Abstract
Background/Objectives: Suicide accounts for 1.4% of global deaths, and the slow-acting nature of traditional treatments for suicide risk underscores the need for alternatives. Psychedelic therapies may rapidly reduce suicide risk. This systematic review evaluates impact of psychedelic therapies on suicide-related outcomes. Methods: A systematic search of MEDLINE, Embase, PsycINFO, and ClinicalTrials.gov was conducted up to November 2024. Results: Four randomized controlled trials (RCTs) evaluated suicidality as a secondary outcome or safety measure, showing significant reductions in suicidal ideation with psilocybin (three studies) and MDMA-assisted therapy (MDMA-AT; one study). Effect sizes, measured by Cohen’s d, ranged from =0.52 to 1.25 (p = 0.01 to 0.005), with no safety issues reported. Five additional RCTs assessed suicidality as a safety measure, showing reductions in suicidal ideation with psilocybin (two studies) and MDMA-AT (three studies; p = 0.02 to 0.04). Among 24 non-randomized and cross-sectional studies, results were mixed. Psilocybin (three studies) reduced suicidal ideation, with odds ratios (OR) of 0.40–0.75. MDMA-AT (five studies in PTSD patients) had a pooled effect size of d = 0.61 (95% CI: 0.32–0.89). LSD (six studies) showed increased odds of suicidality, with odds ratios ranging from 1.15 to 2.08. Studies involving DMT (two studies) and multiple psychedelics (three studies) showed mixed results, with DMT studies not showing significant effects on suicidality and studies involving multiple psychedelics showing varying outcomes, some reporting reductions in suicidal ideation and others showing no significant change. Conclusions: The effect of psychedelic therapies on suicide-related outcomes remains inconclusive, highlighting the need for further trials to clarify safety and therapeutic mechanisms.
1. Introduction
Suicidal thoughts and behaviors, encompassing completed suicide, suicide attempts, and suicidal ideation, represent a major public health challenge and a significant contributor to global mortality [1]. Death by suicide accounts for approximately 1.4% of all deaths worldwide and ranks as the 10th leading cause of death in the United States [2]. Suicidal behavior arises from a complex interplay of factors, broadly categorized into predisposing elements and immediate stressors or triggers [3]. Mental disorders play a predominant role, with over 90% of individuals who die by suicide meeting the criteria for a psychiatric illness as outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [4]. Among psychiatric conditions, mood disorders—primarily major depressive disorder (MDD) and bipolar disorder (BD)—are implicated in approximately 60% of completed suicides [5]. Notably, trauma burden and posttraumatic stress disorder (PTSD) significantly increase the risk of suicidal ideation and behaviors, further compounding the challenge of prevention and treatment [6]. Additional influences include the availability of lethal means, substance use (alcohol and drugs), access to mental health care, cultural and personal attitudes toward suicide, help-seeking behaviors, physical health conditions, marital status, age, and gender [3,7]. Current biological approaches to reduce suicidality remain limited in their effectiveness. Traditional treatments, such as antidepressants, cognitive behavioral therapy (CBT), and electroconvulsive therapy (ECT), have shown to reduce suicidal thoughts and behaviors in individuals with depression [8,9,10]. Additionally, pharmacotherapies such as clozapine and lithium, recognized for their anti-suicidal properties, also played a significant role in managing suicidality [11]. However, these interventions typically require several weeks to take effect, leaving many patients at significant risk of suicide during this period. Recent developments in rapid-acting antidepressants (RAADs), such as ketamine and its FDA-approved derivative esketamine (Spravato), represent promising advances in the treatment of acute suicidal ideation and behaviors, particularly in individuals with depression [12]. While these interventions can reduce symptoms within hours, their effects are limited to this population and do not address suicidality in a broader sense. Additionally, the short duration of effect following a single treatment and the unknown durability of their anti-suicidal properties with repeated administrations pose challenges to their clinical application [13]. Therefore, there is an urgent need for novel efficacious treatments to address this critical issue.
Serotonergic psychedelics, commonly known as “classic” psychedelics, include a range of substances such as psilocybin, dimethyltryptamine (DMT), ayahuasca, mescaline, and lysergic acid diethylamide (LSD) [14]. In contrast, 3,4-methylenedioxymethamphetamine (MDMA) is a distinct psychoactive compound considered an empathogen or entactogen that has also garnered attention for its therapeutic potential [14]. Classic psychedelics are naturally occurring, plant-derived or synthesized psychoactive substances that primarily function as serotonin 2A receptor agonists [15]. These compounds have the potential to induce profound experiences, which, in some cases, can enhance an individual’s perceived quality of life [15]. Similarly, MDMA (3,4-methylenedioxymethamphetamine), though not a classic psychedelic, acts primarily as a serotonin-releasing agent and promotes prosocial effects, emotional openness, and enhanced therapeutic processing, which may also contribute to improvements in perceived quality of life [16,17]. Psychedelics, administered with psychological support, have been investigated in numerous randomized clinical trials (RCTs) for a variety of psychiatric conditions, including MDD, BD, treatment-resistant depression (TRD), substance use disorders (SUDs), and PTSD [18,19,20,21,22]. Psychedelic therapies may also have potential benefits in reducing suicidality due to their ability to enhance emotional processing, facilitate personal insights, and promote a sense of interconnectedness, which can improve mental health and reduce self-destructive thoughts [23]. However, psychedelic use is also linked to adverse events, including heightened anxiety [24], and there have been reports suggesting an increase in suicidal ideations and behaviors in some individuals following LSD use [25].
Despite growing research on psychedelic therapies for psychiatric disorders such as depression, anxiety, substance use disorders, and PTSD, their effects on suicidality remain underexplored. Notably, many clinical trials on psychedelic therapies exclude participants with suicidal ideation, often assessed via the clinician-rated Columbia–Suicide Severity Rating Scale (C-SSRS), or those with borderline personality disorder (BPD)—a population at elevated risk for suicide and comprising a significant portion of psychiatric patients. Given the urgent need for treatments targeting individuals with acute and severe psychiatric symptoms, identifying therapies that can address suicidality in these high-risk groups is of profound importance. A systematic review is needed to evaluate the impact of psychedelic therapies on suicide risk, clarify their safety and therapeutic mechanisms, and guide future research and clinical practice. Such an assessment is crucial for understanding how psychedelic therapies can be safely integrated into treatment for individuals at high suicide risk. Therefore, in this systematic review, we aim to evaluate the effect of psychedelic therapies on suicidal-related outcomes.
