Abstract
Background/Objectives: To systematically review the literature and to characterize the utility of lactate and pH for predicting survival and long-term neurological outcomes after out-of-hospital cardiac arrest (OHCA). Methods: PRISMA guidelines were followed. PubMed, Embase, Web of Science, Cochrane Central, and Academic Search Premier were searched for relevant studies. The population included adults with OHCA. Studies with majority in-hospital cardiac arrest (>50%) and studies predicting return of spontaneous circulation (ROSC) were excluded. Pairs of investigators reviewed the studies for relevance. Data were extracted and risk of bias was assessed using the Newcastle–Ottawa Scale. Meta-analyses were performed to characterize the relationship between lactate and pH with survival and neurological outcomes. Results: We included 21,120 patients over 49 studies. Most studies (78%) included OHCA only. Mean lactate of 7.24 (95%CI:6.05–8.44) was associated with favorable survival (n = 9155; 21 studies), while mean lactate of 7.15 (95%CI:6.37–7.93) was associated with favorable neurological outcome (n = 7534; 21 studies). Mean pH of 7.22 (95%CI:7.10–7.33) was associated with favorable survival (n = 4077; 7 studies), while a mean pH of 7.22 (95%CI:7.17–7.27) was associated with favorable neurological outcome (n = 6701; 13 studies). Poor outcomes were associated with lower pH and higher lactate values. Risk of bias was generally low to medium, while heterogeneity was high. Conclusions: A direct correlation exists between pH with survival and neurological outcome; the likelihood of favorable outcomes increases as pH increases. Conversely, an inverse relationship exists between lactate with survival and neurological outcome; higher lactate is associated with poorer outcomes. For lactate, the threshold for survival was more lenient than for favorable neurological outcome.
1. Introduction
The annual incidence of out-of-hospital cardiac arrest (OHCA) in the United States is 356,000 per year [1,2]. Survival is poor, and less than 10% of emergency medical services-(EMS)-treated nontraumatic OHCA patients survive to hospital discharge [2,3].
Although return of spontaneous circulation (ROSC) is a crucial step in OHCA survival, ROSC is merely the first step in survival and does not necessarily confer other patient-centered outcomes, such as hospital discharge or favorable neurological recovery. Despite ROSC, patients may experience poor neurological recovery, severe neurological or functional deficits, loss of quality of life, delayed withdrawal of care, and significant psychosocial strain on family members [4,5,6,7,8,9].
Post-cardiac arrest care accounts for USD 5.61 billion annually in the United States. Patients who do not survive to hospital discharge account for nearly 58% of this expenditure [10]. Therefore, significant research has been directed towards identifying specific patient cohorts that will benefit from post-cardiac arrest interventions, such as targeted temperature management [11,12], early coronary angiography [13,14,15,16], and extracorporeal cardiopulmonary resuscitation (ECPR) [17].
Identifying specific variables and thresholds associated with favorable versus unfavorable survival and neurological outcomes is essential in guiding clinicians in medical decision-making and meaningful discussions with families regarding evidence-based expectations.
Lactate and pH are two laboratory markers which are readily available in most hospital contexts and are associated with relative hypoxia time. Prior literature has shown a correlation with these markers and survival [18,19,20,21]. The goal of this systematic review and meta-analysis was to identify specific lactate and pH thresholds beyond which meaningful outcomes and survival may become statistically improbable.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The PRISMA checklist is presented in the Supplemental Content. The research protocol is provided in the Supplemental Content and was prospectively registered at the International Prospective Register of Systematic Reviews (PROSPERO no. CRD42024563136).
2.2. Eligibility Criteria and Outcomes
We used the PICO framework (Population, Intervention, Comparison, Outcome) to frame the study question: in adults (≥18 years) with ROSC after nontraumatic OHCA (P), does higher serum pH or lower serum lactate (I), as compared to lower pH or higher lactate (C), have any impact on long-term patient-centered outcomes (O)?
The outcomes included survival and neurological outcomes at and after hospital discharge. Favorable neurological outcome was defined as a Cerebral Performance Category (CPC) score of 1–2, modified Rankin Scale (mRS) of 0–3, or Glasgow outcome scale (GOS) of 4–5. Poor neurological outcome was defined as a CPC of 3–5, mRS of 4–6, or GOS of 1–3. Included time intervals ranged from 24 h to 1 year after OHCA.
Randomized controlled trials, non-randomized controlled trials, and observational studies with a comparison group were included. Animal studies, reviews, abstracts, editorials, comments, and letters to the editor were excluded. Studies using intra-arrest laboratory values to predict ROSC were excluded. Studies evaluating serum pCO2 or analyzing labs prior to ECPR initiation were excluded. Mixed studies containing both OHCA and in-hospital cardiac arrest (IHCA) patients were excluded if IHCA patients represented more than 50% of the study population or if the IHCA:OHCA ratio was not reported. Studies primarily focusing on pediatric patients were excluded. There were no limitations on publication period or manuscript language (provided an English abstract existed).
2.3. Information Sources and Search Strategy
The search terms and strategy were developed in collaboration with a research librarian specializing in systematic reviews. We searched PubMed, Embase, Web of Science, Cochrane Central, and Academic Search Premier databases on 7 December 2022. We performed an updated search using the same search strategy and databases on 15 August 2024 to include papers from December 2022 to August 2024. The search strategy is included in the Supplemental Content.
2.4. Study Selection
Using pre-determined screening criteria, pairs of reviewers independently screened all titles and abstracts retrieved by the query. Kappa statistics were calculated to determine inter-rater agreement. Any discrepancies regarding inclusion and exclusion of screened articles were resolved by discussion between the reviewer pair, with a third reviewer adjudicating unresolved discrepancies. Articles selected for full-text appraisal were assessed by a pair of reviewers and yielded a final selection of articles for data extraction. Disagreements regarding article eligibility were resolved by discussion between the reviewer pair.
2.5. Data Collection
Using a predefined data extraction tool (provided in Supplemental Content), data were independently extracted from each included article by a pair of reviewers. Briefly, extracted data included study design, inclusion and exclusion criteria, population statistics (e.g., age, sex, sample size), and exposure (i.e., pH or lactate) with its respective outcomes (i.e., survival or neurological outcome) and statistics (e.g., mean, median, odds ratios, etc.). Missing data were calculated from provided data if possible. Discrepancies in extracted data were resolved by discussion and consensus decision.
2.6. Risk of Bias for Individual Studies
Given the nature of the PICO, it was projected that most included articles would be nonrandomized studies. The Newcastle–Ottawa scale (NOS) is an instrument developed to systematically assess quality for nonrandomized studies in a systematic review [23]. Therefore, for each included article, two authors independently evaluated the risk of bias using the NOS, and disagreements regarding quality scoring were resolved by discussion.
2.7. Data Synthesis and Analysis
Using the extracted data, the main outcome measures of interest were dichotomized into favorable versus poor (i.e., favorable survival, poor survival, favorable neurological outcome, poor neurological outcome). Means and standard deviations, if available, were extracted and used for effect size. If the mean was available but not the standard deviation, the standard deviation was estimated using the reported p-value or interquartile range. For studies only reporting medians and interquartile ranges, methods from Luo et al. [24] were used to estimate the mean, and methods from Wan et al. [25] were used to estimate the standard deviation.
