Abstract
The recent advances in machine perfusion (MP) technology involve settings ranging between hypothermic, subnormothermic, and normothermic temperatures. Tissue level adenosine triphosphate (ATP) is a long-established marker of viability and functionality and is universal for all organs. In the midst of a growing number of complex clinical parameters for the quality assessment of graft prior to transplantation, a revisit of ATP may shed light on the underlying reconditioning mechanisms of different perfusion temperatures in the form of restoration of metabolic and energy status. This article aims to review and critically analyse animal and preclinical human studies (discarded grafts) during MP of three abdominal organs (liver, kidney, and pancreas) in which ATP was a primary endpoint. A selective review of recent novel reconditioning approaches relevant to mitigation of graft ischaemia-reperfusion injury via MP and for different perfusion temperatures was also conducted. With a current reiterated interest for oxygenation during MP, a re-introduction of tissue ATP levels may be valuable for graft viability assessment prior to transplantation. Further studies may help delineate the benefits of selective perfusion temperatures on organs viability.
1. Introduction
1.1. Current State of Abdominal Transplantation (Liver, Kidneys, and Pancreas)
Organ transplantation remains the most effective treatment of end-stage organ failure. The use and optimisation of protocols in different dynamic MP technologies for abdominal organ preservation (liver, kidney, and pancreas) have been extensively studied in the last decades in single or multi-centre randomised controlled trials (RCTs) [1] and different preclinical human and animal models. In order to expand the current donor pool and match the waitlist demand, there has been an increase in organ retrieval from different donors, such as donation after brain death (DBD), donation after circulatory death (DCD), and extended criteria donor (ECD) [2]. In particular, ECD grafts are associated with poorer transplantation outcomes and there is controversy in terms of greater overall economic costs [3]. Namely, a higher proportion of patients may suffer from early graft dysfunction or delayed graft function (DGF), prolonged hospital stay, and increased morbidity in general: Biliary complications in the case of liver [4], acute renal failure after kidney transplant [5], and the need for insulin therapy in the context of pancreas transplantation [6].
The current major preservation modality is static cold storage (SCS) at 4 °C. Even though SCS is logistically simpler, there is evidence of parenchymal damage by extended periods of ischaemia and lack of oxygen with the use of this modality. If the organ is instead preserved with a dynamic preservation, there is the potential to resuscitate it and restore function, such as the recovery of metabolic energetic status in cell mitochondria, e.g., resynthesis of tissue adenosine triphosphate (ATP), reduction in reactive oxygen species (ROS), and inflammatory cytokines resulted from ischaemia-reperfusion injury (IRI). Viability assessment is also a possibility during ex vivo normothermic machine perfusion (NMP) at 37 °C, which also facilitates the surveillance of perfusion parameters, histological and metabolic analysis of biopsy samples [7] for evaluation of the extent of graft tissue injury, prediction of post-transplantation graft function, and rate of survival.
1.2. ATP Depletion and Ischaemia-Reperfusion Injury
The insights and understanding behind the underlying molecular mechanisms of IRI are important for identification of suitable biomarkers and development of different novel reconditioning approaches such as small interfering RNA (siRNA), drug delivery for attenuating pro-apoptotic molecules, and downstream signalling pathways. These interventions could be adopted during MP to counteract damage due to IRI and reduction of the risk of post-transplantation DGF, one of the well-known clinical manifestations of IRI in transplanted grafts [8].
Ischaemia, defined as ‘hypoperfusion of tissues’, is referred to as the deficiency in oxygen-carrying arterial blood supply to tissues. A depletion in oxygen can lead to a collapse of the electron transport chain in mitochondria, which is important for oxidative phosphorylation/aerobic respiration for generation of cellular ATP. It also induces anaerobic metabolism and an increase in lactic acid with a decrease in both cytoplasmic pH [9] and antioxidative agents, such as superoxide dismutases, which break down the ROS responsible of tissue damage [10]. The depletion of ATP, which is a major energy-rich phosphate source, can have detrimental effects on intracellular synthetic, catabolic, transcriptional, and transport processes and overall cellular viability. This includes clumping of nuclear chromatin, ribosome detachment impairing protein synthesis, and dysfunction of ATP-driven pumps, such as membrane sodium-potassium pumps (Na+/K+ ATPases) and reuptake of Ca2+ ions by calcium pumps (Ca2+ ATPases) on the endoplasmic reticulum and intracellular enzymatic reactions [11]. In normal physiological conditions, the cell gets rid of excess hydrogen ions (H+) by the cooperative activity of two ion pumps. Via the Na+/K+ ATPases and hydrolysis of ATP, sodium ions are pumped out of the cell in exchange for potassium ions (K+). The increase of extracellular sodium ion concentration leads to a flow of Na+ ions down the electrochemical concentration gradient into the cell via the Na+/H+ exchanger, resulting in efflux of H+ ions from the cell. On the contrary, an ischaemia-induced reduction in pH can lead to the accumulation of cellular Na+ ions, Ca2+ ions, H+ ions, with hyperosmolarity, which contributes to cellular oedema, due to flow of water into the cell. Lactic acid produced from anaerobic glucose metabolism can lead to further disruption of lysozyme membranes. Consequently, hydrolases can be released, which can destroy intracellular structures. Opening of the mitochondrial permeability transition pore (MPTP), in part due to calcium overload, is suggested to occur in parallel to these changes, which further impedes on ATP production and leads to a decrease in the metabolic energetic status of the cell [11]. Importantly, the residual tissue ATP level is closely related to the duration of SCS and an extended period of energy substrate depletion could increase the severity of IRI and subsequent clinical manifestations, e.g., acute kidney injury and early graft dysfunction [12].
The automatic solution to ischaemia was thought to be prompt reperfusion, but paradoxically, as Jennings et al. [13] revealed in canine heart models, the re-establishment of blood supply with provision of oxygen-carrying red blood cells to the ischaemic heart appeared to be associated with a greater extent of necrosis. In fact, a reintroduction of oxygen and its reaction with single electron can initiate the formation of ROS, which drives oxidative stress. Still extensively researched, it was believed that multiple events underpin reperfusion injury, for instance, the generation of free radicals (chemical species with one or more unpaired electrons) such as ROS and a decrease in antioxidative agents due to ischaemic injury can contribute to oxidative stress. Altogether, damage to DNA by ROS, induction of MPTP opening, and oxidative stress can activate downstream pro-inflammatory cytokine cascades, which leads to cell death [11]. The strategic use and benefits of oxygenation is certainly one of the most debated in the current use of MP technology, therefore this article aims to critically review the evidence of the effects of temperature on ATP levels in retrieved organs through systematic search of studies on MP in Medline, EMBASE, Cochrane Library, and Transplant Library. Animal and preclinical models discussed in this article were all subjected to oxygenated machine perfusion, unless otherwise stated.