2. Methods
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines [26] and was registered on PROSPERO (registration ID: CRD42024611536).
2.1. Search Strategy
We conducted a comprehensive search in three databases (MEDLINE, Embase, and PsycINFO) via OVID, covering all records from inception through November 2024. We also searched Google Scholar and references of relevant studies. The following keywords were used: psilocybin or psilocibin or psilocybine or silocybin or psiloc* or shrooms or magic mushrooms or mushies or psilocybin-assisted therapy or PAP or psychedelics or MDMA or 3,4-methylenedioxymethamphetamine or ecstasy or molly or ayahuasca or LSD or lysergic acid diethylamide or DMT or dimethyltryptamine or mescaline or peyote or ibogaine or iboga or 5-MeO-DMT or salvinorin A or Salvia or bufotenin or 5-HO-DMT AND suicide or suicidal ideation or suicidality or suicidal thoughts or suicidal behavior or suicide attempt. No language or publication date restrictions were applied. Additionally, a search of registered clinical trials was conducted on ClinicalTrials.gov on 21 November 2024, using the same psychedelic agents paired with suicide-related terms.
2.2. Eligibility Criteria
Two reviewers (SM, TM) independently reviewed the title/abstract and full text of studies based on eligibility criteria. The screening process was conducted in Covidence (https://www.covidence.org/ (accessed on 1 November 2024). Conflicts between reviewers were resolved through discussion, and if disagreements persisted, a third reviewer was consulted to reach a consensus (VB). Studies were included if they met the following criteria: (1) original research of any study design; (2) evaluation of the effect or association of psychedelics or hallucinogens with suicide-related outcomes; (3) published in English. Secondary analyses were included only if their primary focus was on the effects of psychedelics on suicidal-related outcomes. Qualitative studies were also considered. Excluded studies were case reports, non-human studies, systematic reviews, narrative reviews, umbrella reviews, meta-analyses, letters, editorials, posters, conference abstracts, and studies not published in English. We chose to exclude systematic reviews and meta-analyses to ensure that our findings are based on primary data rather than synthesized evidence, thereby avoiding potential duplication of results. Regarding the exclusion of non-English studies, this decision was made to ensure consistency in data extraction and interpretation, as translations may introduce variability or misinterpretation of critical findings.
2.3. Data Extraction
Two reviewers (SM, TM) independently reviewed the full texts of eligible studies and extracted the following variables: author, year of publication, country, study design, participants, intervention, psychotherapy principle, outcome measure, results, and conclusion. Similar to the published studies, the following variables were extracted from the registered clinical trials: study characteristic, intervention, outcome measure, country, diagnosis, and estimated completion date. Additional extracted variables were the ClinicalTrials.gov identifier (i.e., an 11-digit alphanumeric identifier), trial status (i.e., not yet recruiting, recruiting, active, completed, etc.), estimated completion date, and projected sample size.
2.4. Quality Assessment
All studies included in this review were evaluated for quality by two independent assessors (SM, TM) using the JBI Critical Appraisal Tools Checklist for systematic reviews [27]. The specific checklists applied were tailored for RCTs and cohort studies. Lower-quality studies were not excluded; however, their methodological limitations were considered in the interpretation of findings. While a formal sensitivity analysis was not conducted, the influence of these studies was evaluated qualitatively, with particular attention to study limitations (Supplementary Materials Table S1).
3. Results
3.1. Search Results
An electronic OVID database search resulted in a total of 3074 records. We removed duplicate articles (n = 663) and screened the title/abstract of the remaining records (n = 2411). A total of 2346 articles were excluded based on title/abstract. We reviewed the full text of the remaining papers (n = 65) in detail based on our inclusion criteria. Out of 65 records, 26 records were excluded: 14 studies had the wrong study design, 4 did not include suicidal-related outcomes, 3 had insufficient results, 2 had the wrong intervention, 2 were not in English, and 1 had the wrong indication. Finally, 39 articles involving 1,671,773 participants (949 from intervention-based studies, 1,670,409 from population-based studies, and 415 from secondary analysis studies) met the inclusion criteria and were included in this systematic review. We also found one ongoing trial on ClinicalTrials.gov (accessed on 21 November 2024). The study selection details are indicated in Figure 1. The characteristics of the included studies are listed in Table 1 and Table 2 and Figure 2, Figure 3 and Figure 4.
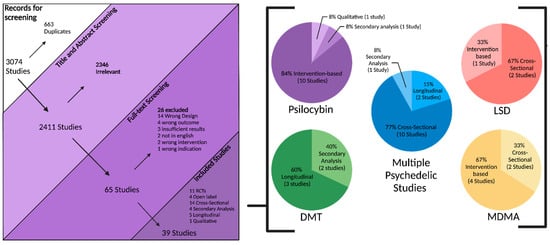
Figure 1.
Study flow diagram.

Table 1.
Characteristics of included intervention-based studies.

Table 2.
Characteristics of included population studies.
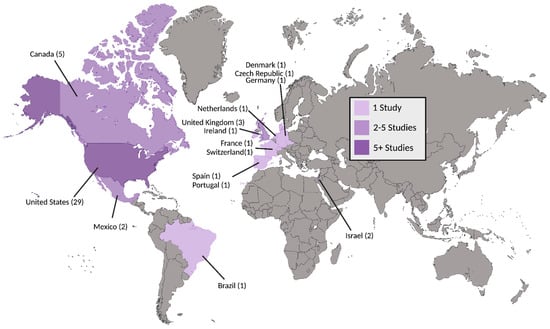
Figure 2.
Countries of included studies. Number of studies for a given country are included in parentheses.