2.7.1. Mean Analysis
A meta-analysis of means (pH and lactate) by outcome type was performed using the meta package in R by pooling the raw means and standard deviations using the restricted maximum-likelihood estimator and the inverse variance method [26,27]. Random-effects models for the comparative outcome groups were run, and the Hartung–Knapp adjustment was applied.
Some degree of heterogeneity was expected as the studies analyzed different outcome measures at varying time intervals. Therefore, a “full” meta-analysis including all studies was performed. A final model excluded outliers [28]. Forest plots for the final models were created using the metafor package [29].
A meta-analysis of standardized mean differences (pH and lactate) between outcomes was performed in a similar fashion.
2.7.2. Odds Ratio Analysis
Using studies that reported pH and lactate odds ratios for outcomes, a meta-analysis was performed for pH and lactate odds ratios. Extracted odds ratios and confidence intervals were log-transformed and pooled using the inverse variance method in random-effects models. The Paule–Mandel estimator was used to calculate τ2.
3. Results
3.1. Identified Studies
The search query identified 5504 unique records, of which 5357 records were excluded after review of the titles and abstracts. The Kappa for the initial screening was 0.55. Of the 147 full-text article reviews, 98 were excluded (Kappa = 0.60) for the reasons listed in Figure 1, leaving a total of 49 articles [9,18,19,20,21,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Several studies passed initial screening, but were excluded during the full-text review because they consisted of a majority IHCA (i.e., >50%) or they included both IHCA and OHCA but did not specify a percentage of each [74,75,76].
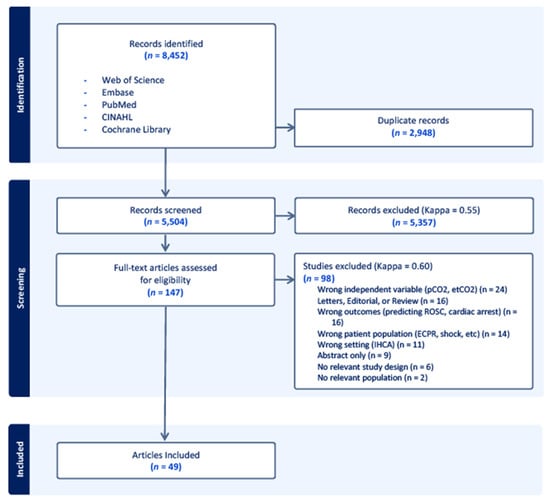
Figure 1.
PRISMA diagram demonstrating the selection of articles during the review process. Out of 5504 screened articles, 147 articles underwent full-text analysis to determine eligibility, and 49 articles were ultimately included for data extraction. Of the included studies, 36 were retrospective cohort studies, 11 were prospective observational cohort studies, one was a combined retrospective and prospective cohort study, and one study was randomized.
3.2. Overview of Included Studies
Of the 49 included articles, 36 articles were retrospective observational cohort studies, 11 were prospective observational cohort studies, one was a prospective randomized controlled trial, and one was a combined retrospective and prospective observational cohort study. Only one study was randomized [71]. The studies included between 32 and 4189 patients, and nine studies included more than 500 patients. Altogether, the studies included 21,120 patients. Studies were conducted in Asia (n = 22), Europe (n = 14), North America (n = 11), Australia (n = 1), and multicontinental (n = 1). All studies were described as including only adult patients. Two studies included ages 16 and above [39,58]; due to the apparent very small number of patients in these studies under the age of 18 and the difficulty in separating these few patients out, the decision was made to include the studies. A brief overview of these studies is provided in Table 1 and detailed information including the results is provided in Appendix A and the Supplemental Content.

Table 1.
Overview of all included studies.
3.2.1. Lactate and Survival Analysis
Twenty-one articles compared lactate levels with survival outcome. Sixteen studies were retrospective cohort studies, while five were prospective observational studies for a total of 9155 patients (favorable survival, 3563; poor survival, 5592). Outcome duration ranged from hospital admission to 6-month survival. Means and standard deviations were deduced for 11 studies. In the final model after outliers were removed (n = 3977), a mean lactate of 10.11 (95%CI: 8.98–11.25, p < 0.0001) was associated with poor survival, while a mean lactate of 7.24 (95%CI: 6.05–8.44, p < 0.0001) was associated with favorable survival (Table 2, Figure 2A).

Table 2.
Post-out-of-hospital-cardiac arrest lactate and pH thresholds for favorable and unfavorable survival and neurological outcomes, outliers removed. Abbreviations: CI, confidence interval; G, pooled effect size (mean); I2, percentage of variability in the effect size not caused by sampling error; τ2, variance of true effect size.
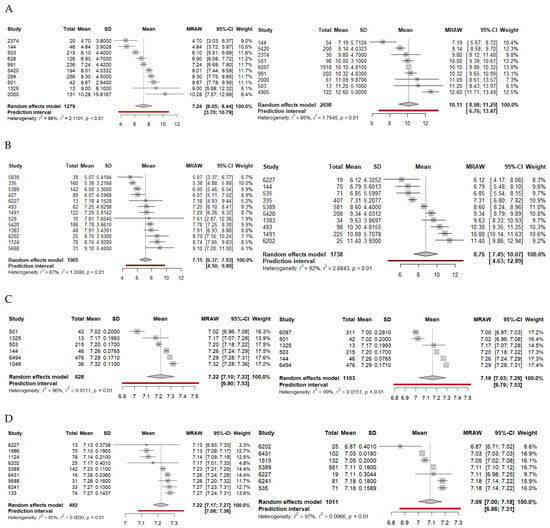
Figure 2.
Forest plots for survival (A) and neurological outcome (B) relative to lactate after outliers are removed. Forest plots for survival (C) and neurological outcome (D) relative to pH after outliers are removed. Favorable outcomes are on the left, and unfavorable outcomes are on the right. Abbreviations: CI, confidence interval; MRAW, raw mean; SD, standard deviation.
The random effects model provided a mean-difference effect size of 2.21 (95%CI: 1.76–2.65, p < 0.0001) after outliers were removed, indicating a lactate difference of nearly 2 between patients with favorable versus poor survival outcomes.
3.2.2. Lactate and Neurological Outcome Analysis
Twenty-one articles compared lactate levels with neurological outcome. Fifteen studies were retrospective studies, five were prospective observational studies, and one was a prospective randomized clinical trial for a total of 7534 patients (favorable neurological outcome, 1730; poor neurological outcome, 5804). Outcome duration ranged from hospital discharge to one-year neurological outcome. Means and standard deviations were deduced for 15 studies. In the final model after outliers were removed (n = 2743), a mean lactate of 8.76 (95%CI: 7.45–10.07, p < 0.0001) was associated with poor neurological outcome, while a mean lactate of 7.15 (95%CI: 6.37–7.93, p < 0.0001) was associated with favorable neurological outcome (Table 2, Figure 2B).