1.3. Preservation Temperature, Metabolism, and Tissue ATP
The picture of abdominal organ transplantation (liver, kidney, and pancreas) is complex: Due to inherent differences in cell types, the resting metabolic activity and tolerance to cold/warm ischaemia of the liver, kidney, and pancreas are different to each other [14] and this needs to be taken into consideration when using ECD organs as a consequence of a broader acceptance criteria. The heterogeneity in donor age, body mass index (BMI), donor starvation due to prolonged hospitalisation in intensive care unit, and inadequate nutritional support and exercise, could be associated with lower than baseline tissue ATP levels and IRI prior to MP preservation and arrival at recipient site.
The current understanding of the relationship of temperature and metabolic rate stemmed from biochemical models, which were derived from ex vivo reactions in test tubes to studies carried out on three-dimensional organ perfusion culture systems. The rationale behind the use of hypothermic MP (HMP) for organ preservation is that by reducing the temperature, cellular metabolism will also decrease, thus reducing the use of oxygen and the rate of depletion in energy substrates, such as ATP. It was proposed that at 4 °C, the rate of metabolism is 10% of that of normal physiological temperature [15]. The van’t Hoff equation in thermodynamics suggested that at hypothermic temperature, e.g., at 4 °C, the rate of chemical reactions of interest will only be 40% of that in organs perfused under normothermic MP (NMP) at 37 °C. Similarly, the Arrhenius relation predicts that as temperature decreases, the thermal excitation of molecules also reduces [16]. The dilemma lies in the possibility that inter- and intra-molecular bonds and structures of proteins and lipids could be altered by hypothermic temperatures, which contributes to ischaemic injuries and decrease in cell viability. Equally, preservation of organs at physiological temperature (37 °C) could accelerate the rate of breakdown of ATP and shift to anaerobic glycolysis due to higher metabolic activity. Thus, there is still a lack of consensus with regards to the most suitable temperature for optimum restoration of metabolic energetic integrity and resuscitation of the organs before transplantation. Figure 1, Figure 2 and Figure 3 represent electron transport during ATP formation in SCS (Figure 1), hypothermic/subnormothermic conditions (Figure 2), and during normothermia (Figure 3).
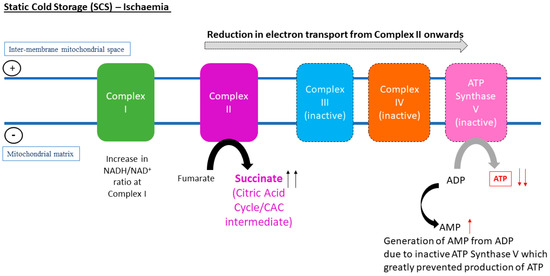
Figure 1.
In vivo metabolomic analysis of murine liver and kidneys is associated with a conserved pathway that occurs during the state of ischaemia, which was the accumulation of succinate at Complex II in the electron transport chain.
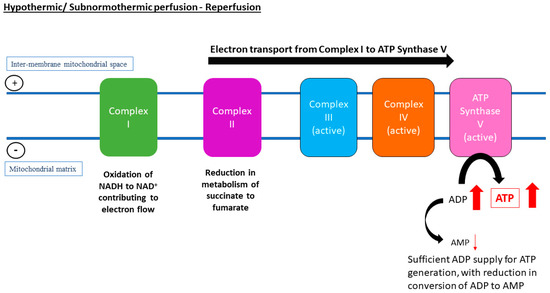
Figure 2.
Despite restoration of oxygen supply, a lower temperature during hypothermic machine perfusion (HMP) or subnormothermic machine perfusion (SNMP) of the liver seems to be associated with slower metabolic rates, thus there is a reduction in conversion of succinate to fumarate and reduction in overall electron flow through Complex I to inter-membrane mitochondrial space. Instead, the electron flow through the complexes is uninterrupted, which drives activity of adenosine triphosphate (ATP) synthase for ATP recovery. Other pathways are suggested to help resynthesise tissue adenosine diPhosphate (ADP) and ATP, such as the purine salvage pathway.
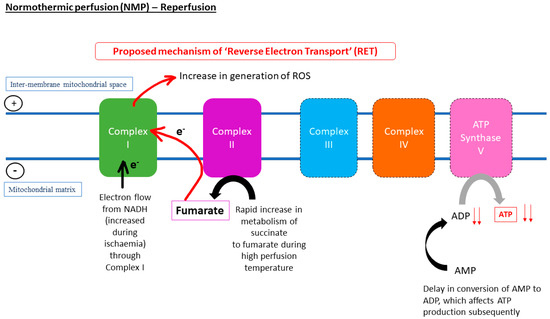
Figure 3.
The increase in metabolism of succinate upon restoration of oxygen during reperfusion might lead to reverse electron transport (RET), absence of electron flow to ATP synthase, and delay in conversion of AMP to ATP. Thus, ADP production might lead to a decline in ATP production. Following from this, in the liver, the absence of ADP due to ischaemia and the high temperature during normothermic machine perfusion (NMP) could increase the metabolism of succinate to fumarate, leading to an increase in the flow of electrons through Complex I, which hastened generation of ROS underlying reperfusion injuries.
The recent technological refinements in dynamic oxygenated machine perfusion techniques and perfusate solutions enable further investigations of the beneficial effects of perfusion at different temperatures and different durations: Hypothermia (4–10 °C), subnormothermia (25–34 °C), normothermia (37 °C), and combined approaches, e.g., hypothermic machine perfusion (HMP) with normothermic machine perfusion (NMP) [16,17] and controlled oxygenated rewarming (COR), which involves a gradual increase of temperature to subnormothermia [18].
ATP is a universal energy-rich phosphate found in cells of every human and animal organ. The expression of tissue ATP is considered to be one of the most sensitive marker for ischaemia and an increase in ATP recovery and total adenine nucleotide levels correlate with better functioning and greater viability of grafts before and after transplantation [19]. With ATP loss being a crucial marker for mitochondrial dysfunction at a cellular level and a critical event during IRI [20], there is the need to revisit the effects of different single or combined perfusion temperatures on ATP levels and novel therapeutic approaches to target IRI injuries and organ reconditioning at these temperatures by compiling findings from recent studies in animal and preclinical models of liver, kidney, and pancreas. These studies are fundamental to the development of MP in human clinical trials and will be immensely beneficial in selecting the optimum temperature for the three types of abdominal organs harvested from healthy to a variety of extended criteria marginal donors.
2. Liver Preservation
There were considerably more small (rat) and large (porcine) animal and human preclinical studies that looked into the effects of temperatures ranging from hypothermia (4, 10–12 °C), subnormothermia (20–33 °C), COR (20 or 35 °C), normothermia (37 °C), and combined perfusion approaches (HMP and NMP) on tissue ATP in the liver (Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6).

Table 1.
Characteristics and outcomes of key pre-clinical and animal study investigating HMP preservation of livers.