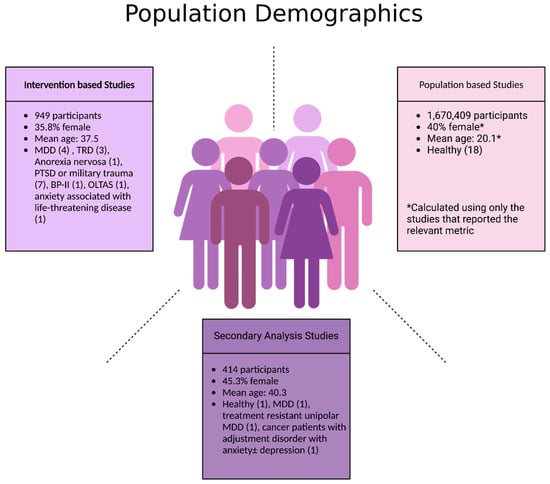
Figure 3.
Population demographics of participants in included studies. Abbreviations: MDD: major depressive disorder; TRD: treatment-resistant depression; PTSD: post-traumatic stress disorder; BP-II: Bipolar II disorder; OLTAS: older long-term acquired immunodeficiency syndrome survivors.
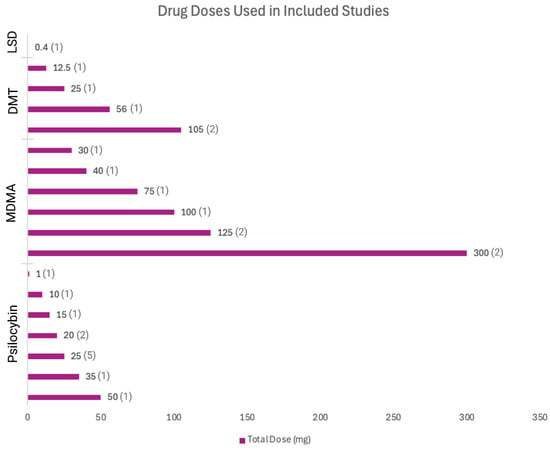
Figure 4.
Drug doses used in included studies.
3.2. Registered Clinical Trial
We found one trial that had the identifier NCT05220410, which is currently recruiting participants. The estimated completion date for the study is April 2024, and it is being conducted in the United States. This is an interventional phase 2 trial with a single-group assignment and no masking. The projected sample size is 20 participants. The intervention involves administering 25 mg of psilocybin, and the primary outcome measure is the Columbia–Suicide Severity Rating Scale (C-SSRS). The trial focuses on individuals with TRD with chronic suicidal ideation.
3.3. Psilocybin
Six RCTs, four open-label trials, one qualitative cross-sectional study, and one secondary analysis investigated the effects of psilocybin on suicidal ideation and attempt.
3.3.1. RCTs with Suicide as Safety or Secondary Outcome
MDD
Carharrt-Harris et al. (2021) examined the effects of psilocybin on suicidal thoughts through the Suicidal Ideation Attributes Scale (SIDAS; out of 50, 5 items out of 10) in MDD patients [28]. Their psilocybin group received two doses of 25 mg psilocybin 3 weeks apart (and daily placebo capsules), whereas their comparator group received two doses of 1 mg psilocybin with 6 weeks of oral escitalopram. All participants also received psychological support. The investigators reported a −2.0 change in the psilocybin group (95% CI = −4.3 to 0.0), a −0.8 change in the placebo group (95% CI = −3.4 to 2.0), and a between-group difference of −1.3 (95% CI = −6.5 to −0.3) from baseline to 6 weeks post dosing, but these were not corrected for multiple comparisons [28]. Davis et al. (2021) administered two doses of psilocybin (20 mg/kg, followed by 30 mg/kg) with psychotherapy to patients with MDD [29]. Patients either received treatment directly after baseline screening (immediate group) or after 8 weeks (delayed group). As a secondary outcome, Davis et al. assessed patients at baseline and clinical endpoints (i.e., time of follow-up) with the clinician-administered C-SSRS intensity of ideation subscale (C-SSRS-ISS; 0 out of 5), and found non-significant reductions in suicidal ideation from baseline (immediate: 1.2, delayed: 1.3; p = ns) to 5 (immediate: 0.2, delayed: 0.6; p = ns) and 8 (immediate: 0.2, delayed: 0.4; p = ns) weeks out [29]. They state that in general, suicidal ideation was low at baseline and trended lower after treatment [29]. Importantly, as participants with medically significant attempts for suicide were excluded from this study, observed C-SSRS-IIS scores may be lower among this population than otherwise. Raison et al. (2023) also investigated MDD patients and conducted an RCT using a single 25 mg dose of psilocybin with psychotherapy [32]. In their safety assessment, they report that no patients showed signs of suicidal behavior at any timepoint during the trial, as measured through the clinician-administered C-SSRS-IIS or the Montgomery–Åsberg Depression Rating Scale-Suicidality Item (MADRS-SI; out of 6) [32]. However, one participant receiving psilocybin and five receiving placebo niacin had an increase in C-SSRS suicidal ideation score from baseline to the end of the trial [32]. von Rotz et al. (2022) gave MDD patients a single 0.215 mg/kg dose of psilocybin with therapy. Although they reported a mean within-group change of −0.35 in patient suicidal ideation as measured via the clinician-administered C-SSRS-ISS (95% CI = −0.10 to 0.71; d = 0.43; p = 0.13), they also report nonsignificant differences between experimental and placebo groups (F(1,50) = 1.40; p = 0.24) [31].