The random effects model provided a mean-difference effect size of 2.28 (95%CI: 1.91–2.64, p < 0.0001) after outliers were removed, indicating a lactate difference of nearly 2 between patients with favorable versus poor neurological outcomes.
3.2.3. pH and Survival Analysis
Seven articles compared pH levels with survival outcome. Six studies were retrospective studies, while one was a prospective observational study for a total of 4077 patients (favorable survival, 1139; poor survival, 2305). Outcome duration ranged from hospital admission to 28-day survival. Means and standard deviations were deduced for four studies. In the final model (n = 1931), a mean pH of 7.16 (95%CI: 7.03–7.29, p < 0.0001) was associated with poor neurological outcome, while a mean pH of 7.22 (95%CI: 7.10–7.33, p < 0.0001) was associated with favorable survival (Table 2, Figure 2C).
The random effects model found a mean-difference effect size of −0.09 (95%CI: −0.16, −0.02; p = 0.18).
3.2.4. pH and Neurological Outcome Analysis
Thirteen articles compared pH levels with neurological outcome. Nine studies were retrospective studies, while four were prospective observational studies for a total of 6701 patients (favorable neurological outcome, 781; poor neurological outcome, 5920). Outcome duration ranged from hospital discharge to 6-month neurological outcome. Means and standard deviations were deduced for seven studies. In the final model after outliers were removed (n = 1493), a mean pH of 7.09 (95%CI: 7.00–7.18, p < 0.0001) was associated with poor neurological outcome, while a mean pH of 7.22 (95%CI: 7.17–7.27, p < 0.001) was associated with favorable neurological outcome (Table 2, Figure 2D).
The random effects model found a mean-difference effect size of −0.13 (95%CI: −0.16, −0.10; p < 0.0001) after outliers were removed, indicating a pH difference of nearly −0.13 between patients with favorable versus poor neurological outcomes.
3.2.5. Risk of Bias
Applying the Newcastle–Ottawa Scale found that out of 49 studies, 8 were found to have a medium risk of bias (7%) [36,37,39,40,42,48,53,57]. The remaining were rated as having a low risk of bias. Details regarding bias assessments are provided in Table 3 and the Supplemental Content.

Table 3.
Overview of risk of bias assessments.
Three studies [9,30,57] did not present means, medians, or odds ratios relative to the measured outcomes. When considering each prognostic marker (i.e., lactate or pH) and their respective outcomes, the five medium risk studies were distributed as follows: lactate and neurological outcome (one included in final mean model, one excluded as an outlier, one included in final odds ratio model), lactate and survival (one included in final mean model, one excluded as an outlier, one included in final odds ratio model), pH and neurological outcome (one excluded as an outlier), pH and survival (zero included, zero excluded).
4. Discussion
Favorable outcomes after OHCA are possible; therefore, it is important to ascertain the potential for recovery to make educated, evidence-based decisions and to guide family discussions. Our meta-analysis found that the likelihood of survival and favorable neurological outcome increases as pH increases. Conversely, an increase in lactate is associated with poorer outcomes.
Another goal of the meta-analysis was to identify specific lactate and pH values beyond which meaningful recovery would be statistically improbable. We found that the pooled mean lactate associated with poor survival was 10.11 (95%CI: 8.98–11.25, p < 0.0001) while the mean lactate associated with favorable survival was 7.24 (95%CI: 6.05–8.44, p < 0.0001). The mean lactate associated with poor neurological outcome was 8.76 (95%CI: 7.45–10.07, p < 0.0001) while the mean lactate associated with favorable neurological outcome was 7.15 (95%CI: 6.37–7.93, p < 0.0001). This would suggest that a lactate of 11.25 is specific for poor survival while 10.07 is specific for poor neurological outcome. A lactate of 8.44 is sensitive for favorable survival, and 7.93 is sensitive for favorable neurological outcome.
Similarly, a pH of 7.03 is specific for poor survival while 7.00 is specific for poor neurological outcome. A pH of 7.10 is sensitive for favorable survival, and 7.17 is sensitive for favorable neurological outcome.
Put differently, a lactate greater than 11.25 or a pH less than 7.03 is highly suggestive of poor long-term survival, and a lactate greater than 10.07 or a pH less than 7.00 is highly suggestive of poor neurological outcome.
For lactate, the thresholds for favorable survival were more lenient than the thresholds for favorable neurological outcome (e.g., lactate of 7.24 vs. 7.15, respectively). The survival–neurological outcome relationship is consistent with Cardiac Arrest Registry to Enhance Survival (CARES) data [2]. The survival–neurological outcome relationship is also consistent with data derived from studies evaluating post-hypoxic organ injury in potential organ donors [77]. A patient may survive a hypoxic insult but have poor neurologic prognosis.
Careful analysis of our data shows that heterogeneity may provide a strong foundation for generalizability. Our I2 values ranged from 71 to 99%, which represents moderately high heterogeneity and classically raises practical questions regarding the effect size. However, pragmatic studies accept all-comers from a particular group of interest, which is found in clinical practice, and are therefore more applicable to broader populations and face fewer issues with external validation.
Sampling differences could explain the variation in our lactate data. Despite the heterogeneity, the τ2 is very low, suggesting that the variation is low despite the wide range of effect sizes between included studies. Furthermore, after removing outliers, the calculated mean demonstrated minimal change in nearly every case, further reinforcing the notion that variability is low. Therefore, the calculated pH and lactate averages are likely comparable to the actual means and can be useful for prognostication.
Similar to our data, Seeger et al. [53] found that high lactate and low pH on admission were associated with death or severe hypoxic brain injury within 30 days after OHCA. Takaki et al. [58] also found significantly higher pH levels in patients with favorable 6-month neurological recovery. In contrast, a 2018 study by Dadeh et al. [66] showed no correlation between initial serum lactate with survival to hospital discharge. This is likely due to the limits of a single-center study with only 207 patients, which limits its generalizability, power, and inability to control confounding factors.
The 49 included studies were dichotomized into two groups using the Newcastle–Ottawa score, ≥7 (low risk of bias) or <7 (medium to high risk of bias). Forty-one out of forty-nine studies (83.7%) received a score ≥7 by independent scoring and majority agreement between primary authors. The results suggest there is low risk of bias within the systematic review and the quality of the original articles is high. Strict control of inclusion criteria ensured the systematic review closely resembles the intended study population. Moreover, outcomes were robustly documented given electronic medical record (EMR) utilization and record linkage. Comparison between cohorts is limited because patient-centered outcomes are dependent on multiple pre-existing patient factors such as age, gender, and comorbidities that are impossible to control; this was the principal contributor of bias.
Prospective validation of our findings is required. This systematic review and meta-analysis demonstrates that a correlation exists between lactate and pH with long-term outcomes. Future studies will need to be performed to validate our findings and to develop a clinical decision tool.
Limitations
Limitations include the retrospective nature and the natural heterogeneity of the meta-analysis, such as varying demographics, comorbidities, and inclusion criteria. A few other limitations warrant discussion. While most included studies exclusively enrolled patients who suffered OHCA, a few studies also included a small portion of IHCA. None of these had >50% IHCA, and in our sample, IHCA represented only a small minority of patients.
