Abstract
Background/Objectives: Tenosynovial giant cell tumor (TGCT), formerly known as pigmented villonodular tenosynovitis (PVNS), is a rare, benign, inflammatory mesenchymal neoplasm originating from the synovium of joints, bursae, or tendon sheaths. Although TGCT can affect any joint, the knee is the most commonly involved site, particularly in cases of diffuse-type TGCT. Bifocal or multifocal involvement is exceedingly uncommon. Methods: Herein, we present a case of localized TGCT with bilateral knee involvement in a 48-year-old female. Results: The patient underwent open arthrotomy with marginal excision of the localized lesions in both knees. Histology and immunohistochemistry staining conformed the diagnosis. At the five-year follow-up, the patient remains asymptomatic and free of recurrence. Conclusions: Given the rarity of bilateral TGCT, clinicians should maintain a high index of suspicion when evaluating patients presenting with bilateral knee pain and swelling and include TGCT in the differential diagnosis. To our knowledge, this represents the fifteenth reported case of bilateral knee TGCT in the literature.
1. Introduction
Tenosynovial giant cell tumor (TGCT) is an uncommon, benign, inflammatory mesenchymal neoplasm arising from the synovium of joints, bursae, or tendon sheaths [1,2]. TGCT is traditionally divided into two subtypes: localized TGCT and diffuse TGCT [3]. However, at an international consensus meeting held in Germany in 2022, the classification was refined into nodular TGCT (N-TGCT)—corresponding to the localized type—and diffuse TGCT (D-TGCT) [4]. According to the 2013 World Health Organization (WHO) classification, the previously distinct terms pigmented villonodular synovitis (PVNS) and giant cell tumor of the tendon sheath (GCTTS) were unified under a single nomenclature: Tenosynovial Giant Cell Tumor [1].
Localized TGCT typically presents as a solitary, lobulated lesion originating from the tendon sheath and, less frequently, from the synovial lining of a joint. The localized type most commonly affects small joints—such as the digits of the hands and feet, or the wrist—and less frequently large joints like the knee. The incidence rate of N-TGCT in the extremities is approximately 11 per million person-years [5,6,7].
In contrast, diffuse TGCT exhibits a more aggressive and locally destructive behavior, involving a large portion or the entirety of the synovium and presenting with a multinodular appearance. The reported incidence of D-TGCT is approximately 5–8.4 per million person-years, with the knee being the most frequently affected joint, followed by the ankle and hip [5,6,7].
TGCT—regardless of subtype—most commonly affects a single joint. Bilateral, bifocal, or multifocal joint involvement is exceedingly rare. Herein, we present a case of bilateral diffuse-type TGCT of the knees in a 48-year-old female, representing the fifteenth reported case of bilateral knee TGCT in the literature. In light of this rare presentation, we discuss relevant diagnostic challenges and treatment considerations.
2. Case Presentation
A 48-year-old woman was referred to our hospital in July 2021 with progressively worsening intermittent pain and swelling in both knees. The symptoms had begun approximately one year earlier and were contemporaneous in onset. There was no history of trauma or concomitant diseases, and her past medical history was unremarkable. Despite the conservative treatment with the use of non-steroidal anti-inflammatory drugs (NSAIDs) and several sessions of physiotherapy, the pain and swelling in both knees persisted and gradually worsened.
On physical examination, tenderness was noted in the suprapatellar region extending from the patellar tendon to the anterolateral joint line. Both knees were swollen, and the range of motion was restricted between 0° and 145°. The McMurray test was negative.
Magnetic Resonance Imaging (MRI) of the left knee demonstrated mild joint effusion, synovial hyperplasia, and a mass-like lesion in continuity with the synovium in the anteromedial patellofemoral joint space, measuring approximately 40 × 26 × 11 mm. The lesion showed homogeneous contrast enhancement and a characteristic “blooming” artifact on gradient-echo sequences. It appeared hypointense on T2-weighted images, consistent with hemosiderin deposition, and iso- to hypointense on T1-weighted images—findings suggestive of localized TGCT (Figure 1A,B).
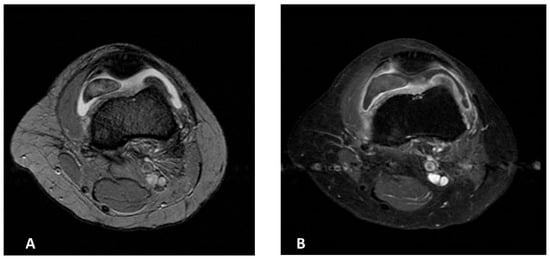
Figure 1.
MR images of the left knee (A,B). (A) Axial T2-weighted MR image of the suprapatellar pouch of the left knee showing intraarticular lesion with cloud-like dark areas due to hemosiderin deposition. (B) Post-contrast T1 fat-saturated MR image of the same region demonstrating synovitis and enhancement of synovial proliferations.