TRD
Goodwin et al. (2022) conducted an RCT to observe the effects of a single, randomized dose of psilocybin at 1 mg (n = 79), 10 mg (n = 75), or 25 mg (n = 79) with psychological support in TRD patients. They used the clinician-administered C-SSRS and considered suicidal ideation with intent or endorsement of the suicidal behavior section of the scale as a serious adverse event (AE) [19]. Throughout all 12 weeks, participants exhibiting suicidal ideation or behavior were higher in the 25 mg and 10 mg groups, relative to the 1 mg group [19]. Twenty-one (27%) patients in the 25 mg group, twenty-seven (36%) in the 10 mg group, and nineteen (24%) in the 1 mg group showed suicidal ideation (passive or active with no intent to plan) at baseline [19]. From baseline to week 3, 11 (13.9%) in the 25 mg group, 13 (17.3%) in the 10 mg group, and 7 (8.9%) in the 1 mg group exhibited increased suicidal risk [19]. Of these patients, some reported serious, suicide-related AEs: two (4.5%) in the 25 mg group (suicidal ideation with intent), two in the 10 mg (7%) group (suicidal ideation with intent), and no patients in the 1 mg group [19]. From week 3 to week 12, 12 (15.2%) in the 25 mg, 12 (16.0%) in the 10 mg, and 12 (15.2%) in the 1 mg dose groups exhibited worsened suicidality. Here, serious suicide-related AEs were reported by three patients in the 25 mg group (three endorsement of suicidal behavior section of C-SSRS) and one in the 10 mg group (one suicidal ideation with intent). As participants at clinically significant risk for suicide were excluded from this study, the adverse events reported here are not part of longstanding behavioral trends [19].
Anorexia Nervosa
Peck et al. (2022) examined the safety of a single 25 mg dose with psychotherapy in 10 women with anorexia nervosa and reported no increases in suicidal risk as per the C-SSRS [30].
3.3.2. Open-Label Trials with Suicide as a Safety or Secondary Outcome
TRD
Carhart-Harris et al. (2018) conducted their own open-label trial with TRD patients, giving them two oral doses of psilocybin at 10 mg and 25 mg. Using the Quick Inventory of Depressive Symptomatology-Suicidality item (QIDS-SI; out of three) and the Hamilton Depression Rating Scale-Suicidality item (HAM-D-SI; out of four), they report significant reductions in QIDS-SI scores for suicide risk from baseline to 1 week (−0.9, 95% CI = −0.4 to −1.4; p < 0.002) and 2 weeks (−0.85, 95% CI = −0.4 to −1.3; p = 0.004) post dosing [34]. Scores at 3 weeks (−0.8, 95% CI = −0.25 to −1.3, p = 0.01) and 5 weeks (−0.7, 95% CI = −0.22 to −1.2, p = 0.01) show trend reductions but were non-significant [34]. Ellis et al. (2024) also conducted an open-label trial using a 25 mg dose with psychotherapy among 15 military veterans with TRD, most (73%, n = 11) of whom had PTSD [37]. Using the clinician-administered C-SSRS as part of their safety assessment, they reported no increases in suicidal ideation from baseline and that no suicidal behavior was present throughout the follow-up period [37].
BD-II
Aaronson et al. (2024) conducted an open-label trial for the use of 25 mg of psilocybin with psychotherapy for major depressive episodes in patients with bipolar type-II disorder (BD-II) [36]. Study clinicians assessed suicidal risk using the C-SSRS-IIS as part of their safety measures and found that the main effect on timepoint was non-significant. They also reported that no patients attempted or completed suicide at any timepoints during the study [36].
OLTAS
Anderson et al. (2020) worked with older long-term acquired immunodeficiency syndrome survivors (OLTAS) with moderate to severe demoralization, as quantified by a score of ≥9 on the Demoralization Scale-II [35]. Patients were given 0.3–0.36 mg/kg doses of psilocybin with group psychotherapy. While the investigators reported no changes in C-SSRS-IIS scores over time nor any suicidal behavior during the intervention period, one patient (out of eighteen) attempted suicide at their 3-month follow-up [35].
3.3.3. Secondary Analyses in Cancer Patients with Adjustment Disorder
Ross et al. (2021) conducted a secondary analysis assessing patient suicidal ideation in their 2016 trial where they administered a single 0.3 mg/kg dose of psilocybin with psychotherapy in cancer patients who were diagnosed with adjustment disorder with anxiety ± depression, acute stress disorder, generalized anxiety disorder (GAD), or anxiety disorder due to cancer [33]. Patients received psilocybin and placebo niacin (250 mg) in a crossover design. They used their own composite SI (suicidal ideation) measure, comprised of item nine of the Beck Depression Inventory-II (BDI-II) and item nine of the Brief Symptom Inventory (BSI) [33]. Ross et al. reported a significant within-group reduction of SI at 6.5 months from baseline (p < 0.001) but did not observe significant between-group effects [33].
3.3.4. Qualitative Studies in Previous Healthy Psilocybin Users
Carbonaro et al. (2016) conducted a cross-sectional study asking previous healthy psilocybin users to rate their most distressing experience while on the drug [47]. Participants responded qualitatively to questions made by the investigators, some of which asked about suicidality. Out of 1993 respondents, six reported complete remission of suicidal thoughts after taking psilocybin (timeline not specified), whereas five reported increased suicidal thinking and behaviors during their experience [47]. Increased suicidality outcomes were reported as attempt to overdose and waking up in an intensive care unit (one participant), attempting to shoot themselves in the head (one participant), pre-existing depression exacerbated by psilocybin and leading to suicide attempt (one participant), and increased salience of suicidal thoughts during the experience (three participants) [47]. The context of psilocybin use (i.e., alone, one other person, small group, large group) was not reported in relation to suicidality outcomes.
3.4. MDMA-Assisted Therapy
Four RCTs and two cross-sectional studies examined the effects of MDMA-assisted therapy (MDMA-AT) on suicide risk as well as different suicide-related outcomes (i.e., ideation intensity, behavior).