One major area of heterogeneity was the follow-up length. Most studies had at least 30-day survival listed as a major outcome, with a few reporting data through 90 days or longer. A smaller number had less common milestones such as 14, 45, or 60-day survival. In total, the resulting 30-day survival marker utilized in our analysis was preserved.
Another source of heterogeneity was time from ROSC to collection of initial samples. “Post-ROSC” times have variable definitions, from immediately after ED arrival to several hours after ROSC. This does not consider pre-hospital down-time, nor laboratory sampling errors, sampling variations between equipment, and variations in laboratory analyses. The above factors could explain the wide range in our data for lactate (~3.8–~12.8). pH, on the other hand, had a tighter range, which can be explained by the body’s homeostasis mechanisms and physiological buffering systems. Nevertheless, many studies included lactate and blood gas measurements within 24 h of ROSC, a critical portion of time in the post-arrest period. The consistent nature of survival having a more lenient threshold than neurological outcome longitudinally across all examined studies speaks to the internal validity of our results.
Additionally, our results show that removing outliers had minimal impact on our results despite the presence of heterogeneity. An interpretation of this could be that many of the unknown confounding factors are self-controlled, and the true population means for pH and lactate lie within our predicted range.
5. Conclusions
Out-of-hospital cardiac arrest accounts for significant morbidity, mortality, and healthcare spending in the United States and around the world. Initial post-ROSC serum lactate and pH values can be powerful prognostic indicators for meaningful recovery as defined by the patient’s wishes and family. Most importantly, these tools can help clinicians educate families and adjudicate resources with an informed approach.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14072244/s1, Document S1: Supplemental Contents; Table S1: Detailed Collected Data for All Studies; Table S2: Detailed Risk of bias.
Author Contributions
Conceptualization, C.T.C.; methodology, C.T.C. and C.W.H.; software, C.T.C., C.W.H., N.T.P. and C.M.H.; validation, C.W.H., C.T.C. and N.T.P.; formal analysis, C.M.H.; investigation, C.W.H., C.T.C. and N.T.P.; resources, C.W.H., C.T.C. and N.T.P.; data curation, C.T.C., C.W.H., N.T.P. and C.M.H.; writing—original draft preparation, C.T.C., C.W.H., N.T.P. and C.M.H.; writing—review and editing, C.T.C., C.W.H., N.T.P. and C.M.H.; visualization, C.T.C. and C.W.H.; supervision, C.W.H.; project administration, C.W.H. and C.T.C.; funding acquisition, C.W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This systematic review was funded by the University of Florida Emergency Medicine Structured Academic Research (STAR) Grant.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to thank Patti McCall-Junkin, MA, MLS, systematic review librarian at the University of Florida College of Medicine, Gainesville, FL, USA, for preparing and conducting the systematic searches.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area under the curve |
| CPR | Cardiopulmonary resuscitation |
| CARES | Cardiac Arrest Registry to Enhance Survival |
| CPC | Cerebral Performance Category |
| ECPR | Extracorporeal cardiopulmonary resuscitation |
| EMR | Electronic medical record |
| EMS | Emergency medical services |
| GOS | Glasgow outcome scale |
| HR | Hazards ratio |
| IHCA | In-hospital cardiac arrest |
| IQR | Interquartile range |
| mRS | Modified Rankin Scale |
| NOS | Newcastle–Ottawa scale |
| OHCA | Out-of-hospital cardiac arrest |
| OR | Odds ratio |
| PICO | Population, intervention, comparison, outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROSC | Return of spontaneous circulation |
| SD | Standard deviation |
Appendix A
This appendix contains the full analysis of pH and lactate with regards to survival and neurological outcome with and without outliers, using the random effects model with Knapp–Hartung adjustment. These data are intended to be compared to the pooled analysis reported in the body of our SRMA.
Appendix A.1. Lactate and Survival Analysis
Twenty-one articles compared lactate levels with survival outcome. Sixteen studies were retrospective cohort studies, while five were prospective observational studies for a total of 9155 patients (favorable survival, 3563; poor survival, 5592). Outcome duration ranged from hospital admission to 6-month survival. Eleven studies reported either medians or IQRs, from which means and standard deviations were deduced. In the pooled analyses for patients with lactate levels drawn after ROSC (n = 9155), a mean lactate threshold of 9.51 (95% CI: 8.36–10.66, I2 = 98.4%, Q = 1222.23 [p < 0.0001]) was associated with poor survival, while a mean lactate threshold of 7.16 (95% CI: 5.87–8.44; I2 = 99.1%, Q = 2184.52 [p < 0.0001]) was associated with favorable survival.
In the final model after outliers were removed (n = 3977), a lactate threshold of 10.11 (95% CI: 8.98–11.25, I2 = 86.1%, Q = 57.6 [p < 0.0001]) was associated with poor survival, while a mean lactate threshold of 7.24 (95% CI: 6.05–8.44, I2 = 88.4%, Q = 77.86 [p < 0.0001]) was associated with favorable survival (Table 3, Figure 2A). Detailed results are included in the Supplemental Content.
The random effects model with Knapp–Hartung Adjustment provided a pooled mean-difference effect size of 2.23 (95% CI: 1.59–2.86, p < 0.0001, τ2 = 1.41), indicating a lactate difference of nearly 2 between patients with favorable versus poor survival outcomes. After outliers were removed, the mean-difference effect size was 2.21 (95% CI: 1.76–2.65, p < 0.0001, τ2 = 0.31).
A meta-analysis of odds ratios is presented in the Supplemental Content.
Appendix A.2. Lactate and Neurological Outcome Analysis
Twenty-one articles compared lactate levels with neurological outcome. Fifteen studies were retrospective studies, five were prospective observational studies, and one was a prospective randomized clinical trial for a total of 7534 patients (favorable neurological outcome, 1730; poor neurological outcome, 5804). Outcome duration ranged from hospital discharge to one-year neurological outcome. Fifteen studies reported either medians or IQRs, from which means and standard deviations were deduced. In the pooled analyses for patients with lactate levels drawn after ROSC (n = 7534), a mean lactate threshold of 8.82 (95% CI: 7.46–10.19, I2 = 99.7%, Q = 6819.2 [p < 0.0001]) was associated with poor neurological outcome, while a mean lactate threshold of 6.74 (95% CI: 5.68–7.80; I2 = 98.7%, Q = 1524.94 [p < 0.0001]) was associated with favorable neurological outcome.
In the final model after outliers were removed (n = 2743), a mean lactate threshold of 8.76 (95% CI: 7.45–10.07, I2 = 92.2%, Q = 114.69 [p < 0.0001]) was associated with poor neurological outcome, while a mean lactate threshold of 7.15 (95% CI: 6.37–7.93, I2 = 86.5%, Q = 88.96 [p < 0.0001]) was associated with favorable neurological outcome (Table 3, Figure 2B). Detailed results are included in the Supplemental Content.
