Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study
Abstract
:1. Introduction
2. Methods
Statistical Methods
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Crizotinib Treatment Patterns
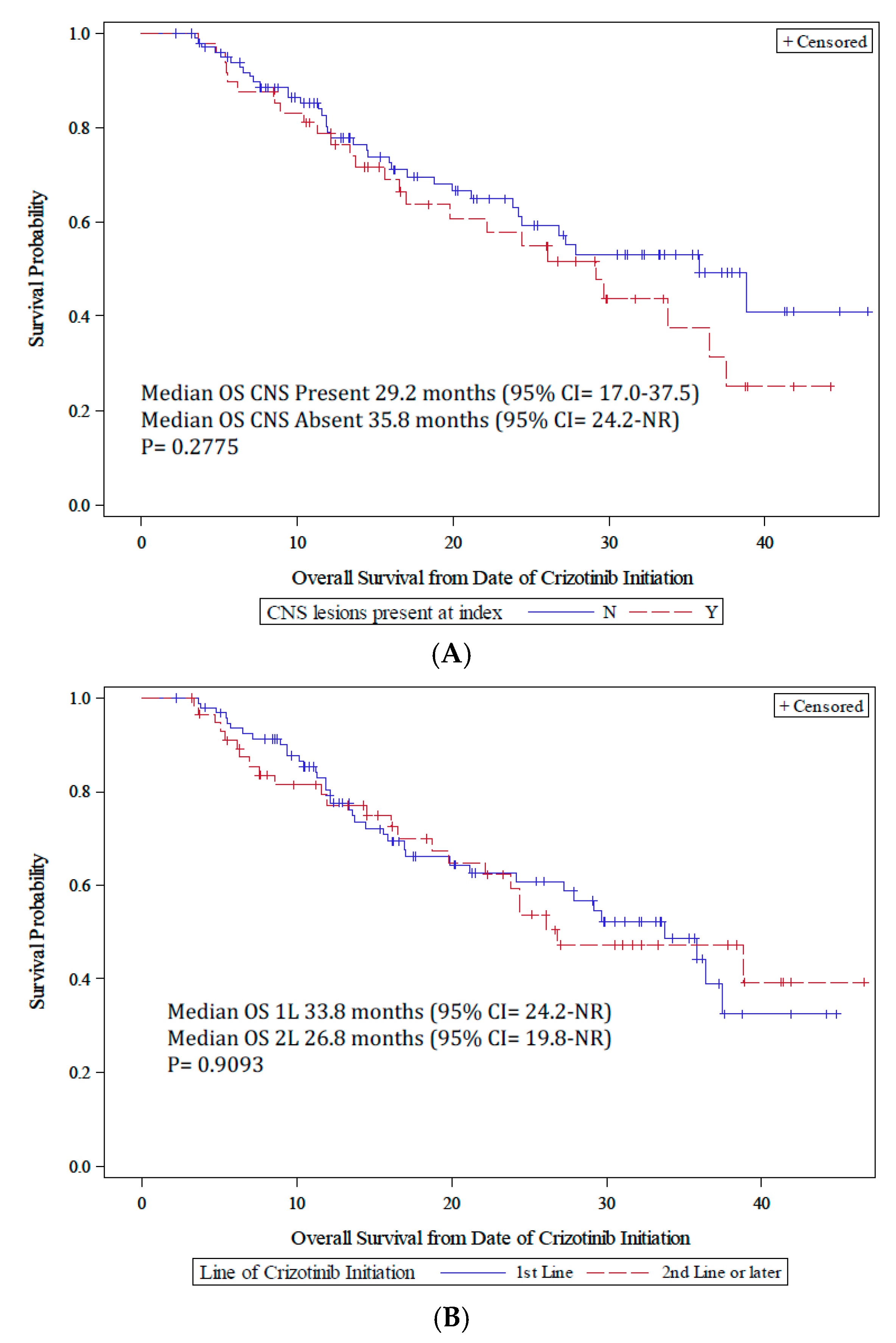
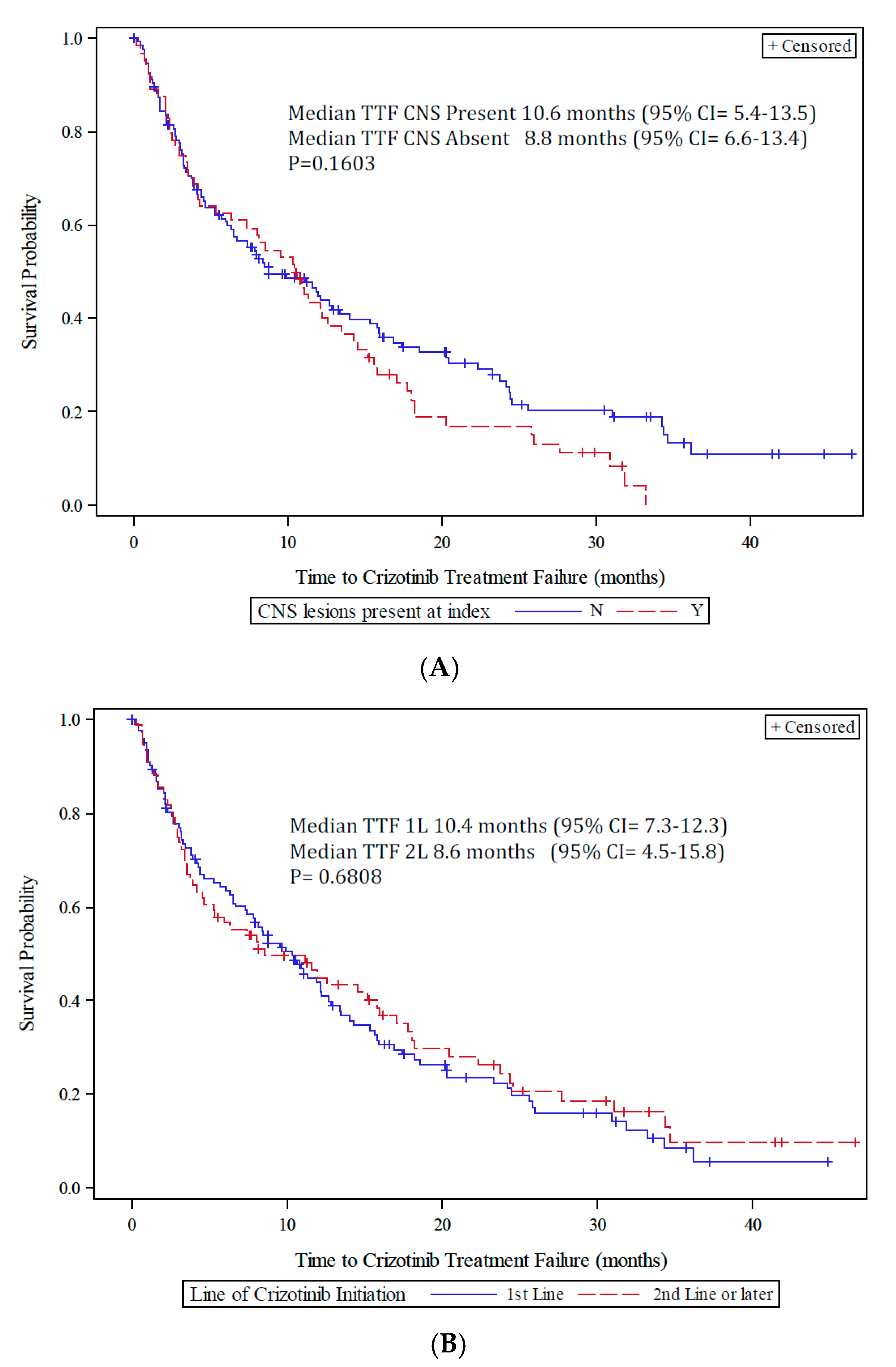
3.3. Crizotinib Clinical Outcomes
3.4. Healthcare Resource Utilization and Cost
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. ALK, lung cancer, and personalized therapy: Portent of the future? J. Natl. Cancer Inst. 2010, 102, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Choi, Y.L.; Soda, M.; Inamura, K.; Togashi, Y.; Hatano, S.; Enomoto, M.; Takada, S.; Yamashita, Y.; Satoh, Y.; et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin. Cancer Res. 2008, 14, 6618–6624. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.H.; Vernersson, E.; Grabbe, C.; Hallberg, B. Anaplastic lymphoma kinase: Signaling in development and disease. Biochem. J. 2009, 420, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Mosse, Y.P.; Wood, A.; Maris, J.M. Inhibition of ALK signaling for cancer therapy. Clin. Cancer Res. 2009, 15, 5609–5614. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Rodig, S.J.; Chirieac, L.R.; Jänne, P.A. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur. J. Cancer 2010, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, J.P.; Mermel, C.; Zejnullahu, K.; Murphy, C.; Lifshits, E.; Holmes, A.J.; Choi, H.G.; Kim, J.; Chiang, D.; Thomas, R.; et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin. Cancer Res. 2008, 14, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Leung, E.L.; So, K.K.; Tam, I.Y.; Sihoe, A.D.; Cheng, L.C.; Ho, K.K.; Au, J.S.; Chung, L.P.; Pik Wong, M. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009, 115, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Schein, P.S.; Scheffler, B. Barriers to efficient development of cancer therapies. Clin. Cancer Res. 2006, 12, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- McDermott, U.; Iafrate, A.J.; Gray, N.S.; Shioda, T.; Classon, M.; Maheswaran, S.; Zhou, W.; Choi, H.G.; Smith, S.L.; Dowell, L.; et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008, 68, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Zou, H.Y.; Arango, M.E.; Li, Q.; Lee, J.H.; McDonnell, S.R.; Yamazaki, S.; Alton, G.R.; Mroczkowski, B.; Los, G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007, 6, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Janne, P.A.; Besse, B.; Solomon, B.J.; Blackhall, F.H. Crizotinib vs. chemotherapy in ALK+ advanced non-small cell lung cancer (NSCLC): Final survival results from PROFILE 1007. J. Clin. Oncol. 2016, 34, 9066. [Google Scholar]
- Karve, S.J.; Price, G.L.; Davis, K.L.; Pohl, G.M.; Smyth, M.N.; Bowman, L. Comparison of demographics, treatment patterns, health care utilization, and costs among elderly patients with extensive-stage small cell and metastatic non-small cell lung cancers. BMC Health Serv. Res. 2014, 14, 555. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients (n = 199) |
|---|---|
| Females | 104 (52.3) |
| Age at crizotinib initiation, years | |
| Median (min, max) | 60.2 (27.1–88.2) |
| 18–35 | 8 (4.0) |
| 36–45 | 26 (13.1) |
| 46–55 | 38 (19.1) |
| 56–65 | 59 (29.7) |
| >65 | 68 (34.2) |
| BMI, kg/m2 | |
| Median (min, max) | 25.6 (15.9–50.3) |
| Census regions (physicians), n (%) | |
| South | 111 (55.8) |
| West | 54 (27.1) |
| Midwest | 24 (12.1) |
| Northeast | 10 (5.0) |
| ECOG PS at advanced NSCLC diagnosis | |
| 0 | 22 (11.1) |
| 1 | 132 (66.3) |
| 2 | 27 (13.6) |
| 3 | 1 (0.5) |
| Unknown | 17 (8.5) |
| Stage at initial NSCLC diagnosis | |
| Early (stage IA, IB, IIA, IIB) | 16 (8.0) |
| Limited/regional (stage IIIA) | 15 (7.5) |
| Locally advanced (stage IIIB) | 22 (11.1) |
| Metastatic (stage IV) | 133 (66.8) |
| Missing/unknown | 13 (6.5) |
| Histology | |