Table 2.
Characteristics and outcomes of key pre-clinical and animal studies investigating SNMP preservation of livers.

Table 3.
Characteristics and outcomes of key pre-clinical and animal studies investigating COR preservation of livers.

Table 4.
Characteristics and outcomes of key pre-clinical and animal studies investigating NMP preservation of livers.

Table 5.
Characteristics and outcomes of key pre-clinical and animal studies investigating the relationship of metabolism and different perfusion temperatures in the liver.

Table 6.
Characteristics and outcomes of key pre-clinical and animal studies comparing HMP versus NMP or combined approach (HMP + NMP) preservations of liver.
2.1. Should a Combined Approach of HMP and NMP Be Adopted for Maximising Resynthesis of Liver Graft ATP?
More than a decade ago, Dutkowski et al. [33] reported the positive effects of oxygenated HMP (or HOPE) at 4 °C on the repair of IRI, possibly through restoration of ATP in HMP perfused rat livers compared to the deleterious effects on ATP by SCS. Similar beneficial effects on hepatic ATP were also reported by de Rougemont et al. in a porcine DCD liver model [34]. Furthermore, in a clinical case-control study (10 dual hypothermic oxygenated MP or dual hypothermic oxygenated machine perfusion (DHOPE) vs. 20 SCS) carried out by van Rijn et al. [35], hepatic ATP was considered as one of the endpoints. Not only did liver tissue ATP increase significantly during MP, but also comparable levels of tissue ATP immediately post-DHOPE and post liver graft reperfusion in recipient patients were reported. Additionally, a significantly lower peak serum alanine aminotransferase (ALT) level and postoperative serum bilirubin concentrations (day 7) were reported in the DHOPE group. The characteristics and outcomes of preclinical human and animal studies involving oxygenated HMP and NMP are shown in Table 1 and Table 4. While HOPE demonstrated potential in the restoration of metabolic energetic status and bile production of human discarded liver grafts, Westerkamp et al. [21] argued that HOPE may not be as effective in the repair of existing hepatobiliary injuries, since there were no differences in the levels of injury markers such as aspartate aminotransferase (AST), ALT, and lactate dehydrogenase (LDH) when compared to the control group (NMP only). In a rat DCD liver model, in which livers were either subjected to 0.5 h or 1 h of warm ischaemia time (WIT), Schlegel et al. [32] reported significantly higher (p < 0.0001) ATP levels in the HOPE group, compared to NMP. Apart from the superiority of HOPE over NMP in terms of protection of mitochondrial function and maintenance of ATP levels, the survival rates were also higher in the two WIT (0.5 h or 1 h) groups with corresponding reduction in parenchymal injury. It is important to note that ex vivo NMP (37 °C) was used in both HOPE (4 h) and NMP (4 h) groups for viability assessment, therefore, it was unclear whether this may imply a longer perfusion time at 37 °C for the NMP group. In a study on discarded DCD and DBD human livers [17], the effect on tissue ATP by a combined HMP (2 h) + NMP (4 h) approach was compared against that of NMP-only group. Even though the end-perfusion tissue ATP levels in viable grafts were similar between the HMP+NMP group and the NMP-only group, during the HMP phase of the combined approach group, a median 1.8-fold increase in tissue ATP was observed. This was in line with the findings of Dutkowski et al. [33], de Rougemont et al. [34], Westerkamp et al. [21], and Schlegel et al. [32], further confirming the beneficial effects of HMP on liver tissue ATP restoration. Moreover, in Boteon et al.’s study [17], there were significant differences in ATP levels between viable and non-viable livers treated by NMP only (2.5 vs. 1.1-fold, p = 0.05), which strongly supports the role of tissue ATP as a marker of viability and further warranting its inclusion in the decision-making process of whether a liver graft should be discarded. Comparing the durations of HMP in Schlegel et al.’s study [32] in rats and Boteon et al.’s study [17] in discarded human grafts (Table 6), whether a longer period of hypothermia may subject the liver graft to a longer state of metabolic quiescence and, thus, compromising the initial increase in ATP remains an open question. Now, there appears to be a clear consensus of the beneficial effects of oxygenated HMP on resynthesis of ATP in liver grafts subjected to cold and/or warm ischaemia. Referring to findings in Schlegel et al.’s study [32], Dutkowski et al. [36] further explained that for the case of DCD livers, which were already subjected to warm ischaemia damage prior to perfusion, oxygenated HMP may facilitate the resynthesis of ATP from accumulated electron donors such as nicotinamide adenine dinucleotide (NADH) and succinate, whilst reducing the rate of electron transfer and minimizing the risk of escape of electrons from the electron transport chain in mitochondria compared to a higher normothermic temperature.
Thirteen years on, there are still limited RCTs on HMP perfused livers compared to subnormothermic machine perfusion (SNMP) and NMP, with results of three ongoing RCTs pending publication in the near future [37]. There is no clear-cut answer to whether oxygenated HMP or NMP is more preferable for liver preservation, since perfusion temperatures in both modalities demonstrated positive effects on metabolic ATP recovery in animal and preclinical models. Whether a certain temperature will be better for a specific graft subjected to long/short periods of cold ischaemia or long/short periods of warm ischaemia, respectively, is still highly debated, as demonstrated in the example of DCD livers.
2.2. Positive Effects of Subnormothermic Perfusion Temperatures (20–30 °C) Liver ATP Levels
The crux of the problem with choosing the optimal perfusion temperature for achieving the maximal benefits in intracellular metabolic status, functionality, and viability of liver grafts lies in weighing up the benefits and shortcomings (e.g., IRI injuries) associated with these temperatures. It was suggested that subnormothermic temperatures may bridge the gap between metabolic retardation during cold hypothermia and hypoxia at ‘hotter’ normal physiological temperatures, enabling the maintenance of an approximate 25% of physiological metabolic levels in the liver graft [37]. In a number of animal and preclinical studies [18,27,28,29,30,38,39] assessing the effects on resynthesis of tissue ATP by SNMP on livers of different extended criteria, DBD or DCD and steatosis were identified (Table 2, Table 3 and Table 4). Additionally, there are studies of a relatively new perfusion technique, controlled oxygenated rewarming (COR) developed by Minor et al. [18] in Essen, Germany. COR involves a slow, step-wise, and progressive increase of perfusion temperature from 4–8 °C to 20 °C [18] or higher (35 °C) [39], which helps the graft to ‘acclimatise’ to an increase in temperature during ex vivo normothermic reperfusion viability assessment (Table 5).