The random effects model with Knapp–Hartung Adjustment provided a pooled mean-difference effect size of 1.99 (95% CI: 1.23–2.76, p < 0.0001, τ2 = 2.32), indicating a lactate difference of nearly 2 between patients with favorable versus poor neurological outcomes. After outliers were removed, the mean-difference effect size was 2.28 (95% CI: 1.91–2.64, p < 0.0001, τ2 = 0.19).
A meta-analysis of odds ratios is presented in the Supplemental Content.
Appendix A.3. pH and Survival Analysis
Seven articles compared pH levels with survival outcome. Six studies were retrospective studies, while one was a prospective observational study for a total of 4077 patients (favorable survival, 1139; poor survival, 2305). Outcome duration ranged from hospital admission to 28-day survival. Four studies reported either medians or IQRs, from which means and standard deviations were deduced. In the pooled analyses for patients with pH levels drawn after ROSC (n = 4077), a mean pH threshold of 7.08 (95% CI: 6.94–7.21, I2 = 98.9%, Q = 549.55 [p < 0.0001]) was associated with poor survival, while a mean pH threshold of 7.18 (95% CI: 7.06–7.30; I2 = 98.3%, Q = 358.60 [p < 0.0001]) was associated with favorable survival.
In the final model after outliers were removed (n = 1931), a mean pH threshold of 7.16 (95% CI: 7.03–7.29, I2 = 98.5%, Q = 335.23 [p < 0.0001]) was associated with poor neurological outcome, while a mean pH threshold of 7.22 (95% CI: 7.10–7.33, I2 = 95.8%, Q = 117.77 [p < 0.0001]) was associated with favorable survival (Table 3, Figure 2C). Detailed results are included in the Supplemental Content.
The random effects model with Knapp–Hartung Adjustment provided a pooled mean-difference effect size of −0.09 (95% CI: −0.16, −0.02; p = 0.18; τ2 = 0.004), indicating a pH difference of −0.09 between patients with favorable versus poor survival outcomes. No outlier studies were identified for the random effects model.
Appendix A.4. pH and Neurological Outcome Analysis
Thirteen articles compared pH levels with neurological outcome. Nine studies were retrospective studies, while four were prospective observational studies for a total of 6701 patients (favorable neurological outcome, 781; poor neurological outcome, 5920). Outcome duration ranged from hospital discharge to 6-month neurological outcome. Seven studies reported either medians or IQRs, from which means and standard deviations were deduced. In the pooled analyses for patients with pH levels drawn after ROSC (n = 6701), a mean pH threshold of 7.07 (95% CI: 6.99–7.14, I2 = 99.4%, Q = 1868.17 [p < 0.0001]) was associated with poor neurological outcome, while a mean pH threshold of 7.21 (95% CI: 7.17–7.26; I2 = 92.7%, Q = 164.84 [p < 0.001]) was associated with favorable neurological outcome.
In the final model after outliers were removed (n = 1493), a mean pH threshold of 7.09 (95% CI: 7.00–7.18, I2 = 97.4%, Q = 230.59 [p < 0.0001]) was associated with poor neurological outcome, while a mean pH threshold of 7.22 (95% CI: 7.17–7.27, I2 = 85.4%, Q = 54.72 [p < 0.001]) was associated with favorable neurological outcome (Table 3, Figure 2D). Detailed results are included in the Supplemental Content.
The random effects model with Knapp–Hartung Adjustment provided a pooled mean-difference effect size of −0.14 (95% CI: −0.20, −0.08; p = 0.0001; τ2 = 0.007), indicating a pH difference of nearly −0.14 between patients with favorable versus poor neurological outcomes. After outliers were removed, the mean-difference effect size was −0.13 (95% CI: −0.16, −0.10; p < 0.0001; τ2 = 0.0005).
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Girotra, S.; Jones, P.G.; McNally, B.; Spertus, J.A.; Chan, P.S.; CARES Surveillance Group. Variation in Out-of-Hospital Cardiac Arrest Survival Across Emergency Medical Service Agencies. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008755. [Google Scholar] [CrossRef] [PubMed]
- Lilja, G.; Nilsson, G.; Nielsen, N.; Friberg, H.; Hassager, C.; Koopmans, M.; Kuiper, M.; Martini, A.; Mellinghoff, J.; Pelosi, P.; et al. Anxiety and Depression among Out-of-Hospital Cardiac Arrest Survivors. Resuscitation 2015, 97, 68–75. [Google Scholar] [CrossRef]
- Wilder Schaaf, K.P.; Artman, L.K.; Peberdy, M.A.; Walker, W.C.; Ornato, J.P.; Gossip, M.R.; Kreutzer, J.S.; Virginia Commonwealth University ARCTIC Investigators. Anxiety, Depression, and PTSD Following Cardiac Arrest: A Systematic Review of the Literature. Resuscitation 2013, 84, 873–877. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical Pathophysiology of Hypoxic Ischemic Brain Injury after Cardiac Arrest: A “Two-Hit” Model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef]
- Hermansen, A.S.; Joshi, V.L.; Wagner, M.K.; Dieperink, K.B.; Zwisler, A.-D.; Borregaard, B.; DANCAS research network. Caregiver Strain among Relatives of Out-of-Hospital Cardiac Arrest Survivors; the DANCAS Relative Survey. Resuscitation 2024, 201, 110298. [Google Scholar] [CrossRef]
- Damluji, A.A.; Al-Damluji, M.S.; Pomenti, S.; Zhang, T.J.; Cohen, M.G.; Mitrani, R.D.; Moscucci, M.; Myerburg, R.J. Health Care Costs After Cardiac Arrest in the United States. Circ. Arrhythm. Electrophysiol. 2018, 11, e005689. [Google Scholar] [CrossRef]
- Marinšek, M.; Sinkovič, A.; Šuran, D. Neurological Outcome in Patients after Successful Resuscitation in Out-of-Hospital Settings. Bosn. J. Basic Med. Sci. 2020, 20, 389–395. [Google Scholar] [CrossRef]
- Mazzeffi, M.; Curley, J.; Gallo, P.; Stombaugh, D.K.; Roach, J.; Lunardi, N.; Yount, K.; Thiele, R.; Glance, L.; Naik, B. Variation in Hospitalization Costs, Charges, and Lengths of Hospital Stay for Coronavirus Disease 2019 Patients Treated with Venovenous Extracorporeal Membrane Oxygenation in the United States: A Cohort Study. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1449–1455. [Google Scholar] [CrossRef]