MRI of the right knee revealed a similar mass-like lesion in the posterior compartment, measuring 19 × 11 × 20 mm, accompanied by mild joint effusion. The signal characteristics on both T2 fat-saturated and T1-weighted images mirrored those of the left knee (Figure 2A,B).
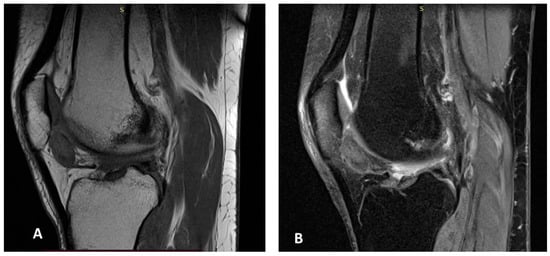
Figure 2.
MR images of the right knee (A,B). Sagittal T1-weighted (A) and gradient-echo (B) MR images of the right knee showing an intraarticular soft-tissue mass with foci of low signal intensity in the anterior compartment, consistent with hemosiderin deposition.
The patient underwent open arthrotomy with marginal excision of the localized lesions in both knees. Grossly, the excised specimens were yellow-brown in color and exhibited an irregular, lobulated surface (Figure 3A–C).
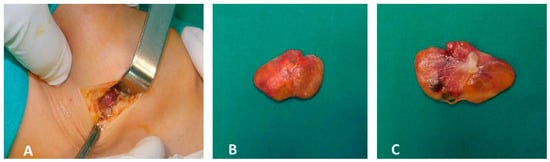
Figure 3.
Intraoperative photographs (A–C). Intraoperative findings following open arthrotomy and marginal resection of the localized lesions in both knees. The excised specimens exhibit a yellow-brown coloration and irregular, lobulated surface.
Histological Findings: Microscopically, the tumor was lobulated, well-circumscribed, and composed of sheets of neoplastic mononuclear cells admixed with multinucleated osteoclast-like giant cells and scattered inflammatory cells, including foamy macrophages. The stroma was collagenous. The mononuclear cells consisted of smaller histiocyte-like cells with pale cytoplasm and round nuclei, and larger epithelioid cells with amphophilic cytoplasm and vesicular nuclei. Hemosiderin granules were frequently observed within the cytoplasm (Figure 4A–C). Mitotic activity was low, and no areas of high-grade morphology—such as nuclear atypia, pleomorphism, increased cellularity, elevated mitotic count, or necrosis—were identified. Immunohistochemistry revealed that the histiocyte-like cells were positive for PGM-1 and negative for SOX-10, S-100, SMA, and CD34 (Figure 5A–C). The morphological and immunohistochemical findings were consistent with the diagnosis of localized tenosynovial giant cell tumor. At the five-year follow-up, the patient remains asymptomatic and free of recurrence (Figure 6A,B). Institutional Review Board Approval was obtained for this study (ΕΒΔ 599/8-11-2021).
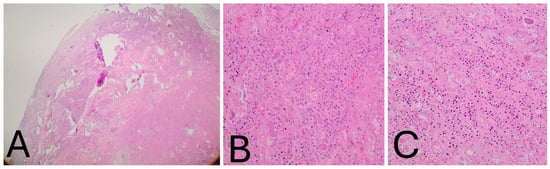
Figure 4.
(A–C). Histological examination. (A) Low-power view showing the well-circumscribed tumor margins (H&E, ×4). (B) High magnification view demonstrating mononuclear histiocyte-like and epithelioid cells containing peripheral hemosiderin granules, interspersed with foamy macro-phages within abundant collagenous stroma. Osteoclast-like giant cells are visible in the upper right field (H&E, ×200). (C) Additional high-power field highlighting multinucleated giant cells (H&E, ×200).

Figure 5.
Immunohistochemistry (IHC) staining (A–C). Immunohistochemistry (IHC) staining of tumor cells showing negativity for (A) SMA (×100). (Β) Low proliferative activity with Ki-67 labeling index of approximately 1% (×100). (C) Diffuse cytoplasmic immunoreactivity of tumor cells for PGM-1 (×100).
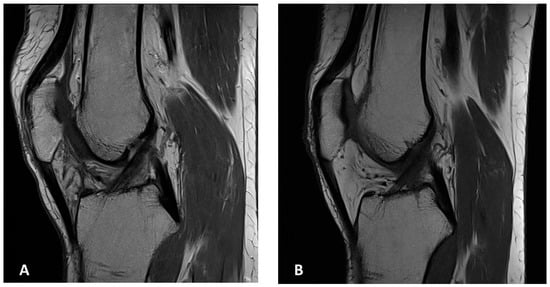
Figure 6.