There is evidence of the positive effects of SNMP on liver tissue ATP in small animal models. The increase in tissue ATP was demonstrated in a rat DCD liver models [22,23] with an associated rise in one-month post-transplantation survival rate (SNMP vs. SCS; 83.3% vs. 0%) [22]. Apart from the ability of SNMP to restore livers subjected to warm ischaemia, the limitations of SNMP in rectifying IRI through regenerating ATP levels to baseline level in rat livers subjected to varying periods of cold ischaemia (24, 48, 72, and 120 h) were shown to be 72 h, equivalent to three days of SCS [24]. Taking the survival rates, a decrease in vessel resistance, and ATP recovery into consideration, 48 h of cold storage may be the limit of the protective effects of SNMP. Extrapolating the studies of SNMP in rat models to discarded human liver grafts with varying degrees of injuries, Bruinsma et al. [25] further demonstrated the effects of SNMP on ATP resynthesis and a reduction in clinical hepatic injury markers (LDH, ALT) in DBD and DCD livers. A negative correlation between tissue ATP and ALT was also demonstrated, suggesting that end-perfusion ATP levels could be effective predictors in determining whether a liver graft is sufficiently ‘repaired’ and ‘reconditioned’ prior to transplantation. In a later model by the same group [26], the underlying metabolic effects and reversal of IRI-induced damage by SNMP (21 °C) on different discarded human livers, namely, DBD, steatotic DCD, non-steatotic DCD with extended WIT (>0.5 h) and control DCD, were analysed with the use of metabolomic profiling and the use of transmission electron microscopy (TEM) for identification of possible mitochondrial ultrastructural changes, together with the monitoring of tissue ATP levels. Overall, there was a significant 4.12-fold increase in liver tissue ATP in the SNMP-treated livers. In terms of the absolute increase in ATP levels, these were highest in the DCD group with WIT < 0.5 h, followed by DCD group with extended WIT and lowest in steatotic DCD group. Since ATP synthesis was closely related to the integrity of mitochondria, the group revealed that a greater severity of structural modifications was indicative of injury, such as changes in appearances of cristae membranes, increase in size due to swelling, and deposition of amorphous material within the mitochondria in DCD (WIT > 0.5 h) and steatotic DCD groups. This study not only demonstrated the emerging role of metabolomic profiling for evaluation of the severity of injury of different extended criteria donor grafts, but also further outlined the underlying mechanisms behind SNMP reconditioning.
So far, SNMP of the liver has shown promising results in targeting major factors that predispose the organ to early graft dysfunction, such as steatosis, extended warm and cold ischaemia, or a combination of these factors. Interestingly, the Vairetti group in Italy suggested that absolute tissue ATP levels in rat liver models were significantly higher in livers perfused at 10 °C (HMP) and 20 °C (SNMP) compared to that in livers perfused at 30 °C and 37 °C (NMP) [31] (Table 5). It was proposed that a depletion in ATP might be reflected in the reduction of bile flow in livers perfused at near or normothermic temperatures. A later study by the same group [38] further demonstrated increases in ATP and glycogen levels in rat livers preserved by SNMP at 20 °C vs. SCS.
2.3. Controlled Oxygenated Rewarming (COR) on Liver Tissue ATP
In Minor et al.’s porcine study [18], COR (8 °C to 20 °C) was compared to SNMP, HMP, with SCS as a control. Comparable ATP levels were detected in COR and SNMP groups, but these were significantly higher than that in the HMP group (p < 0.05). Apart from an increase in bile production and reduction in both AST and ALT levels, the potential reconditioning effects of COR against IRI were further demonstrated in the reduction of expression of pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and enzymes involved in apoptosis, such as caspase-3. The same group [39] revealed, in a rat DCD liver model, that there were no significant differences in the increase and resynthesis of ATP and reduction in ALT between the COR20 group and COR35 group.
2.4. Novel Therapeutic Methods for Targeting IRI and Reconditioning of the Liver during HMP, SNMP, or NMP
In recent years, there was a massive surge in studies that investigated different novel methods for the repair of IRI-related changes and promotion of organ regeneration in liver transplantation, respectively [37]. The following only covers a small picture of the novel modes of treatment against IRI in animal and clinical studies. This ranges from improvements in surgical techniques, targeting downstream targets of peroxisome proliferator-activated receptor-γ (PPAR-γ) in liver [40], to ‘defatting’ steatotic livers [41] and the use of gene therapies, such as RNA interference by siRNA [42]. He et al. [43] presented a case report of an ischaemia-free liver transplantation (IFLT) surgical technique, involving the donation of a fatty liver. This new technique involves the establishment of in situ NMP in the liver during surgical procurement, in which continuous arterial supply to the liver via the coeliac artery was uninterrupted. After NMP for 270 min, the liver graft was transplanted into the recipient. Primary nonfunction, biliary complications were not reported with gradual decrease in AST and ALT throughout one week post-operatively. In mice models, increase in PPAR-γ expression was shown to be protective against cell death. Identification of FAM3A, a target gene of PPAR-γ, was reported to elevate ATP levels and subsequent activation of the Akt signaling pathway, which inhibits apoptosis, mitigating IRI-related damages in liver cells [40].
From previous reports on steatotic livers, there was interest in assessing the abilities of different ‘defatting’ chemical cocktails to stimulate breakdown of lipid droplets, decreasing overall triglyceride concentration in cells and increasing tissue ATP levels in SNMP-treated rat liver models [41].
Apoptosis or cell death is not only a crucial player in organ reperfusion injury, but also an enemy against graft viability and transplant outcome of the liver. A study reported, for the first time this year, the feasibility of introducing siRNA against the Fas receptor in perfusate solutions used during HMP or NMP. Through lipid nanoparticles-mediated transfection and subsequent endocytosis in hepatocytes of end-HMP or end-NMP perfused liver graft during the ischaemic period, there may be a window of opportunity for the repair of graft damage before transplantation [42]. siRNAs are engineered RNA oligonucleotides, and work by hybridizing to the single-stranded mRNAs transcribed from the target gene, in this case, the Fas ligand. This post-transcriptional gene silencing mechanism prevents the Fas ligand from being produced. Therefore, without binding of the Fas ligand to the FAS receptor, activation of downstream apoptotic signalling pathways will be hampered in hepatocytes.
3. Kidney Preservation
In kidneys, there were more studies on HMP compared to other perfusion modalities: Three large animal porcine studies, which researched models with characteristics of different extended criteria donors (e.g., DCD, DBD) [44,45,46] and one preclinical study [47] reporting tissue ATP as one of the primary endpoints between 2010 to 2019 were identified (Table 7).

Table 7.
Characteristics and outcomes of key pre-clinical and animal studies investigating HMP preservation of kidneys.