| Adenocarcinoma (mixed or not) | 177 (88.9) |
| Squamous | 4 (2.0) |
| Not otherwise specified (NOS) | 1 (0.50) |
| Missing/unknown | 17 (8.5) |
| Smokingstatus | |
| Current | 23 (11.6) |
| Former | 67 (33.7) |
| Never | 109 (54.8) |
| Sites of metastases | |
| Adrenal gland | 18 (9.1) |
| Bone | 91 (45.7) |
| Brain | 64 (32.2) |
| Distant lymph nodes | 73 (36.7) |
| Liver | 49 (24.6) |
| Other 1 | 120 (60.3) |
| Line of Therapy for Crizotinib Initiation | CNS Metastases | ||||||
|---|---|---|---|---|---|---|---|
| First-Line (n = 123) | Second/Later-Line (n = 76) | p-Value | Present (n = 64) | Absent (n = 135) | p-Value | ||
| Sex, n (%) | |||||||
| Female | 63 (51.2) | 41 (54.0) | 0.77 | 34 (53.1) | 70 (51.9) | 0.88 | |
| Age (years) at crizotinib initiation | |||||||
| Median (min, max) | 59.3 (27.1, 88.2) | 63.2 (28.3, 86.7) | 59.4 (28.3 81.3) | 61.5 (27.1, 88.2) | |||
| Age distribution (years) | |||||||
| 18–35 | 3 (2.4) | 5 (6.6) | 0.08 | 3 (4.7) | 5 (3.7) | 0.47 | |
| 36–45 | 19 (15.5) | 7 (9.2) | 11 (17.2) | 15 (11.1) | |||
| 46–55 | 29 (23.6) | 9 (11.8) | 12 (18.8) | 26 (19.3) | |||
| 56–65 | 33 (26.8) | 26 (34.2) | 19 (29.7) | 40 (29.6) | |||
| >65 | 39 (31.7) | 29 (38.2) | 19 (29.7) | 49 (36.3) | |||
| Census regions (physicians), n (%) | |||||||
| Midwest | 17 (13.8) | 7 (9.2) | 0.42 | 8 (12.5) | 16 (11.9) | 0.58 | |
| Northeast | 4 (3.3) | 6 (7.9) | 2 (3.1) | 8(5.9) | |||
| South | 69 (56.1) | 42 (55.3) | 33 (51.6) | 78 (57.8) | |||
| West | 33 (26.8) | 21 (27.6) | 21 (32.8) | 33 (24.4) | |||
| BMI | |||||||
| Median (min, max) | 25.7 (15.9, 50.3) | 24.9 (17.3, 49.7) | 25.8 (17.3, 50.3) | 25.2 (15.9,49.7) | |||
| ECOG at crizotinib initiation, n (%) | |||||||
| 0 | 17 (13.8) | 5 (6.6) | 0.23 | 6 (9.4) | 16 (11.9) | 0.23 | |
| 1 | 76 (61.8) | 56 (73.7) | 39 (60.9) | 93 (68.9) | |||
| 2 | 18 (14.6) | 9 (11.8) | 13 (20.3) | 14 (10.4) | |||
| 3 | 1 (0.8) | 0 | 0 (0.0) | 1 (0.7) | |||
| Unknown | 11 (8.9) | 6 (7.9) | 6 (9.4) | 11 (8.2) | |||