3.4.1. RCTs Assessing Suicidality as a Safety Measure in PTSD Patients
Mithoefer et al. (2018) conducted a double-blinded RCT in PTSD patients who received MDMA at a dose of 30 mg, 75 mg, or 125 mg with psychotherapy, followed by a supplemental half dose [38]. After the final RCT endpoint, the 30 mg and 75 mg groups were invited to participate in a 125 mg open-label crossover for three more sessions. The investigators stated in their safety assessment that by all post-treatment endpoints, the percentage of participants reporting suicidal ideation and behavior was reduced compared with baseline lifetime and pretreatment reports, as measured by the clinician-administered C-SSRS [38]. They also reported that there were no treatment-emergent reports of suicidal ideation [38]. Olatora et al. (2018) also ran their own double-blinded RCT with an open-label phase, where they administered to PTSD patients 40 mg, 100 mg, or 125 mg of MDMA with psychotherapy and with a supplemental half dose [39]. After the RCT endpoint, patients received either one (100 mg and 125 mg groups) or three (40 mg group) doses of 100–125 mg MDMA in an open-label crossover. While the investigators reported higher clinician-administered C-SSRS scores in the 100 mg and 125 mg groups, they did not exclude for past suicidal thinking during patient recruitment [39]. Therefore, unlike most similar studies, 28.6% of treatment-receiving participants were already impacted by symptoms of suicidal behavior before participating, and it is unclear to what extent MDMA or their medical history attributed to trends in C-SSRS scores [39]. Mitchell et al.’s (2021) RCT administered two doses of MDMA to PTSD patients through two sessions (first session: 80 + 40 mg half dose; second session: 120 + 60 mg half dose) with psychotherapy [40]. MDMA, when compared to a placebo, did not increase suicidal risk in patients with PTSD at all follow-up timepoints, as measured by a study clinician using the C-SSRS intensity of ideation subscale in their safety assessment [40]. Mitchell et al. ran another RCT in 2023 administering MDMA to PTSD patients using the same dosing regimen and found that from baseline to end of intervention, MDMA-AT did not increase suicidal risk relative to placebo in patients as scored by the clinician-administered C-SSRS [22].
3.4.2. Cross-Sectional Studies: Survey Data in Non-Clinical Populations
Celine et al. (2019) looked at France’s national survey on health and drug use, ESCAPAD, and found that among French adolescents, ecstasy use is associated with increased suicide risk relative to non-users ([odds ratio] OR = 2.42, [adjusted OR] aOR = 2.74; [no p-values reported]) [48]. Here, adjusting the OR is accounting for covariates that influence the outcome variable, i.e., suicide risk, and is therefore controlling for confounding effects. Kim et al. (2011) conducted their own cross-sectional study using multinomial logistic regression analysis on data from the United States National Survey on Drug Use and Health (NSDUH), where, while not adjusted for confounders, they found that ecstasy use among American adolescents was associated with higher suicidal ideation and attempt compared to non-use (ideation OR: 1.5 [p ≤ 0.05], attempt OR: 5.5 [p < 0.001]) [49].
3.5. DMT and Ibogaine
In total, two prospective longitudinal (one cohort and one single-case), one retrospective longitudinal, and two secondary analyses (one of a RCT and one of an open-label trial) provided reports on the effect of DMT and ibogaine on suicidal behavior.
3.5.1. Observational Studies in Persons with Military Trauma or PTSD
Davis et al. (2023) collected prospective data from a clinical treatment program about an open-label trial treating trauma-exposed military veterans. Patients were treated with 10 mg/kg ibogaine, followed by three doses of 5-Meo-DMT, at 5 mg, 10 mg, and 15 mg, accompanied by psychotherapy [41]. Fourth (30 mg) and fifth doses (45 mg) were administered if the patient did not reach an altered state of consciousness. The investigators reported a −0.53 (p < 0.01) decrease in the DSI-SS (Depressive Symptom Inventory-Suicide Subscale; out of 0 to 12) between baseline and one month post treatment [41]. Davis et al. (2020) also conducted a longitudinal, retrospective study using the same treatment regimen and found a −2.3 (p < 0.0001) decrease in DSI-SS scores from one month pretreatment to one month post treatment in military veterans with cognitive and physical trauma [42]. Ragnhildstveit et al. (2023) followed a 23-year-old woman with PTSD, administering to her a bufotoxin extract containing 10–15 mg of DMT. Immediately, within 24 h and for 12 months following treatment, the patient’s Beck Hopelessness Scale (BHS; out of 20) scores dropped below the cut-off for suicidal ideation and behavior (> 9) [43]. The investigators considered this drop a significant and large reduction in the patient’s suicide risk.
3.5.2. Secondary Analyses in MDD Patients
Zeifman et al. (2019) conducted a secondary analysis of an RCT for treatment-resistant patients with unipolar MDD [44]. They assessed baseline patient characteristics using the MADRS-SI for a population that either received a placebo or a single 1 mL/kg dosing of ayahuasca (containing 0.36 mg/mL of DMT) with no psychotherapy. They report moderate between-group effects for decreases in MADRS-SI scores at one day (d = 0.58, 95% CI = −1.32 to 0.17), two days (d = 0.56, 95% CI = −1.30 to 0.18), and seven days (d = 0.67, 95% CI = −1.42 to 0.08) after intervention [44]. Within the ayahuasca group, there were large effect sizes for decreases in MADRS-SI at one day (d = 1.33; 95% CI 1.25 to 3.18; n = 14), two days (d = 1.42; 95% CI 1.50 to 3.74; n = 13), and seven days (d = 1.19; 95% CI 1.21 to 3.50; n = 14) [44]. Zeifman et al. (2021) conducted another secondary analysis on an open-label trial for MDD patients receiving 2.2 mL/kg dose of ayahuasca (itself containing 0.8 mg/mL of DMT) with no psychotherapy and found rapid reductions in suicidality, as measured through MADRS-SI scores, that remained 21 days after intervention [45].
3.6. LSD
One RCT and two cross-sectional studies examined LSD effects in patient and non-clinical populations, respectively.
3.6.1. RCT for Patients with Anxiety Associated with Life-Threatening Disease
Gasser et al. (2015) ran an RCT for patients with anxiety associated with life-threatening disease, giving them two doses of 200 μg LSD with psychotherapy in 4-to-6-week intervals. At their 12-month follow up, 33% of patients reported “no more suicidal thoughts” and “less depressed feelings” [46].