3.1. Oxygenated HMP (4 to 10 °C) Could Restore Synthesis of ATP in Kidneys
Buchs et al. [44] compared the reconditioning effects of oxygenated HMP or HOPE at 4 °C vs. SCS on porcine DCD grafts (Maastricht I and II) subjected to varying lengths of WIT (0 or 0.5 h) and CIT (0 or 4 or 8 or 18 h). In this study, the team designed an MP device that was compatible with magnetic resonance, which facilitates real-time, non-invasive, and in-line 31P NMR spectroscopy for monitoring ATP resynthesis in the graft during MP. In groups where grafts were only subjected to WIT or CIT (4 or 8 h), respectively, ATP levels were detectable after HOPE, albeit lower than baseline control level. However, it was shown that after an extended period of SCS, equivalent to 18 h of CIT, ATP levels were almost undetectable, despite the application of HOPE. Cold pulsatile perfusion did not result in ATP resynthesis in grafts subjected to both warm and cold ischaemia (4 h), with only precursors ((phosphomonoesther (PME) and inorganic phosphorus (oPi)) being detected. Extending these findings, Buch et al. proposed that immediate HOPE in mobile perfusion machines for WIT-treated DCD grafts could restore the energetic status of the grafts. Thus, this could restrict the use of SCS and maintain graft viability during transport. However, the reanimation of grafts that were subjected to CIT damage by HOPE may be limited to 8 h of cold storage and not beyond that. The limitations of the study were that no data about the rate of DGF and survival were available, since grafts were not surgically transplanted into the animals after these assessments.
The role of ATP as an essential marker for viability of kidneys, a prerequisite for satisfactory transplantation outcome, was further demonstrated in a porcine study utilizing the same NMR imaging method for tissue ATP measurement in DCD kidneys subjected to pulsatile HOPE (4 °C) by Lazeyras et al. [45]. In order to obtain an overall picture of the energetic status of the graft, ATP (alpha-, beta-, gamma-ATP resonances) and precursors (PME, oPi) were all taken into account. Further findings through this non-invasive ATP assessment method included the superiority of perfusion pO2 of 100 kPa over 50 or 20 kPa in resynthesis of ATP. This suggested that the high level of precursors detected may be due to the reduction of cell’s ability to undergo re-phosphorylation of AMP in PME to energy-rich ATP, but this speculation might be inaccurate due to technical obstacles like overlapping resonances. Nevertheless, findings from both studies highlighted the possibility of encompassing 31P NMR spectroscopy with clinical MRI in future studies of the effects of preservation conditions on the viability of graft and a tool to the decision-making process of expanding the donor pool, which is currently subjective and heterogeneous, involving visual inspection (e.g., colour, patchiness, physical injuries) and renal resistance.
The importance of ATP tissue measurements as a quantitative index to be added on to the current restricted panel of clinical evaluation criteria of kidneys was also put forward by Kaminski et al. [48] in a porcine study, which revealed that cortical ATP synthesis was significantly restored in WI-injured grafts treated by HOPE at 8 °C compared to SCS (5.8 vs. 0.06 mmol/L, p < 0.01). A 90% reduction of tissue ATP was noted upon 1 h of WIT, and interestingly, the renal cortical tissue ATP levels in HOPE-treated, WIT-injured grafts exceeded that in control grafts (3.3 mmol/L tissue) that were not subjected to WIT.
The metabolic protective effects of HOPE at 4 °C on tissue ATP were also demonstrated in discarded human DBD kidneys subjected to >20 h of cold ischaemia by Ravaioli et al. [47] compared to the SCS group, in which the team investigated different oxygenation parameters (hyperbaric and normobaric oxygen). Taking the similarities of kidneys between porcine and humans in terms of size and function into account, ATP resynthesis appears to be possible in grafts challenged by CIT exceeding 8 h, a limit suggested in Buchs et al.’s study [43]. The significance of this study and the three animal models supported the hypothesis that oxygen delivery HMP (4–10 °C) might favour improvements in cellular ATP levels in grafts subjected to IRI due to prolonged CIT and short WIT (0.5 h). The association between ATP levels in organs prior to transplantation and the reduction in risk of DGF were also previously discussed by Wijemars et al. [49].
3.2. The Effects of Higher Perfusion Temperatures (>4–10 °C) on Kidney Viability are Currently Unclear
Quantitative changes to ATP in porcine kidneys perfused at normothermic temperature (30 °C) were only reported in one study [45], in which HMP (4 °C) and SCS were also tested as preservation methods (Table 7). Two hours of CIT on these kidneys was associated with an increase in ADP:ATP ratio prior to preservation, and after perfusion, the ADP:ATP ratios were found to be comparable between NMP, HMP, and SCS groups. Contrary to other studies on HMP of kidneys, Kay et al. did not observe significant changes in tissue ATP even in the HMP group.
A recent porcine transplant study by Bhattacharjee et al. [50] reported that a perfusion temperature of 22 °C resulted in significant improvements on IRI injuries in SCS treated kidneys in terms of histology, suppression of expression of IRI related Toll-like receptor signalling molecules (MyD88, NF-kB and HMGB1), and graft functions, such as increase in urinary output and maximal renal blood flow compared to MP at 15 °C and 37 °C. While it was accepted that a hypothermic temperature may be optimal for the protection of metabolic energetic status and viability of kidney grafts, effects of subnormothermia on tissue ATP remains to be established in comparison to other more extensively studied temperatures.
Current novel treatment methods tested in animal models are centred around ameliorating HMP-induced injuries of kidney grafts [51]. A minimally invasive method called rapid sampling microdialysis (rsMD), which was developed in a porcine HMP model [52], may help monitor tissue ATP levels and other metabolic markers during MP in real-time, promoting further investigations into the relationship of MP temperature and renal tissue ATP. Proteolytic enzymes like matrix metalloproteinases (MMPs) are known to be related to the pathophysiology of fibrotic renal diseases. Tissue MMP-2 was, in particular, implicated in patients suffering from antibody-mediated active renal graft vs. host rejection [53]. In a rat DCD kidney model, Moser et al. [54] tested two inhibitory agents on levels MMPs; these include a siRNA against MMP-2 and together with doxycycline. Additionally, high levels of MMP-2 and MMP-9 were identified from perfusates of human kidney grafts, suggesting that an elevation of MMPs might be related to cold hypothermic perfusion. Apart from a decrease in MMPs, a reduction in inflammatory markers such as LDH was also noted. Identification of natural inhibitors, TIMP-1 and TIMP-2 of MMP-9 and MMP-2, respectively, was useful in the study of biomarkers for predicting early and long-term delayed graft function. Paradoxically, a detection and elevation of urinary TIMP-1 and TIMP-2 at day 1 and 3 were correlated with unsatisfactory eGFR at post-operative day 1, 7, and 14. The authors reasoned that this increase might be due to elevation of MMP-9 and MMP-2 in patients who have undergone kidney transplantation [55]. TIMP-2, also a marker of acute kidney injury, was recently shown to be predictive of delayed graft function in patients having received DCD kidney transplantations [56]. Borrowing the idea from Gilloidy et al.’s [42] report on the use of siRNA to protect the end-perfusion liver graft from end-ischaemia, although currently still elusive, the transfection of MMP-2 siRNA during cold hypothermic perfusion of kidney grafts may be useful for prevention of tubular damage and IRI.