| Disease stage at initial NSCLC diagnosis, n (%) | |||||||
| Early (stage IA, IB, IIA, IIB) | 7 (5.7) | 9 (11.8) | <0.01 | 2 (3.1) | 14 (10.4) | <0.01 | |
| Limited/regional (stage IIIA) | 6 (4.9) | 9 (11.8) | 6 (9.4) | 9 (6.7) | |||
| Locally advanced (stage IIIB) | 8 (6.5) | 14 (18.4) | 2 (3.1) | 20 (14.8) | |||
| Metastatic (stage IV) | 94 (76.4) | 39 (51.3) | 52 (81.3) | 81 (60.0) | |||
| Unknown | 8 (6.5) | 5 (6.6) | 2 (3.1) | 11 (8.2) | |||
| Histology at crizotinib initiation (%) | |||||||
| Adenocarcinoma (mixed or not) | 113 (91.9) | 64 (84.2) | 0.42 | 57 (89.1) | 120 (88.9) | 0.11 | |
| Squamous | 2 (1.6) | 2 (2.6) | 3 (4.7) | 1 (0.7) | |||
| Not otherwise specified (NOS) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (0.7) | |||
| Missing/Unknown | 8 (6.5) | 9 (11.8) | 4 (6.3) | 13 (9.6) | |||
| Smokingstatus (closest to crizotinib initiation), n (%) | |||||||
| Current smoker | 11 (8.9) | 12 (15.8) | 0.30 | 7 (10.9) | 16 (11.9) | 0.31 | |
| Former smoker | 41 (33.3) | 26 (34.2) | 17 (26.6) | 50 (37.0) | |||
| Never smoked | 71 (57.7) | 38 (50.0) | 40 (62.5) | 69 (51.1) | |||
| Site(s) of distant metastases at crizotinib initiation, n (%) | |||||||
| Adrenal gland | 11 (8.9) | 7 (9.2) | 1.00 | 6 (9.4) | 12 (8.9) | 1.00 | |
| Bone | 56 (45.5) | 35 (46.1) | 1.00 | 31 (48.4) | 60 (44.4) | 0.65 | |
| Brain | 43 (35.0) | 21 (27.6) | 0.35 | 64 (100.0) | 0(0.0) | <0.01 | |
| Distant lymph nodes | 46 (37.4) | 27 (35.5) | 0.88 | 16 (25.0) | 57 (42.2) | 0.02 | |
| Liver | 34 (27.6) | 15 (19.7) | 0.24 | 18 (28.1) | 31 (23.0) | 0.48 | |
| Other | 75 (61.0) | 45 (59.2) | 0.88 | 35 (54.7) | 85 (63.0) | 0.28 | |
| Treatment Patterns | |||||||
| Overall (n = 199) | Line of Therapy for Crizotinib Initiation | p-Value | CNS Metastases | ||||
| First-Line (n = 123) | Second/Later-Line (n = 76) | Present (n = 64) | Absent (n = 135) | p-Value | |||
| Total duration of crizotinib treatment | |||||||