MR images of both knees. Sagittal MR images of the right (A) and left (B) knee at the latest follow-up showing no evidence of local recurrence.
3. Discussion
The first documented case of tenosynovial giant cell tumor (TGCT) was reported by Chassaignac in 1852, describing involvement of the flexor tendons of the fingers [8]. Simon subsequently defined the localized type in 1865 [9], and Moser, in 1909, described the diffuse form of the disease affecting the knee [10]. In 1941, Jaffe et al. presented 12 cases, outlining the pathological, radiological, and clinical features of TGCT and unifying the various forms of the disease (focal, nodular, diffuse, intra- and extra-articular) under a single entity, proposing a reactive or inflammatory origin [11]. Granowitz et al. later classified TGCT into two distinct clinical forms—localized and diffuse—in 1976 [3]. More recently, the 2022 International Consensus Meeting in Germany redefined the terminology as nodular TGCT (N-TGCT), corresponding to the localized form, and diffuse TGCT (D-TGCT) [4].
Historically, several terms have been used to describe this tumor, including synovial xanthoma, synovial fibroendothelioma, chronic hemorrhagic villous synovitis, fibrohaemosideric sarcoma, and fibrous xanthoma of the synovial membrane [11,12,13,14].
In 2006, West et al. demonstrated the neoplastic nature of TGCT by identifying chromosomal abnormalities, including trisomy of chromosomes 5 and 7 and translocations involving 1p11–13 with 2q37 [15]. The translocation t(1;2)(p13;q37) results in overexpression of the colony-stimulating factor 1 (CSF1) gene through formation of a COL6A3–CSF1 fusion product, which occurs in approximately 2–16% of tumor cells. These neoplastic cells overexpress CSF1, promoting tumor growth via an autocrine loop and recruitment of non-neoplastic cells through a paracrine “landscape effect” [15,16,17].
Macroscopically, N-TGCT typically presents as a well-circumscribed, encapsulated or pedunculated lesion measuring 0.5–4 cm, while D-TGCT exhibits a villous (intra-articular) or multinodular (extra-articular) pattern, often exceeding 5 cm and infiltrating a large portion or the entirety of the synovium. Microscopically, both subtypes share similar histopathological features, including mononuclear histiocyte-like cells, siderophages (macrophages containing hemosiderin granules), multinucleated giant cells, foamy macrophages, fibroblast-like synoviocytes, stromal hyalinization, and a variable lymphocytic infiltrate [1,2,18,19,20,21,22].
Plain radiographs, typically performed as the first-line imaging modality, may appear normal or demonstrate nonspecific findings such as soft tissue swelling, joint effusion, or well-defined bone erosions with sclerotic margins due to cortical pressure effects. Bone mineralization and joint space are usually preserved, and calcification is absent [12,23,24,25]. Radiography remains useful for excluding other entities such as degenerative disease, synovial chondromatosis, or aggressive neoplasms [12].
Magnetic resonance imaging (MRI) is the modality of choice for evaluating TGCT. In D-TGCT, MRI reveals multinodular synovial thickening with low-to-intermediate signal intensity on both T1- and T2-weighted images, and a characteristic “blooming” artifact on gradient-echo sequences due to hemosiderin deposition—a nearly pathognomonic finding [12,26,27]. In N-TGCT, MRI demonstrates a well-defined mass with similar signal characteristics, though blooming is less prominent [12].
Clinical presentation depends on lesion location and disease extent but commonly includes joint pain, swelling, stiffness, locking, and restricted range of motion [28]. According to Mastboom et al., both TGCT subtypes show a predilection for the knee joint (46% for N-TGCT and 64% for D-TGCT), followed by the hand/wrist (localized form) and ankle/hip (diffuse form) [5]. Van der Heijden et al. further reported that the knee is involved in approximately 75% of D-TGCT cases [29]. TGCT typically affects adults aged 30–50 years, with recurrence rates ranging from 9 to 14% for localized TGCT and 23–72% for diffuse TGCT, the latter being 2.6 times higher [1,27,30].
TGCT is predominantly monoarticular; bilateral or multifocal disease is exceedingly rare. The present case represents the fifteenth reported instance of bilateral knee TGCT.
Historically, the first bilateral knee case was described by Kelikian and Lewis (1949) in a 15 year-old girl and her brother, both with diffuse TGCT and possible familial predisposition [31]. Subsequent bilateral knee cases include those reported by Greenfield and Wallace (1950) [32], Gehweiler and Wilson (1969) [33], Sharma et al. (2009) [34], Kim et al. (2010) [35], Soubai et al. (2010) [36], Cho et al. (2012) [37], Klammer et al. (2013) [38], Shah et al. (2015) [39], Meftah et al. (2016) [40], Fernandes et al. (2018) [41], Okamura et al. (2022) [42], and Lachkar et al. (2024) [43] (Table 1).