- De Fazio, C.; Skrifvars, M.B.; Søreide, E.; Grejs, A.M.; Di Bernardini, E.; Jeppesen, A.N.; Storm, C.; Kjaergaard, J.; Laitio, T.; Rasmussen, B.S.; et al. Quality of Targeted Temperature Management and Outcome of Out-of-Hospital Cardiac Arrest Patients: A Post Hoc Analysis of the TTH48 Study. Resuscitation 2021, 165, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Filipiak, K.J.; Mosteller, L.; Jaguszewski, M.; Smereka, J.; Ruetzler, K.; Ahuja, S.; Ladny, J.R. Survival, Neurological and Safety Outcomes after out of Hospital Cardiac Arrests Treated by Using Prehospital Therapeutic Hypothermia: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2021, 42, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Harhash, A.A.; May, T.; Hsu, C.-H.; Seder, D.B.; Dankiewicz, J.; Agarwal, S.; Patel, N.; McPherson, J.; Riker, R.; Soreide, E.; et al. Incidence of Cardiac Interventions and Associated Cardiac Arrest Outcomes in Patients with Nonshockable Initial Rhythms and No ST Elevation Post Resuscitation. Resuscitation 2021, 167, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, N.I.; Netherton, S.; Welsford, M.; Drennan, I.R.; Nation, K.; Belley-Cote, E.; Torabi, N.; Morrison, L.J.; International Liaison Committee on Resuscitation Advanced Life Support Task Force (ILCOR). A Systematic Review and Meta-Analysis of the Effect of Routine Early Angiography in Patients with Return of Spontaneous Circulation after Out-of-Hospital Cardiac Arrest. Resuscitation 2021, 163, 28–48. [Google Scholar] [CrossRef]
- Kern, K.B.; Radsel, P.; Jentzer, J.C.; Seder, D.B.; Lee, K.S.; Lotun, K.; Janardhanan, R.; Stub, D.; Hsu, C.-H.; Noc, M. Randomized Pilot Clinical Trial of Early Coronary Angiography Versus No Early Coronary Angiography After Cardiac Arrest Without ST-Segment Elevation: The PEARL Study. Circulation 2020, 142, 2002–2012. [Google Scholar] [CrossRef]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.D.; Dubois, E.A.; Meuwissen, M.M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography After Cardiac Arrest Without ST Segment Elevation: One-Year Outcomes of the COACT Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 1358–1365. [Google Scholar] [CrossRef]
- Downing, J.; Al Falasi, R.; Cardona, S.; Fairchild, M.; Lowie, B.; Chan, C.; Powell, E.; Pourmand, A.; Tran, Q.K. How Effective Is Extracorporeal Cardiopulmonary Resuscitation (ECPR) for out-of-Hospital Cardiac Arrest? A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2022, 51, 127–138. [Google Scholar] [CrossRef]
- Carr, C.; Carson, K.A.; Millin, M.G. Acidemia Detected on Venous Blood Gas After Out-of-Hospital Cardiac Arrest Predicts Likelihood to Survive to Hospital Discharge. J. Emerg. Med. 2020, 59, e105–e111. [Google Scholar] [CrossRef]
- Cocchi, M.N.; Salciccioli, J.; Yankama, T.; Chase, M.; Patel, P.V.; Liu, X.; Mader, T.J.; Donnino, M.W. Predicting Outcome After Out-of-Hospital Cardiac Arrest: Lactate, Need for Vasopressors, and Cytochrome c. J. Intensive Care Med. 2020, 35, 1483–1489. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Giberson, T.; Gaieski, D.; Abella, B.; Peberdy, M.A.; Rittenberger, J.C.; Callaway, C.W.; Ornato, J.; Clore, J.; et al. Initial Lactate and Lactate Change in Post-Cardiac Arrest: A Multi-Center Validation Study. Crit. Care Med. 2014, 42, 1804–1811. [Google Scholar] [CrossRef]
- Momiyama, Y.; Yamada, W.; Miyata, K.; Miura, K.; Fukuda, T.; Fuse, J.; Kikuno, T. Prognostic Values of Blood pH and Lactate Levels in Patients Resuscitated from Out-of-Hospital Cardiac Arrest. Acute Med. Surg. 2017, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 18 March 2025).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 18 March 2025).
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. Companion R Package for the Guide Doing Meta-Analysis in R. Available online: https://dmetar.protectlab.org/ (accessed on 18 March 2025).
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Al Assil, R.; Singer, J.; Heidet, M.; Fordyce, C.B.; Scheuermeyer, F.; van Diepen, S.; Sekhon, M.; Leung, K.H.B.; Stenstrom, R.; Christenson, J.; et al. The Association of pH Values during the First 24 h with Neurological Status at Hospital Discharge and Futility among Patients with Out-of-Hospital Cardiac Arrest. Resuscitation 2021, 159, 105–114. [Google Scholar] [CrossRef]
- Cocchi, M.N.; Miller, J.; Hunziker, S.; Carney, E.; Salciccioli, J.; Farris, S.; Joyce, N.; Zimetbaum, P.; Howell, M.D.; Donnino, M.W. The Association of Lactate and Vasopressor Need for Mortality Prediction in Survivors of Cardiac Arrest. Minerva Anestesiol. 2011, 77, 1063–1071. [Google Scholar]
- Dell’Anna, A.M.; Sandroni, C.; Lamanna, I.; Belloni, I.; Donadello, K.; Creteur, J.; Vincent, J.-L.; Taccone, F.S. Prognostic Implications of Blood Lactate Concentrations after Cardiac Arrest: A Retrospective Study. Ann. Intensive Care 2017, 7, 101. [Google Scholar] [CrossRef]
- Düring, J.; Dankiewicz, J.; Cronberg, T.; Hassager, C.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; Nielsen, N.; Pellis, T.; Stammet, P.; et al. Lactate, Lactate Clearance and Outcome after Cardiac Arrest: A Post-Hoc Analysis of the TTM-Trial. Acta Anaesthesiol. Scand. 2018, 62, 1436–1442. [Google Scholar] [CrossRef]
- Freire Jorge, P.; Boer, R.; Posma, R.A.; Harms, K.C.; Hiemstra, B.; Bens, B.W.J.; Nijsten, M.W. Early Lactate and Glucose Kinetics Following Return to Spontaneous Circulation after Out-of-Hospital Cardiac Arrest. BMC Res. Notes 2021, 14, 183. [Google Scholar] [CrossRef]
- Han, K.S.; Kim, S.J.; Lee, E.J.; Park, K.Y.; Lee, J.Y.; Lee, S.W. Impact of Rapid Lactate Clearance as an Indicator of Hemodynamic Optimization on Outcome in Out-of-Hospital Cardiac Arrest: A Retrospective Analysis. PLoS ONE 2019, 14, e0214547. [Google Scholar] [CrossRef] [PubMed]