| Mean (SD), months | 11.5 (10.6) | 11.0 (9.9) | 12.3 (11.6) | 0.66 | 11.4 (9.4) | 11.5 (11.1) | 0.64 |
| Median (range), months | 8.5 (0.2–48.3) | 8.5 (0.2–46.6) | 8.4 (0.3–48.3) | 10.5 (0.2–33.2) | 7.9 (0.3–48.3) | ||
| Total duration of crizotinib treatment, n (%) | |||||||
| <3 months | 49 (24.6) | 30 (24.4) | 19 (25.0) | 1.00 | 16 (25.0) | 33 (24.4) | 1.00 |
| ≥3 months | 150 (75.4) | 93 (75.6) | 57 (75.0) | 48 (75.0) | 102 (75.6) | ||
| Cancer treatment received within 30 days before crizotinib start date, n (%) † | 52 (26.1) | 26 (21.1) | 26 (34.2) | 0.03 | 13 (20.3) | 39 (28.9) | 0.94 |
| Platinum doublet 1,± | 17 (32.7) | 13 (50.0) | 4 (15.4) | 5 (38.5) | 12 (30.8) | ||
| Platinum triplet 2,± | 13 (25.0) | 7 (26.9) | 6 (23.1) | 7 (53.8) | 6 (15.4) | ||
| Pemetrexed ± | 8 (15.4) | 1 (3.8) | 7 (26.9) | 3 (23.1) | 5 (12.8) | ||
| Erlotinib ± | 3 (5.8) | 1 (3.8) | 2 (7.7) | 0 (0.0) | 3 (7.7) | ||
| Bevacizumab ± | 2 (3.9) | 0 (0.0) | 2 (7.7) | 0 (0.0) | 2 (5.1) | ||
| Other 3,± | 9 (17.3) | 4 (15.4) | 5 (19.2) | 2 (15.4) | 7 (17.9) | ||
| Cancer treatment received within 30 days post crizotinib end date, n (%) † | 71 (35.7) | 50 (40.7) | 21 (27.6) | 0.21 | 24 (37.5) | 47 (34.8) | 0.99 |
| Platinum doublet 4,± | 16 (22.5) | 12 (24.0) | 4 (19.0) | 6 (25.0) | 10 (21.3) | ||
| Platinum triplet 5,± | 2 (2.8) | 2 (4.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | ||
| Ceritinib ± | 29 (40.9) | 22 (44.0) | 7 (33.3) | 10 (41.7) | 19 (40.4) | ||
| Pemetrexed ± | 5 (7.0) | 2 (4.0) | 3 (14.3) | 2 (8.3) | 3 (6.4) | ||
| Alectinib ± | 2 (2.8) | 2 (4.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | ||
| Docetaxel ± | 2 (2.8) | 0 (0.0) | 2 (9.5) | 1 (4.2) | 1 (2.1) | ||
| Other 6,± | 15 (21.1) | 10 (20.0) | 5 (23.8) | 5 (20.8) | 10 (21.3) | ||
| Initial crizotinib total daily dose, n (%) | |||||||
| 250 mg QD | 11 (5.5) | 5 (4.1) | 6 (7.9) | 0.04 | 3 (4.7) | 8 (5.9) | 1.0 |