3.6.2. Cross-Sectional Studies: Retrospective Survey Data in Non-Clinical Populations
Han et al. (2022) conducted multivariable analyses using NSDUH data from 2015 to 2019 and found that past-year LSD use, relative to non-use, was associated with mildly elevated suicidal ideation among adolescents (OR = 2.4 [no p-values reported]). They controlled for confounding variables and reported an adjusted OR of 1.2 (no p-value reported) [50]. Yockey et al. (2019) examined the 2017 NSDUH results and also used multivariable analyses to conclude that lifetime LSD use among adolescents was significantly associated with more frequent thoughts about suicide (OR = 2.46 [p < 0.001], aOR = 1.381 [p < 0.001]) [51].
3.7. Not Specified/Multiple Psychedelic Studies
From our search, ten cross-sectional, two longitudinal, and one secondary analysis (of two longitudinal studies) examined the effects of psychedelics but either used multiple psychedelics or did not specify which psychedelics they assessed.
3.7.1. Longitudinal Studies
Argento et al. conducted two overlapping longitudinal studies using a cohort of women belonging to Vancouver, Canada’s An Evaluation for Sex Workers Health Access (AESHA) cohort [54,55]. At baseline, they excluded any participants who reported previous suicidal thoughts or attempts in this analysis. They then followed participants annually for several years and later tested for associations between changes in suicidality and psychedelic use (e.g., LSD, MDMA, and psilocybin) [54,55]. Argento et al. (2017) used AESHA data from January 2010 to August 2014 and found that lifetime psychedelic use was independently associated with reduced suicidality in a population of sex workers after adjusting for covariates (OR = 1.00 [p = 0.995], aOR = 0.40 [p = 0.036]) [54]. In their follow-up study, Argento et al. (2018), using data from January 2010 to February 2017, looked at associations between psychedelic use and suicidal behaviors in users of different drugs [55]. They observed that psychedelic use moderated suicidal ideation in prescription opioid users ([OR = psychedelic use vs. no psychedelic use]; OR = 0.69 vs. 2.91 [p = 0.016]; aOR = 0.36 vs. 2.59 [p = 0.036]) and cocaine users (OR = 0.39 vs. 4.69 [p = 0.001] [55]. While not significant, they also tested for the effect of psychedelic use in crack users (OR = 1.31 vs. 4.08 [p = 0.326]), crystal meth users (OR = 1.98 vs. 4.51 [p = 0.206]), and heroin users (OR = 0.99 vs. 2.15 [p = 0.170]) [55].
3.7.2. Cross-Sectional Studies: Retrospective Survey Data in Non-Clinical Populations
Guigovaz et al. (2024) used regression analyses on NSDUH data from 2008 to 2020 and found that hallucinogen use, (e.g., MDMA, LSD, psilocybin) among adolescents is not significantly associated with increased suicidal ideation, planning, or attempt (OR = 0.46, 0.56, and 1.14 respectively; [no p-values reported]) [62]. Sexton et al. also conducted analyses using the NSDUH [56,57]. In 2019, Sexton et al. looked at the NSDUH from 2008 to 2016 and found that lifetime novel psychedelic use (e.g., 2-[4-Bromo-2,5-dimethoxyphenyl]-ethanamine; 2C-B, 2,5-dimethoxy-4-iodophenethylamine; 2CI) compared to lifetime classic psychedelic use (e.g., LSD, mescaline, DMT, psilocybin) was associated with increased odds of suicide ideation (OR = 1.4 [p = 0.0180]), planning (OR = 1.6 [p = 0.0285]), and attempt (OR = 1.2 [p = 0.6310]) [56]. They also found that lifetime novel psychedelic use, compared to no lifetime psychedelic use, was associated with increased odds of suicide ideation (OR = 1.3 [p = 0.0749]) and planning (OR = 1.4 [p = 0.1196]) but not attempt (OR = 0.9 [p = 0.8813]) [56]. Novel psychedelics were defined as serotonin 2A receptor (5HT2AR) agonists with similar pharmacokinetics and pharmacodynamics to classic psychedelics [56]. In 2020, Sexton et al. analyzed the NSDUH from 2008 to 2017 [57]. They focused on associations between suicide and either lifetime classic tryptamine or novel phenylethylamine use. When adjusting for covariates, while they found that increased past-year suicidal thinking was not associated with lifetime classic tryptamine use (aOR = 0.79 [no p-value reported]), they did observe an association with novel phenylethylamine use (aOR = 1.60 [no p-value reported]) [57]. Hendricks et al. (2015) also looked at the NSDUH and found that in 2008–2012, lifetime classic psychedelic use, relative to no use, was associated with reduced past-year suicidal thinking (OR = 0.86 [no p-value reported]), planning (OR = 0.71 [no p-value reported]), and attempt (OR = 0.64 [no p-value reported]) [53]. Here, odds ratios are not adjusted for confounders. Jones et al. (2022) looked at the NSDUH from 2008 to 2019 and reported that neither MDMA or psilocybin are associated with increased suicidal planning (OR = 0.9 [p = 0.01]), thinking (MDMA: OR = 0.88 [p = 0.05]; psilocybin: OR = 0.88 [p = 0.08]), or attempt (MDMA: OR = 1.00 [p = non-significant; ns]; psilocybin: OR = 0.85 [p = 0.07]) [25]. However, unlike MDMA and psilocybin, LSD was associated with increased odds of suicidal thinking (OR = 1.07 [p = 0.05]) [25]. Again, odds ratios were not adjusted for confounders. Another article published by Jones et al. in 2022 looked at NSDUH data from 2004 to 2019 [60]. They report that while psilocybin use is associated with reduced suicidal thoughts (aOR = 0.84 [p ≤ 0.05]) and behaviors (suicidal planning: aOR = 0.78 [p ≤ 0.05]; suicidal attempt: aOR = 0.77 [p ≤ 0.05]), LSD use is associated with increased suicidal thinking (aOR = 1.19 [p ≤ 0.05]) and behaviors (suicidal planning: aOR = 1.36 [p ≤ 0.05]; suicidal attempt: aOR = 1.23 [p ≤ 0.05]) [60]. MDMA, peyote, and mescaline were examined as well but were nonsignificant and not distinctly associated with suicidal ideation or behavior [60]. Yang et al. (2022) looked at associations of LSD, tryptamine (DMT/AMT [a-methyltryptamine]/Foxy [5-methoxy-diisopropyltryptamine]), Salvia divinorum, and MDMA use with suicidal