Table 1.
Bilateral tenosynovial giant cell tumor (TGCT) cases in the literature.
Bilateral TGCT has also been described in other joints, including the shoulders [44], wrists [45], thumbs [46], hips [47,48,49], ankles [22,50,51], and Achilles tendons [52,53]. Multifocal or bifocal presentations have been rarely documented.
Surgical excision remains the gold standard for TGCT management, using either open or arthroscopic techniques depending on the disease extent. In extra-articular N-TGCT, complete excision usually results in low recurrence rates [29]. For intra-articular N-TGCT of the knee, open surgery appears superior to arthroscopy: Mastboom et al. reported recurrence rates of 9% after open excision versus 18% following arthroscopic removal [54]. In D-TGCT, van der Heijden et al. noted recurrence rates of 14% after open versus 40% after arthroscopic synovectomy [29].
Adjuvant radiotherapy, including external beam radiation (EBR) and radiosynoviorthesis (RSO), has been employed for extensive or recurrent disease, although its use remains controversial due to risks of infection, radionecrosis, and secondary malignancy [55,56].
Molecular advances identifying the CSF1–CSF1R signaling pathway as central to TGCT pathogenesis have led to the development of targeted therapies. Pexidartinib, a selective CSF1R inhibitor, is the first FDA-approved systemic therapy for advanced or unresectable TGCT [57,58,59,60]. Other investigational or selective inhibitors include emactuzumab, cabiralizumab, lacnotuzumab, and sotuletinib [61,62,63,64,65,66], while non-selective inhibitors such as nilotinib, imatinib, and pimicotinib have shown variable efficacy [67,68,69,70]. Most recently, the FDA accepted a New Drug Application (NDA) for vimseltinib, another selective CSF1R inhibitor, offering a promising therapeutic option for patients with TGCT [71,72].
4. Conclusions
Tenosynovial giant cell tumor (TGCT) is a rare, benign, and typically monoarticular neoplasm. Among patients with diffuse TGCT (D-TGCT), the knee joint is the most frequently affected site. Bilateral TGCT—either diffuse or localized—is exceptionally uncommon, with only 14 cases of bilateral knee involvement previously reported in the literature. The present case represents the fifteenth such report and the fifth involving bilateral diffuse-type TGCT of the knees. Our patient exhibited classic clinical, radiologic, and histopathologic features of D-TGCT in both knees. Surgical excision remains the gold standard of treatment, and the choice between open and arthroscopic approaches should be guided by the lesion’s extent and anatomical location. Given the rarity of bilateral TGCT, clinicians should maintain a high index of suspicion when evaluating patients presenting with bilateral knee pain and swelling and include TGCT in the differential diagnosis.
Author Contributions
Conceptualization, V.D.D. and P.J.P.; methodology, P.J.P. and D.P.P.; validation, V.D.D. and P.J.P. data curation, V.D.D., P.J.P. and N.A.S.; writing—original draft preparation, V.D.D., D.P.P., I.T., M.P. and O.P.; writing—review and editing, N.A.S., D.P.P., M.P., O.P. and P.J.P.; supervision, P.J.P. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethics Committee of ATTIKON University General Hospital. Institutional Review Board Approval was obtained for this study (8 December 2021 approval date) (ΕΒΔ 599/8-11-2021).
