± 0.45 | 1.14 ± 0.85 | 1.52 ± 0.89 | 0.139 a | 0.76 ± 0.83 |
| Mean follow-up period, months | 17.8 ± 19.4 | 17.7 ± 15.1 | 16.2 ± 12.9 | 0.963 a | 16.2 ± 17.4 |
| Complete displacement or regression of SMH from the fovea (%) | 37/45 (82.2) | 13/21(61.9) | 8/11(72.7) | 0.200 b | |
| Anti-VEGF monotherapy | 18/23 (78.3) | 2/6 (33.3) | – | 20/29 (69.0) | |
| Intravitreal anti-VEGF with C3F8 gas | 9/12 (75.0) | 6/9 (66.7) | 1/4 (25.0) | 16/25 (64.0) | |
| PPV with a subretinal tPA and SF6 gas | 10/10 (100.0) | 5/6 (83.3) | 6/7 (85.7) | 21/23 (91.3) | |
| Additional intravitreal anti-VEGF injections | |||||
| No. of eyes treated with anti-VEGF (%) | 30 (66.7) | 15 (71.4) | 8 (72.7) | 0.886 b | 53 (68.8) |
| No. of injections for 12 months | 3.27 ± 2.06 | 2.60 ± 1.45 | 3.00 ± 1.85 | 0.526 a | 3.04 ± 1.83 |
| Breakthrough hemorrhage (%) | 3 (6.7) | 1 (4.8) | 4 (36.4) | 0.009 b | 8 (1.04) |
| Recurrent SMH | 0 | 1 | 0 | 1 | |
| Complications | |||||
| RPE rip | 0 | 1 | 0 | 1 | |
| Macular hole | 0 | 1 | 0 | 1 | |
| Retinal detachment | 0 | 0 | 0 | 0 |
| Small-Sized SMH Group | Medium-Sized SMH Group | Large-Sized SMH Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-VEGF Monotherapy | C3F8/Anti-VEGF | PPV/tPA/SF6 | p-Value | Anti-VEGF Monotherapy | C3F8/Anti-VEGF | PPV/tPA/SF6 | p-Value | C3F8/Anti-VEGF | PPV/tPA/SF6 | p-Value | |
| (n = 23) | (n = 12) | (n = 10) | (n = 6) | (n = 9) | (n = 6) | (n = 4) | (n = 7) | ||||
| Mean size of SMH, ODD | 2.00 ± 0.80 | 2.56 ± 0.70 | 3.34 ± 0.59 | 0.001 a | 5.93 ± 2.00 | 4.73 ± 0.84 | 4.77 ± 0.39 | 0.148 a | 8.46 ± 2.23 | 7.03 ± 2.30 | 0.345 b |
| Mean thickness of SMH, μm | 431.6 ± 131.0 | 541.4 ± 194.7 | 493.3 ± 100.7 | 0.088 a | 669.6 ± 470.0 | 794.6 ± 420.3 | 910.0 ± 625.2 | 0.551 a | 1006.4 ± 259.6 | 921.4 ± 458.7 | 0.450 b |
| Duration of SMH, days | 21.4 ±39.1 | 8.3 ± 9.2 | 16.4 ± 27.3 | 0.370 a | 28.6 ± 35.8 | 4.7 ± 1.9 | 4.5 ± 3.4 | 0.067 | 17.8 ± 14.2 | 9.3 ± 10.2 | 0.214 b |
| LogMAR visual acuity (No.) | |||||||||||
| Baseline | 0.95 ± 0.62 (23) | 0.95 ± 0.59 (12) | 1.41 ± 0.89 (10) | 0.282 a | 1.47 ± 1.22 (6) | 1.46 ± 0.93 (9) | 1.61 ± 0.49 (6) | 0.703 a | 1.96 ± 1.09 (4) | 1.91 ± 0.76 (7) | 0.923 b |
| 1 month | 0.72 ± 0.57 (23) | 0.91 ± 0.62 (12) | 1.03 ± 0.31 (10) | 0.036 a | 1.42 ± 1.18 (6) | 1.23 ± 0.85 (9) | 1.40 ± 0.39 (6) | 0.756 a | 2.38 ± 1.03 (4) | 2.50 ± 0.97 (7) | 0.879 b |
| 3 months | 0.49 ± 0.41 (20) | 0.82 ± 0.77 (9) | 0.99 ± 0.85 (9) | 0.266 a | 1.51 ± 1.30 (5) | 1.02 ± 0.66 (7) | 1.35 ± 0.47 (5) | 0.679 a | 2.42 ± 0.95 (4) | 1.74 ± 1.13 (5) | 0.421 b |
| 6 months | 0.50 ± 0.45 (16) | 0.73 ± 0.65 (6) | 0.71 ± 0.79 (6) | 0.850 a | 1.42 ± 1.39 (5) | 1.17 ± 0.70 (6) | 1.12 ± 0.54 (4) | 0.779 a | 2.45 ± 0.63 (3) | 1.60 ± 1.22 (5) | 0.417 b |
| 12 months | 0.21 ± 0.22 (10) | 0.39 ± 0.41 (6) | 0.83 ± 0.62 (5) | 0.209 a | 1.47 ± 1.25 (4) | 0.93 ± 0.75 (5) | 1.08 ± 0.62 (4) | 0.818 a | 2.45 ± 0.95 (3) | 1.05 ± 0.58 (4) | 0.064 b |
| Mean follow-up period, months | 16.3 ± 15.6 | 15.3 ± 19.9 | 24.1 ± 26.6 | 0.512 a | 20.3 ± 20.9 | 14.3 ± 13.0 | 20.2 ± 12.8 | 0.695 a | 18.0 ± 13.7 | 15.1 ± 13.4 | 0.744 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Park, D.-G.; Sagong, M. Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities. J. Clin. Med. 2020, 9, 3088. https://doi.org/10.3390/jcm9103088
Jeong S, Park D-G, Sagong M. Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities. Journal of Clinical Medicine. 2020; 9(10):3088. https://doi.org/10.3390/jcm9103088
Chicago/Turabian StyleJeong, Seongyong, Dong-Geun Park, and Min Sagong. 2020. "Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities" Journal of Clinical Medicine 9, no. 10: 3088. https://doi.org/10.3390/jcm9103088
APA StyleJeong, S., Park, D.-G., & Sagong, M. (2020). Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities. Journal of Clinical Medicine, 9(10), 3088. https://doi.org/10.3390/jcm9103088