- Hope Kilgannon, J.; Hunter, B.R.; Puskarich, M.A.; Shea, L.; Fuller, B.M.; Jones, C.; Donnino, M.; Kline, J.A.; Jones, A.E.; Shapiro, N.I.; et al. Partial Pressure of Arterial Carbon Dioxide after Resuscitation from Cardiac Arrest and Neurological Outcome: A Prospective Multi-Center Protocol-Directed Cohort Study. Resuscitation 2019, 135, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Suzuki, M.; Yonemoto, N.; Hori, S.; Tamura, T.; Sakurai, A.; Tahara, Y.; Nagao, K.; Yaguchi, A.; Morimura, N.; et al. Early Lactate Clearance Is Associated with Improved Outcomes in Patients with Postcardiac Arrest Syndrome: A Prospective, Multicenter Observational Study (SOS-KANTO 2012 Study). Crit. Care Med. 2017, 45, e559–e566. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Amuthan, R.; Adams, M.P.; Love, T.E.; Enfield, K.B.; Gimple, L.W.; Cantillon, D.J.; Menon, V. Initial Arterial pH as a Predictor of Neurologic Outcome after Out-of-Hospital Cardiac Arrest: A Propensity-Adjusted Analysis. Resuscitation 2019, 139, 76–83. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, B.K.; Lee, D.H.; Jung, Y.H.; Cho, Y.S.; Lee, S.M.; Lee, S.J.; Park, C.H.; Jeung, K.W. Association between Lactate Clearance during Post-Resuscitation Care and Neurologic Outcome in Cardiac Arrest Survivors Treated with Targeted Temperature Management. Clin. Exp. Emerg. Med. 2017, 4, 10–18. [Google Scholar] [CrossRef]
- Kliegel, A.; Losert, H.; Sterz, F.; Holzer, M.; Zeiner, A.; Havel, C.; Laggner, A.N. Serial Lactate Determinations for Prediction of Outcome after Cardiac Arrest. Medicine 2004, 83, 274–279. [Google Scholar] [CrossRef]
- Laurikkala, J.; Skrifvars, M.B.; Bäcklund, M.; Tiainen, M.; Bendel, S.; Karhu, J.; Varpula, T.; Vaahersalo, J.; Pettilä, V.; Wilkman, E.; et al. Early Lactate Values After Out-of-Hospital Cardiac Arrest: Associations with One-Year Outcome. Shock 2019, 51, 168–173. [Google Scholar] [CrossRef]
- Lee, D.H.; Cho, I.S.; Lee, S.H.; Min, Y.I.; Min, J.H.; Kim, S.H.; Lee, Y.H.; Korean Hypothermia Network Investigators. Correlation between Initial Serum Levels of Lactate After Return of Spontaneous Circulation and Survival and Neurological Outcomes in Patients Who Undergo Therapeutic Hypothermia after Cardiac Arrest. Resuscitation 2015, 88, 143–149. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yu, S.-H.; Chen, C.-Y.; Huang, F.-W.; Chen, W.-K.; Shih, H.-M. Early Blood pH as an Independent Predictor of Neurological Outcome in Patients with Out-of-Hospital Cardiac Arrest. Medicine 2021, 100, e25724. [Google Scholar] [CrossRef]
- Lonsain, W.S.; De Lausnay, L.; Wauters, L.; Desruelles, D.; Dewolf, P. The Prognostic Value of Early Lactate Clearance for Survival after Out-of-Hospital Cardiac Arrest. Am. J. Emerg. Med. 2021, 46, 56–62. [Google Scholar] [CrossRef]
- Orban, J.-C.; Novain, M.; Cattet, F.; Plattier, R.; Nefzaoui, M.; Hyvernat, H.; Raguin, O.; Kaidomar, M.; Kerever, S.; Ichai, C. Association of Serum Lactate with Outcome after Out-of-Hospital Cardiac Arrest Treated with Therapeutic Hypothermia. PLoS ONE 2017, 12, e0173239. [Google Scholar] [CrossRef]
- Park, J.H.; Wee, J.H.; Choi, S.P.; Oh, J.H.; Cheol, S. Assessment of Serum Biomarkers and Coagulation/Fibrinolysis Markers for Prediction of Neurological Outcomes of out of Cardiac Arrest Patients Treated with Therapeutic Hypothermia. Clin. Exp. Emerg. Med. 2019, 6, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Peluso, L.; Belloni, I.; Calabró, L.; Dell’Anna, A.M.; Nobile, L.; Creteur, J.; Vincent, J.-L.; Taccone, F.S. Oxygen and Carbon Dioxide Levels in Patients after Cardiac Arrest. Resuscitation 2020, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Lichtenauer, M.; Schwaiger, P.; Seelmaier, C.; Pretsch, I.; Ausserwinkler, M.; Reichle, J.; Jirak, P.; Jung, C.; Strohmer, B.; et al. Thinking Fast and Slow: Lactate and MELD-XI (Model for End-Stage Liver Disease Excluding INR) Are Useful for Estimating Mortality after Cardiopulmonary Resuscitation. Minerva Anestesiol. 2021, 87, 1017–1024. [Google Scholar] [CrossRef]
- Rosenberg, R.D.; Guo, C.-Y.C.; Chatterjee, S.; Schreyer, K.E.; Bashir, R.; O’Murchu, B.; Aggarwal, V.; DeAngelis, M.; Edmundowicz, D.; O’Neill, B.P. The Prognostic Value of Initial Serum Lactate for Survival in Postcardiac Arrest Patients Undergoing Cardiac Catheterization. Catheter. Cardiovasc. Interv. 2021, 97, 228–234. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Kim, Y.-J.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Kim, W.Y. Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and Their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. J. Clin. Med. 2020, 9, 159. [Google Scholar] [CrossRef]
- Sarıaydın, T.; Çorbacıoğlu, Ş.K.; Çevik, Y.; Emektar, E. Effect of Initial Lactate Level on Short-Term Survival in Patients with out-of-Hospital Cardiac Arrest. Turk. J. Emerg. Med. 2017, 17, 123–127. [Google Scholar] [CrossRef]
- Sauter, T.C.; Iten, N.; Schwab, P.R.; Hautz, W.E.; Ricklin, M.E.; Exadaktylos, A.K. Out-of-Hospital Cardiac Arrests in Switzerland: Predictors for Emergency Department Mortality in Patients with ROSC or on-Going CPR on Admission to the Emergency Department. PLoS ONE 2017, 12, e0188180. [Google Scholar] [CrossRef]
- Seeger, F.H.; Toenne, M.; Lehmann, R.; Ehrlich, J.R. Simplistic Approach to Prognosis after Cardiopulmonary Resuscitation-Value of pH and Lactate. J. Crit. Care 2013, 28, 317.e13–317.e20. [Google Scholar] [CrossRef]
- Shin, J.; Lim, Y.S.; Kim, K.; Lee, H.J.; Lee, S.J.; Jung, E.; You, K.M.; Yang, H.J.; Kim, J.J.; Kim, J.; et al. Initial Blood pH during Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest Patients: A Multicenter Observational Registry-Based Study. Crit. Care 2017, 21, 322. [Google Scholar] [CrossRef]