Informed Consent Statement
The participant provided written informed consent prior to inclusion.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de St Aubain, S.; Van de Rijn, M. Tenosynovial giant cell tumour, localized type. In WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; Fletcher, C.D.M.B.J., Hogendoorn, P.C.W., Mertens, F., Eds.; IARC: Lyon, France, 2013; Volume 5, pp. 100–101. [Google Scholar]
- de St Aubain, S.; Van de Rijn, M. Tenosynovial giant cell tumour, diffuse type. In WHO Classification of Tumours of Soft Tissue and Bone; Fletcher, C.D.M.B.J., Hogendoorn, P.C.W., Mertens, F., Eds.; IARC: Lyon, France, 2013; Volume 5, pp. 102–103. [Google Scholar]
- Granowitz, S.P.; D’Antonio, J.; Mankin, H.L. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin. Orthop. Relat. Res. 1976, 114, 335–351. [Google Scholar]
- Stacchiotti, S.; Dürr, H.R.; Schaefer, I.-M.; Woertler, K.; Haas, R.; Trama, A.; Caraceni, A.; Bajpai, J.; Baldi, G.G.; Bernthal, N.; et al. Best clinical management of tenosynovial giant cell tumor (TGCT): A consensus paper from the community of experts. Cancer Treat. Rev. 2022, 112, 102491. [Google Scholar] [CrossRef] [PubMed]
- Mastboom, M.J.L.; Verspoor, F.G.M.; Verschoor, A.J.; Uittenbogaard, D.; Nemeth, B.; Mastboom, W.J.B.; Bovée, J.V.M.G.; Dijkstra, P.D.S.; Schreuder, H.W.B.; Gelderblom, H.; et al. Higher incidence rates than previously known in tenosynovial giant cell tumors: A nationwide study in the Netherlands. Acta Orthop. 2017, 88, 688–694. [Google Scholar] [CrossRef] [PubMed]
- De St Aubain, S.; van de Rijn, M. Tenosynovial Giant Cell Tumour. In World Health Organization (WHO) Classification of Soft Tissue and Bone Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2020; pp. 133–136. [Google Scholar]
- Ehrenstein, V.; Andersen, S.L.; Qazi, I.; Sankar, N.; Pedersen, A.B.; Sikorski, R.; Acquavella, J.F. Tenosynovial Giant Cell Tumor: Incidence, prevalence, patient characteristics and recurrence. A registry-based cohort study in Denmark. J. Rheumatol. 2017, 44, 1476–1483. [Google Scholar] [CrossRef]
- Chassaignac, E.P. Cancer de la gaine des tendons. Gaz. Des Hop. Civ. Mil. 1852, 25, 185–186. [Google Scholar]
- Simon, G. Exstirpation einer sehr grossen, mit dickem Stiele angewachsenen Kniegelenkmaus mit glucklichem Erfolge. Arch. Klin. Chir. 1865, 6, 573. [Google Scholar]
- Moser, E. Primares sarkom der fussgelenkkapsel. Dtsch. Z. Chir. 1909, 98, 306–310. [Google Scholar] [CrossRef]
- Jaffe, H.L.; Lichenstein, L.; Sutro, C.J. Pigmented: Villonodular synovitis, bursitis and tendosynovitis. Arch. Pathol. 1941, 31, 731–765. [Google Scholar]
- Murphey, M.D.; Rhee, J.H.; Lewis, R.B.; Fanburg-Smith, J.C.; Flemming, D.J.; Walker, E.A. Pigmented villonodular synovitis: Radiologic-pathologic correlation. Radiographics 2008, 28, 1493–1518. [Google Scholar] [CrossRef]
- Hughes, T.H.; Sartoris, D.J.; Schweitzer, M.E.; Resnick, D.L. Pigmented villonodular synovitis: MRI characteristics. Skelet. Radiol. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Flandry, F.; Hughston, J.C. Pigmented villonodular synovitis. J. Bone Jt. Surg. Am. 1987, 69, 942–949. [Google Scholar] [CrossRef]
- West, R.B.; Rubin, B.P.; Miller, M.A.; Subramanian, S.; Kaygusuz, G.; Montgomery, K.; Zhu, S.; Marinelli, R.J.; De Luca, A.; Downs-Kelly, E.; et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc. Natl. Acad. Sci. USA 2006, 103, 690–695. [Google Scholar] [CrossRef]
- Cupp, J.S.; Miller, M.; Montgomery, K.; Nielsen, T.O.; O’Connell, J.X.; Huntsman, D.; van de Rijn, M.; Gilks, C.B.; West, R.B. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am. J. Surg. Pathol. 2007, 31, 970–976. [Google Scholar] [CrossRef]
- Moller, E.; Mandahl, N.; Mertens, F.; Panagopoulos, I. Molecular identification of COL6A3-CSF1 fusion transcript in tenosynovial giant cell tumors. Genes Chromosomes Cancer 2008, 47, 21–25. [Google Scholar] [CrossRef]
- Monaghan, H.; Salter, D.M.; AL-Nafussi, A. Giant cell tumour of tendon sheath(localized nodular tenosynovitis): Clinicopathological features of 71 cases. J. Clin. Pethol. 2001, 54, 404–407. [Google Scholar] [CrossRef]
- Darling, J.M.; Goldring, S.R.; Harada, Y.; Handel, M.L.; Glowacki, J.; Gravallese, E.M. Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am. J Pathol. 1997, 150, 1383–1393. [Google Scholar]
- de saint Aubain Somerhausen, N.; Fletcher, C.D.M. Diffuse type giant cell tumor: Clinikopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am. J. Surg. Pathol. 2000, 24, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, W.; Uzuki, M.; Kurose, A.; Yoshida, M.; Nishida, J.; Shimamura, T.; Sawai, T. Cell characterization of mononuclear and giant cells constiuning pigmented villonodular synovitis. Hum. Pathol. 2003, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.D.; Cotton, R.E.; Deacon, O.W.; Lowy, M.; Newman, P.H.; Sissons, H.A.; Thomson, A.D. The diagnosis and treatment of pigmented villonodular synovitis. J. Bone Jt. Surg. Br. 1968, 50, 290–305. [Google Scholar] [CrossRef]
- Available online: https://emedicine.medscape.com/article/1253223-overview (accessed on 12 April 2024).