4. Pancreas Preservation
With the advent of MP technology in the preservation of liver and kidneys, there is growing interest in its application in pancreas transplantation. The major goal is the maintenance of viability of islet cells including insulin producing beta cells in the graft, which is highly susceptible to IRI damage [57]. Existing challenges include the lack of recognised viability markers, and technical obstacles like susceptibility of vascular endothelial lining to MP flow. ATP generation as a means to evaluate cellular metabolism and as part of the functional assessment of the organ were once again highlighted [58]. The characteristics of two studies on HMP and NMP that reported tissue ATP as one of the primary endpoints are shown in Table 8 and Table 9, respectively.

Table 8.
Characteristics and outcomes of key pre-clinical study investigating HMP preservation of pancreas

Table 9.
Characteristics and outcomes of key animal study investigating NMP preservation of pancreas.
In a pilot study, dual arterial HMP (4–7 °C) was shown to be effective in increasing tissue ATP by 6.8-fold in 10 human discarded DCD pancreata and by 2.6-fold in 10 DBD pancreata. All pancreata were subjected to 3 to 5 h of cold preservation prior to HMP or SCS. It was noted that prior to HMP, ATP level was significantly lower in DCD pancreata compared to DBD pancreata, possibly related to IRI sustained from WI in the DCD group. However, after HMP, ATP level in DCD pancreata was comparable to that of DBD pancreata. The improvements in viability of HMP-treated pancreata were accompanied by absence of oedema, but an increase in amylase, which was also reported in the SCS group. A higher level of amylase was believed to be associated with poorer transplantation outcomes and may mean acinar and parenchymal damage. However, the authors speculated that the increase in amylase levels after both dynamic and static preservation could be related to experimental factors, such as injuries induced by biopsy collections. Among the 20 samples, viable islets were isolated from 2 DCD pancreata [57]. The relationship between tissue ATP and better transplantation outcome was also reported in earlier studies involving a two-layer preservation method of pancreata, rather than MP [60].
Instead of directly measuring pancreata tissue ATP levels, Kumar et al. [59] studied the effects of ex vivo NMP (37 °C) and high- or low-pressure perfusion on ATP Synthetase Complex V, a marker of viability and an enzyme essential for ATP production in a porcine DCD pancreata model. It was found that low-pressure ex vivo NMP treatment of pancreata resulted in the highest levels of ATP Synthetase Complex V, indicating active metabolic ATP production to support ATP-dependent pathways underlying insulin secretion after glucose stimulation. Amylase was also found to be lower in low pressure NMP treated pancreata compared to SCS. As mentioned above, even though a higher amylase is suggestive of a higher risk of organ dysfunction, more studies will be needed to assess the benefits of a lower temperature (4 °C) over a higher temperature as high as 37 °C. The limitation of the study lies in the use of a grading system for measuring the positive immunohistochemical staining of biopsied samples, instead of measuring the tissue ATP concentration by a commercial bio-illuminescence kit used in most animal and preclinical studies apart from NMR spectroscopy. Careful interpretation of these results is necessary, and a further validation of the beneficial effects of normothermic perfusion temperature on ATP resynthesis in donor pancreata will be needed with the use of kits or NMR spectroscopy. However, the two studies strongly suggested that more research and possible clinical trials in MP of pancreata from different types of ECDs are warranted, and changes in tissue ATP could be considered as part of the criteria for determining viability and functionality of pancreata graft.
5. Conclusions
This review analysed the studies in the last decade that had tissue ATP in animal and preclinical models as an endpoint (Table 10).

Table 10.
Preclinical and animal MP studies that reported ATP or ATP-related parameters as one of the end points (2009–2019).
ATP acts as a convenient mean for addressing one of the major challenges in transplantation: What is the effect of temperature on organ metabolism and how does this correlate with post-transplantation outcomes, such as DGF and survival rate? In the liver (Table 11), MPs of all temperatures were shown to increase tissue ATP levels, but there is less agreement over the temperature range that gives rise to the highest absolute increase in end-perfusion tissue ATP.

Table 11.
MP studies that reported liver tissue ATP (2009–2019).
In the kidneys (Table 12), with fewer published literature on SNMP and NMP to date, HOPE appeared to be most effective in increasing graft ATP resynthesis.

Table 12.
MP studies that reported tissue ATP in kidneys (2009–2019).
More evidence is needed on the effects of preservation temperature on donor pancreatic ATP levels (Table 13).

Table 13.
MP studies that reported tissue ATP in pancreas (2009–2019).
In conclusion, tissue ATP is a useful quantitative index for informing clinicians in donor organ evaluation and a marker for the extent of resuscitation of end-ischaemic grafts by pharmacological and genetic interventions.
Author Contributions
M.I.B. designed the study, analysed the data, and wrote the paper; J.Y. performed the study, collected the data, and wrote the paper; M.N. performed the study and collected the data; V.P. designed the study, analysed the data, and reviewed the paper.