- Shinozaki, K.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Watanabe, E.; Tateishi, Y.; Nakanishi, K.; Kitamura, N.; Sato, Y.; et al. Blood Ammonia and Lactate Levels on Hospital Arrival as a Predictive Biomarker in Patients with Out-of-Hospital Cardiac Arrest. Resuscitation 2011, 82, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sivaraju, A.; Gilmore, E.J.; Wira, C.R.; Stevens, A.; Rampal, N.; Moeller, J.J.; Greer, D.M.; Hirsch, L.J.; Gaspard, N. Prognostication of Post-Cardiac Arrest Coma: Early Clinical and Electroencephalographic Predictors of Outcome. Intensive Care Med. 2015, 41, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Starodub, R.; Abella, B.S.; Grossestreuer, A.V.; Shofer, F.S.; Perman, S.M.; Leary, M.; Gaieski, D.F. Association of Serum Lactate and Survival Outcomes in Patients Undergoing Therapeutic Hypothermia after Cardiac Arrest. Resuscitation 2013, 84, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Takaki, S.; Kamiya, Y.; Tahara, Y.; Tou, M.; Shimoyama, A.; Iwashita, M. Blood pH Is a Useful Indicator for Initiation of Therapeutic Hypothermia in the Early Phase of Resuscitation after Comatose Cardiac Arrest: A Retrospective Study. J. Emerg. Med. 2013, 45, 57–64. [Google Scholar] [CrossRef]
- Tetsuhara, K.; Kato, H.; Kanemura, T.; Okada, I.; Kiriu, N. Severe Acidemia on Arrival Not Predictive of Neurologic Outcomes in Post-Cardiac Arrest Patients. Am. J. Emerg. Med. 2016, 34, 425–428. [Google Scholar] [CrossRef]
- Isenschmid, C.; Kalt, J.; Gamp, M.; Tondorf, T.; Becker, C.; Tisljar, K.; Locher, S.; Schuetz, P.; Marsch, S.; Hunziker, S. Routine Blood Markers from Different Biological Pathways Improve Early Risk Stratification in Cardiac Arrest Patients: Results from the Prospective, Observational COMMUNICATE Study. Resuscitation 2018, 130, 138–145. [Google Scholar] [CrossRef]
- Tolins, M.L.; Henning, D.J.; Gaieski, D.F.; Grossestreuer, A.V.; Jaworski, A.; Johnson, N.J. Initial Arterial Carbon Dioxide Tension Is Associated with Neurological Outcome after Resuscitation from Cardiac Arrest. Resuscitation 2017, 114, 53–58. [Google Scholar] [CrossRef]
- Von Auenmueller, K.I.; Christ, M.; Sasko, B.M.; Trappe, H.-J. The Value of Arterial Blood Gas Parameters for Prediction of Mortality in Survivors of Out-of-Hospital Cardiac Arrest. J. Emerg. Trauma Shock 2017, 10, 134–139. [Google Scholar] [CrossRef]
- Williams, T.A.; Martin, R.; Celenza, A.; Bremner, A.; Fatovich, D.; Krause, J.; Arena, S.; Finn, J. Use of Serum Lactate Levels to Predict Survival for Patients with Out-of-Hospital Cardiac Arrest: A Cohort Study. Emerg. Med. Australas. 2016, 28, 171–178. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Sakamoto, T.; Sato, H. Relationship between Laboratory Findings and the Outcome of Cardiopulmonary Arrest. Am. J. Emerg. Med. 2009, 27, 308–312. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Yu, Y.; An, L.; Qi, Z.; Li, C. Effects of Early Hemodynamics, Oxygen Metabolism, and Lactate Dynamics on Prognosis of Post-Cardiac Arrest Syndrome. Chin. Med. J. 2022, 135, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Dadeh, A.; Nuanjaroan, B. Using Initial Serum Lactate Level in the Emergency Department to Predict the Sustained Return of Spontaneous Circulation in Nontraumatic Out-of-Hospital Cardiac Arrest Patients. Open Access Emerg. Med. 2018, 10, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Soloperto, R.; Magni, F.; Farinella, A.; Bogossian, E.G.; Peluso, L.; De Luca, N.; Taccone, F.S.; Annoni, F. A Comparison of Prognostic Factors in a Large Cohort of In-Hospital and Out-of-Hospital Cardiac Arrest Patients. Life 2024, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, H.; Rhee, B.; Song, H.; Kim, H. Predicting Survival Outcomes in Post-Cardiac Arrest Syndrome: The Impact of Combined Sequential Organ Failure Assessment Score and Serum Lactate Measurement. Med. Sci. Monit. 2023, 29, e942119-1–e942119-9. [Google Scholar] [CrossRef]
- Kandilcik, M.; Arslan, M.; Öksüz, H.; Gişi, G.; Yavuz, C.; Öksüz, G.; Doğaner, A. Evaluation of the Factors Affecting Mortality after Cardiac Arrest—Do Lactate and Procalcitonin Concentrations Have Any Implications? Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3430–3438. [Google Scholar] [CrossRef]
- Imamura, S.; Miyata, M.; Tagata, K.; Yokomine, T.; Ohmure, K.; Kawasoe, M.; Otsuji, H.; Chaen, H.; Oketani, N.; Ogawa, M.; et al. Prognostic Predictors in Patients with Cardiopulmonary Arrest: A Novel Equation for Evaluating the 30-Day Mortality. J. Cardiol. 2023, 82, 146–152. [Google Scholar] [CrossRef]
- Dusik, M.; Rob, D.; Smalcova, J.; Havranek, S.; Karasek, J.; Smid, O.; Brodska, H.L.; Kavalkova, P.; Huptych, M.; Bakker, J.; et al. Serum Lactate in Refractory Out-of-Hospital Cardiac Arrest: Post-Hoc Analysis of the Prague OHCA Study. Resuscitation 2023, 192, 109935. [Google Scholar] [CrossRef]
- Choi, S.Y.; Oh, S.H.; Park, K.N.; Youn, C.S.; Kim, H.J.; Park, S.H.; Lim, J.Y.; Kim, H.J.; Bang, H.J. Association between Early Lactate-Related Variables and 6-Month Neurological Outcome in out-of-Hospital Cardiac Arrest Patients. Am. J. Emerg. Med. 2024, 78, 62–68. [Google Scholar] [CrossRef]
- Chen, D.-L.; Chung, C.-M.; Wang, G.-J.; Chang, K.-C. Lactate-to-Albumin Ratio and Cholesterol Levels Predict Neurological Outcome in Cardiac Arrest Survivors. Am. J. Emerg. Med. 2024, 83, 9–15. [Google Scholar] [CrossRef]
- Mueller, M.; Jankow, E.; Grafeneder, J.; Schoergenhofer, C.; Poppe, M.; Schriefl, C.; Clodi, C.; Koch, M.; Ettl, F.; Holzer, M.; et al. The Difference between Arterial pCO2 and etCO2 after Cardiac Arrest—Outcome Predictor or Marker of Unfavorable Resuscitation Circumstances? Am. J. Emerg. Med. 2022, 61, 120–126. [Google Scholar] [CrossRef]
- Riveiro, D.F.M.; de Oliveira, V.M.; Braunner, J.S.; Vieira, S.R.R. Evaluation of Serum Lactate, Central Venous Saturation, and Venous-Arterial Carbon Dioxide Difference in the Prediction of Mortality in Postcardiac Arrest Syndrome. J. Intensive Care Med. 2016, 31, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, Y.; Yue, X.; Gao, Q. Serum pH and Lactate Predict Outcomes after Cardiopulmonary Resuscitation. Acta Medica Mediterr. 2020, 36, 801–804. [Google Scholar]
- George, S.; Thomas, M.; Ibrahim, W.H.; Abdussalam, A.; Chandra, P.; Ali, H.S.; Raza, T. Somatic Survival and Organ Donation among Brain-Dead Patients in the State of Qatar. BMC Neurol. 2016, 16, 207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).