- Gaillard, F. Pigmented Villonodular Synovitis (PVNS). Case Study. Available online: https://radiopaedia.org/ (accessed on 5 September 2024).
- Botez, P.; Sirbu, P.D.; Grierosu, C.; Mihailescu, D.; Savin, L.; Scarlat, M.M. Adult multifocal pigmented villonodular Synovitis clinical review. Int. Orthop. 2013, 37, 729–733. [Google Scholar] [CrossRef]
- Masih, S.; Antebi, A. Imaging of pigmented villonodular synovitis. Semin. Musculoskelet. Radiol. 2003, 7, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, S.; Ayral, X.; Dougados, M.; Gossec, L. Pigmented Villonodular Synovitis: A retrospective single-Center study of 122 cases and review of the literature. Semin. Arthritis Rheum. 2011, 40, 539–546. [Google Scholar] [CrossRef]
- Gelhorn, H.L.; Tong, S.; McQuarrie, K.; Vernon, C.; Hanlon, J.; Maclaine, G.; Lenderking, W.; Ye, X.; Speck, R.M.; Lackman, R.D.; et al. Patient-reported Symptoms of Tenosynovial Giant Cell Tumors. Clin Ther. 2016, 38, 778–793. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, L.; Gibbons, C.L.; Hassan, A.B.; Kroep, J.R.; Gelderblom, H.; van Rijswijk, C.S.; Nout, R.A.; Bradley, K.M.; Athanasou, N.A.; Dijkstra, P.D.; et al. A multidisciplinary approach to giant cell tumors of tendon sheath and synovium—A critical appraisal of literature and treatment proposal. J. Surg. Oncol. 2013, 107, 433–445. [Google Scholar] [CrossRef]
- Verspoor, F.G.M.; Zee, A.A.G.; Hannink, G.; van der Geest, I.C.M.; Veth, R.P.H.; Schreuder, H.W.B. Long term follow up results of primary and recurrent pigmented villonodular synovitis. Rheumatology 2014, 53, 2063–2070. [Google Scholar] [CrossRef]
- Kelikian, H.; Lewis, E.K. Arthrograms. Radiology 1949, 52, 465–487. [Google Scholar] [CrossRef]
- Greenfield, M.M.; Wallace, K.M. Pigmented villonodular synovitis. Radiology 1950, 54, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Gehweiler, J.A.; Wilson, J.W. Diffuse biarticular pigmented villonodular synovitis. Radiology 1969, 93, 845–851. [Google Scholar] [CrossRef]
- Sharma, V.; Cheng, E.Y. Outcomes after excision of pigmented villonodular synovitis of the knee. Clin. Orthop. Relat. Res. 2009, 467, 2852–2858. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, J.W.; Ahn, J.H.; Chang, M.J.; Cho, E.Y. Localized tenosynovial giant cell tumor in both knee joints. Skelet. Radiol. 2010, 39, 923–926. [Google Scholar] [CrossRef]
- Soubai, R.B.; Tahiri, L.; Ibrahimi, A. Bilateral pigmented villonodular synovitis of the knee. Jt. Bone Spine 2011, 78, 219–221. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, S.H.; Han, S.B.; Lee, D.K.; Kim, C.H.; Lee, D.H. Bilateral tenosynovial giant cell of the knee accompanied by chronic ACL tear. J. Orthop. Sci. 2012, 17, 93–97. [Google Scholar] [CrossRef]
- Klammer, G.; Betz, M.; Delaloye, B.; Farshad, M.; Peter, K.P. Bilateral diffuse pigmented villonodular synovitis of the knee. J. Knee Surg. 2013, 26, S67–S71. [Google Scholar]
- Shah, S.H.; Porrino, J.A.; Green, J.R.; Chew, F.S. Bilateral pigmented villonodular synovitis of the knee. Rad. Case Rep. 2015, 10, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Meftah, A.; Moumen, A.; Eljadi, H.; Guerboub, A.A.; Elmoussaoui, S.; Belmejdoub, G. Synovite villondulaire bilatérale du genou chez un patient diabétique. Presse Med. 2016, 45, 465–467. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Videira, L.D.; Sasaki, S.U.; Natalino, R.J.M.; DE Almeida, A.M.; Pedrinelli, A.; Hernandez, A.J. Bilateral localized pigmented villonodular synovitis of the knee: Case report and review. Acta Ortop. Bras. 2018, 26, 183–186. [Google Scholar] [CrossRef]