thinking, planning, and attempt from NSDUH data (2015 to 2020) [61]. Compared to non-users, LSD was associated with greater suicidal ideation (OR = 1.21 [p < 0.001]), planning (OR = 1.14 [p = ns]), and attempt (OR = 1.27 [p = ns]) [61]. Where Salvia divinorum use was associated with even greater ideation (OR = 1.41 [p < 0.05]), planning (OR = 1.74 [p = ns]), and attempt (OR = 2.05 [p = ns]) than LSD, ecstasy was associated with lesser ideation (OR = 0.86 [p < 0.05]), planning (OR = 0.80 [p = ns]), and attempt (OR = 0.83 [p = ns]) [61]. Tryptamine effects on suicidal ideation (OR = 1.14 [p = ns]) and attempt (OR = 1.16 [p = ns]) were comparable to LSD, but tryptamines were associated with increased suicidal planning (OR = 1.81 [p < 0.01]) [61]. Desai et al. (2022) used data from the Youth Risk Behavior Surveillance System (YRBSS) by the United States Centers for Disease Control and Prevention, which targets school-going adolescents [59]. The investigators found that relative to non-users, hallucinogen-(e.g., LSD, PCP [phencyclidine], mescaline, or mushrooms)-using high schoolers more often considered suicide (OR = 1.31 [p = 0.03]), made a suicide plan (OR = 1.44 [p = 0.03]), attempted suicide (OR = 1.15 [p = 0.395]), or had an injurious suicide attempt (OR = 1.39 [p = 0.118]) [59]. Importantly, these associations were not adjusted for confounders. Wong et al. used the YRBSS from 2001 to 2009 to examine if hallucinogen and ecstasy use were associated with suicidal behaviors [52]. They found that hallucinogen-using high schoolers, compared to non-users, exhibited higher suicide ideation (OR = 3.3, aOR = 1.8; [both p ≤ 0.0001]), planning (OR = 4.0, aOR = 1.0; [both p ≤ 0.0001]), suicide attempt (OR = 4.9, aOR = 2.1; [both p ≤ 0.0001]), or severe suicide attempt (OR = 10.4, aOR = 3.4; [both p ≤ 0.0001]) [52]. Interestingly, ecstasy use was also positively associated with such parameters, but to a reduced extent (ideation: OR = 3.1, aOR = 1.6 [both p > 0.0001]; planning: OR = 4.3, aOR = 1.5 [both p > 0.0001]; attempt: OR = 5.0, aOR = 1.9 [both p > 0.0001]; severe attempt: OR = 10.7, aOR = 2.9 [both p > 0.0001]) [52]. Soboka et al. (2024) examined Nova Scotia’s Mental Health and Addiction (MHA) intake program data from 2020 to 2021 and tested participant data for associations with suicide risk [63]. They considered past suicide attempts, suicidal thoughts two weeks before the interview, and suicidal ideation during the interview in defining suicide risk. They report that psychedelic use, when adjusted for covariates, was positively associated with mild (aOR = 2.04 [p < 0.001]) and even moderate/high (aOR = 3.54 [p < 0.001]) suicide risk [63].
3.7.3. Secondary Analyses
Zeifman et al. (2020) performed a secondary analysis on two longitudinal, observational studies [58]. They used a composite suicidal ideation score (composite SI) made of the single-item QIDS-SI and the five-item Suicidal Ideation Attributes Scale (SIDAS; out of 50), ranging from 1 to −1. In their first study, Zeifman et al. used a convenience sample of participants planning to use a psychedelic of choice (e.g., psilocybin, LSD, ayahuasca, 5-Meo-DMT, Salvia divinorum, mescaline, or ibogaine) and reported that relative to baseline, psychedelic use was associated with significant reductions in suicidal ideation at 2 weeks (0.48 to −0.19 [p < 0.001]) and 4 weeks (0.48 to −0.47 [p < 0.001]) [58]. In the second study, participants planned to use psychedelics in the presence of a facilitator: an individual who would guide them through the process. Once again, psychedelic use was associated with a significant reduction in suicidal ideation from baseline to 4 weeks post psychedelic use (0.16 to −0.28 [p < 0.001]) [58].
4. Discussion
In this systematic review, we evaluated the effects of psychedelics on suicidal-related outcomes using 39 studies. Psilocybin was studied in multiple trials focusing on its effects on suicidal ideation and behavior, primarily in patients with depression and other psychiatric conditions. In summary, while clinical trials have supported the potential of psychedelics, especially psilocybin, in reducing suicidality in patients with psychiatric conditions, findings from observational and cross-sectional studies on general or adolescent populations suggested mixed or negative associations. However, these studies are limited by unknown confounding variables, such as the potential for increased substance use among individuals with mental health conditions or the reverse association, where those with suicidality may engage in greater substance use. Additionally, the lack of baseline data or detailed population characteristics severely limits the interpretability of these findings regarding the effects of psychedelics on suicidality, underscoring the need for further rigorous investigation.
Suicidality is a multifaceted and complex phenomenon that can manifest across a range of psychiatric disorders, including mood disorders, anxiety, PTSD, substance use disorders, and chronic pain [64]. While most pharmacological treatments are designed to target the primary disorder with the assumption that improving the underlying condition will also alleviate suicidality, this approach may be overly reductive. Suicidality may persist even in the absence of other symptoms, suggesting that treating the primary disorder may not always be sufficient to address the risk of suicide [65]. Consequently, it is critical to reconsider the assumption that treating the underlying condition inherently resolves suicidality. A more nuanced approach that evaluates suicidality as a distinct clinical target, independent of the underlying disorder, may offer more effective and tailored therapeutic interventions. This is analogous to the use of hypnotic medications to address sleep disturbances, irrespective of the underlying psychiatric condition, and could lead to more focused strategies for managing suicidality in clinical practice.