| 200 mg BID | 10 (5.0) | 3 (2.4) | 7 (9.21) | 3 (4.7) | 7 (5.2) | ||
| 250 mg BID | 178 (89.5) | 115 (93.5) | 63 (82.9) | 58 (90.6) | 120 (88.9) | ||
| Crizotinib total daily dose changes, n (%) | |||||||
| ≥1 dose escalation | 3 (1.5) | 1 (0.8) | 2 (2.6) | 0.77 | 0 (0.0) | 3 (2.2) | 0.67 |
| ≥1 dose reduction | 26 (13.1) | 16 (13.0) | 10 (13.2) | 7 (10.9) | 19 (14.1) | ||
| ≥1 dose reduction and ≥1 dose escalation | 12 (6.0) | 7 (5.7) | 5 (6.6) | 3 (4.7) | 9 (6.7) | ||
| No changes | 158 (79.4) | 99 (80.5) | 59 (77.6) | 54 (84.4) | 104 (77.0) | ||
| Other cancer treatment during active crizotinib treatment, n (%) | |||||||
| Radiotherapy | 37 (18.6) | 24 (19.5) | 13 (17.1) | 0.71 | 23 (35.9) | 14 (10.4) | <0.01 |
| Other | 49 (24.6) | 33 (26.8) | 16 (21.1) | 0.40 | 22 (34.4) | 27 (20.0) | 0.03 |
| Primary reason(s) for final d/c of crizotinib 7, n (%) | |||||||
| Death | 26 (16.8) | 16 (16.7) | 10 (17.0) | 0.79 | 9 (15.8) | 17 (17.4) | 0.86 |
| Disease progression | 91 (58.7) | 60 (62.5) | 31 (52.5) | 33 (57.9) | 58 (59.2) | ||
| Treatment-related toxicity or side effects | 5 (3.2) | 2 (2.1) | 3 (5.1) | 1 (1.8) | 4 (4.1) | ||
| Physician preference | 6 (3.9) | 3 (3.1) | 3 (5.1) | 3 (5.3) | 3 (3.1) | ||
| Patient preference | 8 (5.2) | 4 (4.2) | 4 (6.8) | 3 (5.3) | 5 (5.1) | ||
| Cost | 1 (0.7) | 1 (1.0) | 0 (0.0) | 1 (1.8) | 0 (0.0) | ||
| Other reason | 10 (6.5) | 6 (6.3) | 4 (6.8) | 3 (5.3) | 7 (7.1) | ||
| Unknown/missing | 8 (5.2) | 4 (4.2) | 4 (6.8) | 4 (7.0) | 4 (4.1) | ||
| Overall (n = 199) | Line of Therapy for Crizotinib Initiation | |||
|---|---|---|---|---|
| First-Line (n = 123) | Second/Later-Line (n = 76) | p-Value | ||
| Visits to an emergency room (on outpatient basis) 1 | ||||
| Had ≥ 1 visit, n (%) | 28 (14.1) | 19 (15.5) | 9 (11.8) | 0.53 |
| Hospital admissions (overnight stay or day admission excluding ER visits) for reasons directly related to NSCLC 1 | ||||
| Had ≥ 1 admission, n (%) | 61 (30.7) | 41 (33.3) | 20 (26.3) | 0.34 |