- Okamura, H.; Ishikawa, H.; Ohno, T.; Fujita, S.; Inagaki, K. Localized Pigmented Villonodular Synovitis of the Bilateral Knee, A case report. J. Orthop. Case Rep. 2022, 12, 18–21. [Google Scholar] [CrossRef]
- Lachkar, A.; Najib, A.; Yacoubi, H. Bilateral knee pigmented villonodular synovitis in a young adult: Radiologic diagnosis and surgical approach. Radial. Case Rep. 2024, 20, 145–150. [Google Scholar] [CrossRef]
- Graf, J.; Bernd, L.; Pauschert, R.; Niethard, F.U. Rare occurrence of villonodular synovitis of both shoulder joints. Z Rheumatol. 1991, 50, 46–48. [Google Scholar] [PubMed]
- Jamieson, T.W.; Curran, J.J.; Desmet, A.A.; Cotelingam, J.D.; Kimmich, H. Bilateral pigmented villonodular synovitis of the wrists. Orthop. Rev. 1990, 19, 432–436. [Google Scholar] [PubMed]
- Dalal, S.; Bostock, S.H. Bilateral symmetrical giant cell tumours of the thumb. Hand Surg. 2003, 8, 237–238. [Google Scholar] [CrossRef]
- Eisenberg, R.L.; Hedgcock, M.W. Bilateral pigmented villonodular synovitis of the hip. Br. J. Radiol. 1978, 51, 916–917. [Google Scholar] [CrossRef]
- Cotten, A.; Flipo, R.M.; Chastanet, P.; Desvigne-Noulet, M.C.; Duquesnoy, B.; Delcambre, B. Pigmented villonodular synovitis of the hip: Review of radiographic features in 58 patients. Skelet. Radiol 1995, 24, 1–6. [Google Scholar] [CrossRef]
- Patkar, D.; Prasad, S.; Shah, J.; Patankar, T.; Kothari, S. Pigmented villonodular synovitis: Magnetic resonance features of an unusual case of bilateral hip joint involvement. Australas. Radiol. 2000, 44, 458–459. [Google Scholar] [CrossRef]
- Pavlica, L.; Nikolić, D.; Tadić, J.; Tatić, V.; Bralović, S.; Panajotović, L. Pigmented villonodular synovitis—Analysis of 50 patients. Vojnosanit. Pregl. 1997, 54, 209–216. [Google Scholar]
- Krause, F.G.; Wroblewski, J.A.; Younger, A.S. Pigmented villonodular synovitis in both hindfeet. Can. J. Surg. 2009, 52, E38–E39. [Google Scholar]
- Mohapatra, N.C.; Samal, P.; Mylarappa, A.; Mishra, J. Bilateral giant cell tumor of tendo achilles: A case series on reconstruction by peroneus brevis-tibialis posterior tendon. Foot 2021, 48, 101813. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Ghosh, S.; Chaudhuri, A.; Moulik, S.G. Bilateral giant cell tumor of tendon sheath of tendoachilles. Med. J. Dr. Patil. Univ. 2014, 7, 211–214. [Google Scholar]
- Mastboom, M.J.L.; Staals, E.L.; Verspoor, F.G.M.; Rueten-Budde, A.J.; Stacchiotti, S.; Palmerini, E.; Schaap, G.R.; Jutte, P.C.; Aston, W.; Leithner, A.; et al. Surgical Treatment of Localized-Type Tenosynovial Giant Cell Tumors of Large Joints: A Study Based on a Multicenter-Pooled Database of 31 International Sarcoma Centers. J. Bone Jt. Surg. Am. 2019, 101, 1309–1318, Erratum in J. Bone Jt. Surg. Am. 2020, 102, e49. [Google Scholar] [CrossRef] [PubMed]
- Kampen, W.U.; Boddenberg-Patzold, B.; Fischer, M.; Gabriel, M.; Klett, R.; Konijnenberg, M.; Kresnik, E.; Lellouche, H.; Paycha, F.; Terslev, L.; et al. The EANM guideline for radiosynoviorthesis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Sewerin, P.; Ohlerth, S.M.; Pomykala, K.L.; Freudenberg, S.; Rischpler, C.; Lützen, U. The future of radiosynoviorthesis: Bright or bleak? Q. J. Nucl. Med. Mol. Imaging 2022, 66, 345–351. [Google Scholar] [CrossRef]
- Tap, W.D.; Wainberg, Z.A.; Anthony, S.P.; Ibrahim, P.N.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Staddon, A.P.; Cohn, A.L.; Shapiro, G.I.; et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N. Engl. J. Med. 2015, 373, 428–437. [Google Scholar] [CrossRef]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.-Y.; Alcindor, T.; Ganjoo, K.; Martín-Broto, J.; Ryan, C.W.; et al. Pexidartinb versus placebo for advanced tenosynovial cell tumour (ENLIVEN): A randomized phase 3 trial. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef]