Funding
No external resource funded this research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADP | Adenosine DiPhosphate |
| AMP | Adenosine MonoPhosphate |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Transaminase |
| ATP | Adenosine TriPhosphate |
| BMI | Body Mass Index |
| CIT | Cold Ischaemia Time |
| COR | Controlled Oxygenated Rewarming |
| DBD | Donation after Brain Death |
| DCD | Donation after Circulatory Death |
| DGF | Delayed Graft Function |
| DHOPE | Dual Hypothermic Oxygenated Machine Perfusion |
| ECD | Extended Criteria Donor |
| GGT | Gamma-Glutamyl Transferase |
| GLDH | Glutamate Dehydrogenase |
| HMP | Hypothermic Machine Perfusion |
| HOPE | Oxygenated Hypothermic Machine Perfusion |
| IRI | Ischaemia-Reperfusion Injury |
| IFLT | Ischaemia-free liver transplantation |
| LDH | Lactate De-Hydrogenase |
| MP | Machine Perfusion |
| MPS | Machine Perfusion Solution |
| MPTP | Mitochondrial Permeability Transition Pore |
| NADH | Nicotinamide Adenine Dinucleotide |
| NMP | Normothermic Machine perfusion |
| pCO2 | Partial Carbon dioxide pressure |
| PME | Phosphomonoester |
| pO2 | Partial Oxygen Pressure |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| SCS | Static Cold Storage |
| siRNA | Small Interfering RNA |
| SNMP | Subnormothermic Machine Perfusion |
| TBARS | ThioBarbituric Acid Reactive Substances |
| TEM | Transmission Electron Microscopy |
| TNF-alpha | Tumour Necrosis Factor-Alpha |
| UW | University of Wisconsin Solution |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| vWF | Von Willebrand Factor |
| WIT | Warm Ischaemia Time |
References
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine Perfusion for Abdominal Organ Preservation: A Systematic Review of Kidney and Liver Human Grafts. J. Clin. Med. 2019, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, D.; Oberbauer, R.; Heemann, U.; Viklicky, O.; Peruzzi, L.; Mariat, C.; Crespo, M.; Budde, K.; Oniscu, G.C. Recent advances in kidney transplantation: A viewpoint from the Descartes advisory board. Nephrol. Dial. Transplant. 2018, 33, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Dziodzio, T.; Jara, M.; Hardt, J.; Weiss, S.; Viktor Ritschl, P.; Denecke, C.; Biebl, M.; Gerlach, U.; Reinke, P.; Pratschke, J.; et al. Effects of expanded allocation programmes and organ and recipient quality metrics on transplant-related costs in kidney transplantation—An institutional analysis. Transpl. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J. An update on liver transplantation: A critical review. J. Autoimmun. 2016, 66, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mannon, R.B. Delayed Graft Function: The AKI of Kidney Transplantation. Nephron 2018, 140, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, R.W.; Gruessner, A.C.; Tan, M.; Kandaswamy, R.; Sutherland, D.E.R.; Humar, A. Risk Factors and Impact of Delayed Graft Function after Pancreas Transplants. Arab. Archaeol. Epigr. 2004, 4, 758–762. [Google Scholar]
- Santangelo, M.; Furian, L.; Kessaris, N.; Hadaya, K.; Kimenai, D.; Bellini, M.I. Renal Transplantation: What Has Changed in Recent Years. BioMed Res. Int. 2019, 2019, 3618104. [Google Scholar]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold Pulsatile Machine Perfusion versus Static Cold Storage in Kidney Transplantation: A Single Centre Experience. BioMed Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Ishikawa, J.; Oshima, M.; Iwasaki, F.; Suzuki, R.; Park, J.; Nakao, K.; Matsuzawa-Adachi, Y.; Mizutsuki, T.; Kobayashi, A.; Abe, Y.; et al. Hypothermic temperature effects on organ survival and restoration. Sci. Rep. 2015, 5, 9563. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar] [PubMed]
- Bellini, M.I.; D’Andrea, V. Organ preservation: Which temperature for which organ? J. Int. Med Res. 2019, 47, 2323–2325. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, H.F.L.; Nicholson, M.L. Oxygenated Kidney Preservation Techniques. Transplant. 2012, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and Advances in Kidney Preservation. BioMed Res. Int. 2018, 2018, 9206257. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hübscher, S.; Perera, M.T.P.R.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transplant. 2018, 24, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Efferz, P.; Fox, M.; Wohlschlaeger, J.; Lüer, B. Controlled Oxygenated Rewarming of Cold Stored Liver Grafts by Thermally Graduated Machine Perfusion Prior to Reperfusion. Arab. Archaeol. Epigr. 2013, 13, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, C.J.; Farid, W.R.; De Jonge, J.; Metselaar, H.J.; Kazemier, G.; Van Der Laan, L.J. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J. Hepatol. 2014, 61, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Vajdová, K.; Graf, R.; Clavien, P.-A.; Clavien, P. ATP-supplies in the cold-preserved liver: A long-neglected factor of organ viability. Hepatology 2002, 36, 1543–1552. [Google Scholar] [CrossRef]
- Westerkamp, A.C.; Karimian, N.; Matton, A.P.; Mahboub, P.; Van Rijn, R.; Wiersema-Buist, J.; De Boer, M.T.; Leuvenink, H.G.; Gouw, A.S.; Lisman, T.; et al. Oxygenated Hypothermic Machine Perfusion After Static Cold Storage Improves Hepatobiliary Function of Extended Criteria Donor Livers. Transplantation 2016, 100, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Rizzo, V.; Boncompagni, E.; Bianchi, A.; Gringeri, E.; Neri, D.; Richelmi, P.; Freitas, I.; Cillo, U.; Vairetti, M. Machine perfusion at 20 degrees C reduces preservation damage to livers from non-heart beating donors. Cryobiology 2011, 62, 152–158. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Lee, J.; D’Andrea, V.; Liu, Q.; Izamis, M.-L.; Uygun, K.; Yarmush, M.L. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant. Res. 2012, 1, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruinsma, B.G.; Berendsen, T.A.; Izamis, M.L.; Yarmush, M.L.; Uygun, K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int. J. Artif. Organs 2013, 36, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Yeh, H.; Özer, Ş.; Martins, P.N.; Farmer, A.; Wu, W.; Saeidi, N.; Dries, S.O.D.; Berendsen, T.A.; Smith, R.N.; et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 2014, 14, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Sridharan, G.V.; Weeder, P.D.; Avruch, J.H.; Saeidi, N.; Özer, S.; Geerts, S.; Porte, R.J.; Heger, M.; Van Gulik, T.M.; et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 2016, 6, 22415. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Rizzo, V.; Mannucci, B.; Richelmi, P.; Croce, A.C.; Vairetti, M. Liver Graft Susceptibility during Static Cold Storage and Dynamic Machine Perfusion: DCD versus Fatty Livers. Int. J. Mol. Sci. 2017, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Von Horn, C.; Baba, H.A.; Hannaert, P.; Hauet, T.; Leuvenink, H.; Paul, A.; Minor, T.; COPE Consortium Partners. Controlled oxygenated rewarming up to normothermia for pretransplant reconditioning of liver grafts. Clin. Transplant. 2017, 31, e13101. [Google Scholar] [CrossRef]
- Xu, H.; Berendsen, T.; Kim, K.; Soto-Gutierrez, A.; Bertheium, F.; Yarmush, M.L.; Hertl, M. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J. Surg. Res. 2012, 173, e83–e88. [Google Scholar] [CrossRef]