| Outpatient visits | ||||
| Had ≥ 1 claim, n (%) | 170 (85.4) | 104 (84.6) | 66 (86.8) | 0.84 |
| Median Cost (min, max) | $108.65 (9.30, 336.88) | $110.50 (9.30, 334.99) | $99.38 (18.86, 336.88) | |
| Laboratory procedures | ||||
| Had ≥ 1 claim, n (%) | 153 (76.9) | 90 (73.2) | 63 (82.9) | 0.12 |
| Median Cost (min, max) | $26.12 (1.08, 153.49) | $25.24 (1.08, 153.49) | $26.29 (2.32, 127.76) | |
| Radiotherapy | ||||
| Had ≥ 1 claim, n (%) | 37 (18.6) | 24 (19.5) | 13 (17.1) | 0.71 |
| Median Cost (min, max) | $268.71 (7.63, 1613.79) | $276.09 (7.63, 1613.79) | $219.90 (54.65, 576.38) | |
| Imaging 2 | ||||
| Had ≥ 1 claim, n (%) | 53 (26.6) | 31 (25.2) | 22 (29.0) | 0.76 |
| Median Cost (min, max) | $69.84 (4.43, 206.86) | $64.17 (4.43, 180.72) | $71.72 (6.97, 206.86) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, C.; Masters, E.T.; Black-Shinn, J.; Boyd, M.; Mardekian, J.; Espirito, J.L.; Chioda, M. Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study. J. Clin. Med. 2018, 7, 129. https://doi.org/10.3390/jcm7060129
Reynolds C, Masters ET, Black-Shinn J, Boyd M, Mardekian J, Espirito JL, Chioda M. Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study. Journal of Clinical Medicine. 2018; 7(6):129. https://doi.org/10.3390/jcm7060129
Chicago/Turabian StyleReynolds, Craig, Elizabeth T. Masters, Jenny Black-Shinn, Marley Boyd, Jack Mardekian, Janet L. Espirito, and Marc Chioda. 2018. "Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study" Journal of Clinical Medicine 7, no. 6: 129. https://doi.org/10.3390/jcm7060129
APA StyleReynolds, C., Masters, E. T., Black-Shinn, J., Boyd, M., Mardekian, J., Espirito, J. L., & Chioda, M. (2018). Real-World Use and Outcomes of ALK-Positive Crizotinib-Treated Metastatic NSCLC in US Community Oncology Practices: A Retrospective Observational Study. Journal of Clinical Medicine, 7(6), 129. https://doi.org/10.3390/jcm7060129