- Gelderblom, H.; van de Sande, M. Pexidartinib: First approved systemic therapy for patients with tenosynovial giant cell tumor. Future Oncol. 2020, 16, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Wagner, A.J.; Tap, W.D.; Palmerini, E.; Wainberg, Z.A.; Desai, J.; Healey, J.H.; van de Sande, M.A.J.; Bernthal, N.M.; Staals, E.L.; et al. Long-term outcomes of pexidartinib in tenosynovial giant cell tumors. Cancer 2021, 127, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.A.; Le Tourneau, C.; Toulmonde, M.; Cannarile, M.A.; Ries, C.; Brillouet, A.; Müller, C.; Jegg, A.-M.; et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.; Bröske, A.M.; Rüttinger, D.; Mueller, C.; Phipps, A.; Walz, A.; Ries, C.; Baehner, M.; Cannarile, M.; Meneses-Lorente, G. PK/PD Mediated Dose Optimization of Emactuzumab, a CSF1R Inhibitor, in Patients with Advanced Solid Tumors and Diffuse-Type Tenosynovial Giant Cell Tumor. Clin. Pharmacol. Ther. 2020, 108, 616–624. [Google Scholar] [CrossRef]
- Sankhala, K.K.; Blay, J.-Y.; Ganjoo, K.N.; Italiano, A.; Hassan, A.B.; Kim, T.M.; Ravi, V.; Cassier, P.A.; Rutkowski, P.; Sankar, N.; et al. A phase I/II dose escalation and expansion study of cabiralizumab (cabira; FPA-008), an anti-CSF1R antibody, in tenosynovial giant cell tumor (TGCT, diffuse pigmented villonodular synovitis D-PVNS). J. Clin. Oncol. 2017, 35, 11078. [Google Scholar] [CrossRef]
- MCS110 in Patients with Pigmented Villonodular Synovitis (PVNS). ClinicalTrials.gov. IDNCT01643850. Available online: https://clinicaltrials.gov/study/NCT01643850?tab=results (accessed on 29 October 2023).
- Available online: https://www.creativebiolabs.net/lacnotuzumab-overview.htm (accessed on 15 December 2023).
- Thongchot, S.; Duangkaew, S.; Yotchai, W.; Maungsomboon, S.; Phimolsarnti, R.; Asavamongkolkul, A.; Thuwajit, P.; Thuwajit, C.; Chandhanayingyong, C. Novel CSF1R-positive tenosynovial giant cell tumor cell lines and their pexidartinib (PLX3397) and sotuletinib (BLZ945)-induced apoptosis. Hum. Cell 2022, 36, 456–467. [Google Scholar] [CrossRef]
- Gelderblom, H.; Cropet, C.; Chevreau, C.; Boyle, R.; Tattersall, M.; Stacchiotti, S.; Italiano, A.; Piperno-Neumann, S.; Le Cesne, A.; Ferraresi, V.; et al. Nilotinib in locally advanced pigmented villonodular synovitis: A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2018, 19, 639–648. [Google Scholar] [CrossRef]
- Spierenburg, G.; Grimison, P.; Chevreau, C.; Stacchiotti, S.; Piperno-Neumann, S.; Le Cesne, A.; Ferraresi, V.; Italiano, A.; Duffaud, F.; Penel, N.; et al. Long-term follow-up of nilotinib in patients with advanced tenosynovial giant cell tumours. Eur. J. Cancer 2022, 173, 219–228. [Google Scholar] [CrossRef]
- Verspoor, F.G.M.; Mastboom, M.J.L.; Hannink, G.; Maki, R.G.; Wagner, A.; Bompas, E.; Desai, J.; Italiano, A.; Seddon, B.M.; van der Graaf, W.T.A.; et al. Long-Term Efficacy of Imatinib Mesylate in Patients with Advanced Tenosynovial Giant Cell Tumor. Sci. Rep. 2019, 9, 14551. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhou, Y.; Li, B.; Xie, X.; Du, X.; Yuan, T.; Chen, B.; Zou, Q.; Guo, Q.; Liang, P.; et al. Efficacy and safety profile of pimicotinib (ABSK021) in Tenosynovial Giant Cell Tumor (TGCT): Phase 1b update. J. Clin. Oncol. 2023, 41, 11559. [Google Scholar] [CrossRef]
- Available online: https://www.onclive.com/view/vimseltinib-improves-25-week-orr-vs-placebo-in-tgct (accessed on 16 November 2023).
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-vimseltinib-symptomatic-tenosynovial-giant-cell-tumor (accessed on 2 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.