- Maida, K.; Akamatsu, Y.; Hara, Y.; Tokodai, K.; Miyagi, S.; Kashiwadate, T.; Miyazawa, K.; Kawagishi, N.; Ohuchi, N. Short Oxygenated Warm Perfusion with Prostaglandin E1 Administration Before Cold Preservation as a Novel Resuscitation Method for Liver Grafts From Donors After Cardiac Death in a Rat In Vivo Model. Transplantation 2016, 100, 1052–1058. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Bianchi, A.; Richelmi, P.; Vairetti, M. Metabolic shift in liver: Correlation between perfusion temperature and hypoxia inducible factor-1alpha. World J. Gastroenterol. 2015, 21, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.-A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Graf, R.; Clavien, P.A. Rescue of the Cold Preserved Rat Liver by Hypothermic Oxygenated Machine Perfusion. Arab. Archaeol. Epigr. 2006, 6, 903–912. [Google Scholar] [CrossRef] [PubMed]
- De Rougemont, O.; Breitenstein, S.; Leskosek, B.; Weber, A.; Graf, R.; Clavien, P.A.; Dutkowski, P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann. Surg. 2009, 250, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.; Karimian, N.; Matton, A.P.M.; Burlage, L.C.; Westerkamp, A.C.; van den Berg, A.P.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Dutkowski, P.; Guarrera, J.V.; De Jonge, J.; Martins, P.N.; Porte, R.J.; Clavien, P.-A.; De Jonge, J. Evolving Trends in Machine Perfusion for Liver Transplantation. Gastroenterology 2019, 156, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Lurje, I.; Tolba, R.H.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs-New kids on the block. Liver Int. 2019, 39, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Berardo, C.; Di Pasqua, L.G.; Siciliano, V.; Rizzo, V.; Richelmi, P.; Ferrigno, A.; Vairetti, M. Machine Perfusion at 20 degrees C Prevents Ischemic Injury and Reduces Hypoxia-Inducible Factor-1alpha Expression During Rat Liver Preservation. Ann. Transplant. 2017, 22, 581–589. [Google Scholar] [CrossRef]
- Okamura, Y.; Hata, K.; Tanaka, H.; Hirao, H.; Kubota, T.; Inamoto, O.; Kageyama, S.; Tamaki, I.; Yermek, N.; Yoshikawa, J.; et al. Impact of Subnormothermic Machine Perfusion Preservation in Severely Steatotic Rat Livers: A Detailed Assessment in an Isolated Setting. Am. J. Transplant. 2017, 17, 1204–1215. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Meng, Y.; Chen, Z.; Yang, J. Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver. Int. J. Mol. Sci. 2018, 19, 1302. [Google Scholar] [CrossRef]
- Karimian, N.; Yeh, H. Opportunities for Therapeutic Intervention During Machine Perfusion. Curr. Transplant. Rep. 2017, 4, 141–148. [Google Scholar] [CrossRef]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of siRNA Uptake (for RNA Interference) During Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Guo, Z.; Zhao, Q.; Ju, W.; Wang, D.; Wu, L.; Yang, L.; Ji, F.; Tang, Y.; Zhang, Z.; et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am. J. Transplant. 2018, 18, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Buchs, J.B.; Lazeyras, F.; Ruttimann, R.; Nastasi, A.; Morel, P. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion 2011, 26, 159–165. [Google Scholar] [CrossRef]
- Lazeyras, F.; Buhler, L.; Vallée, J.-P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.-B. Detection of ATP by “in line” 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magma 2012, 25, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.D.; Hosgood, S.A.; Harper, S.J.; Bagul, A.; Waller, H.L.; Nicholson, M.L. Normothermic Versus Hypothermic Ex Vivo Flush Using a Novel Phosphate-Free Preservation Solution (AQIX) in Porcine Kidneys. J. Surg. Res. 2011, 171, 275–282. [Google Scholar] [CrossRef]
- Ravaioli, M.; Baldassare, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischemia Time in Human Marginal Kidney Grafts. Ann. Transplant. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Kaminski, J.; Delpech, P.O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86. [Google Scholar] [CrossRef]
- Wijermars, L.G.; Schaapherder, A.F.; De Vries, D.K.; Verschuren, L.; Wüst, R.C.; Kostidis, S.; Mayboroda, O.A.; Prins, F.; Ringers, J.; Bierau, J.; et al. Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int. 2016, 90, 181–191. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Ruthirakanthan, A.; Sun, Q.; Richard-Mohamed, M.; Luke, S.; Jiang, L.; Aquil, S.; Sharma, H.; Tun-Abraham, M.E.; Alharbi, B.; et al. Subnormothermic Oxygenated Perfusion Optimally Preserves Donor Kidneys Ex Vivo. Kidney Int. Rep. 2019, 4, 1323–1333. [Google Scholar] [CrossRef]
- Krzywonos-Zawadzka, A.; Franczak, A.; Moser, M.A.J.; Olejnik, A.; Sawicki, G.; Bil-Lula, I. Pharmacological Protection of Kidney Grafts from Cold Perfusion-Induced Injury. BioMed Res. Int. 2019, 2019, 9617087. [Google Scholar] [CrossRef]
- Hamaoui, K.; Gowers, S.; Damji, S.; Rogers, M.; Leong, C.L.; Hanna, G.; Darzi, A.; Boutelle, M.; Papalois, V. Rapid sampling microdialysis as a novel tool for parenchyma assessment during static cold storage and hypothermic machine perfusion in a translational ex vivo porcine kidney model. J. Surg. Res. 2016, 200, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Sui, W.; Wang, B.; Zou, H.; Zou, G.; Luo, H. Expression of MMP-2 and TIMP-1 in Renal Tissue of Patients with Chronic Active Antibody-mediated Renal Graft Rejection. Diagn. Pathol. 2012, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.J.; Arcand, S.; Lin, H.-B.; Wojnarowicz, C.; Sawicka, J.; Banerjee, T.; Luo, Y.; Beck, G.R.; Luke, P.P.; Sawicki, G. Protection of the Transplant Kidney from Preservation Injury by Inhibition of Matrix Metalloproteinases. PLoS ONE 2016, 11, e0157508. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Domanski, L.; Bober, J.; Safranow, K.; Romanowski, M.; Pawlik, A.; Kwiatkowski, S.; Ciechanowski, K. Urinary Metalloproteinases-9 and -2 and Their Inhibitors TIMP-1 and TIMP-2 are Markers of Early and Long-Term Graft Function After Renal Transplantation. Kidney Blood Press. Res. 2016, 41, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Bank, J.R.; Ruhaak, R.; Soonawala, D.; Mayboroda, O.; Romijn, F.P.; van Kooten, C.; Cobbaert, C.M.; de Fijter, J.W. Urinary TIMP-2 Predicts the Presence and Duration of Delayed Graft Function in Donation After Circulatory Death Kidney Transplant Recipients. Transplantation 2019, 103, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Leemkuil, M.; Lier, G.; Engelse, M.A.; Ploeg, R.J.; De Koning, E.J.P.; Hart, N.A.T.; Krikke, C.; Leuvenink, H.G.D. Hypothermic Oxygenated Machine Perfusion of the Human Donor Pancreas. Transplant. Direct 2018, 4, e388. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, K.; Papalois, V. Machine Perfusion and the Pancreas: Will It Increase the Donor Pool? Curr. Diabetes Rep. 2019, 19, 56. [Google Scholar] [CrossRef]
- Kumar, R.; Chung, W.Y.; Runau, F.; Isherwood, J.D.; Kuan, K.G.; West, K.; Garcea, G.; Dennison, A.R. Ex vivo normothermic porcine pancreas: A physiological model for preservation and transplant study. Int. J. Surg. 2018, 54, 206–215. [Google Scholar] [CrossRef]
- Kuroda, Y.; Fujino, Y.; Morita, A.; Ku, Y.; Saitoh, Y. Correlation between high adenosine triphosphate tissue concentration and good posttransplant outcome for the canine pancreas graft after preservation by the two-layer cold storage method. Transplantation 1991, 52, 989–991. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).