Several RCTs have reported positive effects of psychedelic administration on suicide risk, with most trials finding moderate between-group effect sizes for reductions in suicidality. Some studies reported rapid effects of psychedelics on suicidal ideations, potentially similar to ketamine [66], which is an important advantage given the limitations of current treatments due to their delayed onset of action. However, the limited reporting on the timing of suicide outcome assessments prevents definitive conclusions. Antidepressants and ECT typically take up to two weeks to show therapeutic effects [67,68], making psychedelics a critical alternative for patients with suicidal ideations without intent or plan, offering a potentially life-saving intervention when time is of the essence. The mechanisms by which psychedelics may reduce suicidality are complex and multifaceted, with one primary mechanism being the promotion of neuroplasticity, enhancing the brain’s ability to process and reframe negative beliefs and emotional experiences associated with suicidality [69,70]; however, these effects are closely influenced by factors such as set (mindset), setting (environment), and therapeutic integration [69,70]. Importantly, combining psychedelics with evidence-based therapies such as CBT may offer additional benefits [71]. Meta-analytic evidence suggests that CBT reduces suicide attempts by half in those who attempted suicide in the previous six months, making it a valuable adjunctive or follow-up intervention [72]. Moreover, psychedelics could be leveraged to address potentially modifiable factors associated with higher suicide risk, such as hopelessness, anxiety, impulsivity, psychotic symptoms, and the impact of stressful life events (e.g., financial stress, victimization) [73]. By promoting a shift in perspective on life stresses and alleviating emotional burdens, psychedelics, when combined with therapies like CBT, may more effectively target these underlying factors, further supporting reductions in suicidality [74]. Future studies should investigate the efficacy of integrated psychedelic-assisted therapy approaches to maximize therapeutic outcomes in this high-risk population.
Psychedelics also increase connectivity in brain regions involved in emotional regulation and self-reflection, facilitating emotional processing and alleviating feelings of hopelessness and despair [75]. Additionally, reductions in experiential avoidance—identified as an indirect effect in a clinical trial and as a correlate in two naturalistic studies—may contribute to decreases in suicidality by enabling individuals to confront and process difficult thoughts and emotions rather than suppressing them [58,76]. Psychedelics enable profound emotional experiences that allow individuals to confront unresolved trauma and suppressed emotions, potentially leading to emotional catharsis [77]. This is thought to be mediated by serotonin system interactions, particularly through the 5-HT2A receptor, which plays a crucial role in mood regulation [78]. The therapeutic context, which typically involves supportive, guided environments, also contributes to the reduction in suicidality by fostering trust, safety, and emotional processing, potentially leading to shifts in perspective and enhanced meaning or connectedness. The combination of psychedelics’ unique mechanisms of action, along with their rapid therapeutic effects, presents a promising option for individuals at high risk of suicide [79]. However, it is important to note that individuals at high suicide risk, including those with a history of attempts reported during screening, have been systematically excluded from included studies, limiting the generalizability of current findings to this population. Additionally, BPD has also been underrepresented in these trials, despite the elevated suicide risk associated with this group [79]. Further, while psychedelics offer rapid reductions in symptoms, the inconsistent reporting of safety outcomes across trials represents a significant limitation, particularly in studies addressing suicide outcomes. Specifically, many trials rely on scales designed to measure broader psychiatric disorders rather than directly assessing suicidality. When suicidality is evaluated, it is often through single-item measures or scales with only 2–3 questions, which fail to capture the complexity of suicidality, including its chronicity, acuity, risk level, and predisposing factors [72]. Additionally, the timing of these measurements is not always consistent, leaving uncertainty about when suicidal outcomes were assessed in relation to the psychedelic intervention. Another concern is the ambiguous categorization of suicidal outcomes—whether they are treated as adverse effects or primary outcomes [72]. If considered adverse effects, suicidality may not be systematically monitored or directly inquired about, risking underreporting when left to participant-initiated disclosures. This lack of standardization in assessing and reporting suicidal outcomes undermines a comprehensive evaluation of the risk-benefit profile of psychedelics. For comparison, ketamine and esketamine demonstrate significant short-term benefits, but their effects are often transient, requiring repeated dosing, and come with their own safety considerations [13]. Therefore, further research is essential to identify which individuals are most likely to benefit from psychedelics, to evaluate their safety in high-risk populations, and to address the caveats associated with their clinical application. Our search of clinicaltrials.gov yielded only one trial with a primary outcome focused on suicide prevention, underscoring the need for additional studies in this area.
Suicidal ideation and attempts are assessed in clinical trials as a safety measure due to concerns about the potential for increased suicidal thoughts following psychedelic administration. The C-SSRS is the most commonly used tool to evaluate suicidal ideation in these studies. While the majority of trials report no increase in suicidal risk during the study period, some severe adverse events, including suicidal ideation following psychedelic use, have been documented [79]. Additionally, one study has reported death by suicide following psilocybin administration [80]. Prior to their death by suicide, the participant exhibited no signs of behavioral impairment and showed no adverse effects during follow-up later that day or in the subsequent days [80]. However, the authors noted that this was not attributed to the intervention. In clinical trials, the controlled environment and support from trained professionals are intended to mitigate these risks, yet the unpredictable nature of psychedelic experiences means that some individuals may react in ways that are difficult to anticipate. As such, ongoing monitoring and individualized care are crucial components of ensuring participant safety, particularly for those with a history of mental health issues. Increased suicidal ideations, planning, and attempts have also been reported in population-based studies, though these findings have been inconsistent. Some studies suggest that psychedelics were associated with increased odds of suicidal behaviors in certain individuals, while others reported no significant association, and some demonstrated reduced suicidal behaviors following psychedelic use. These mixed results may be due to several factors, such as differences in study design, participant characteristics, and the specific contexts in which psychedelics are used. The intensity of the psychedelic experience itself—often involving profound emotional and psychological shifts—can vary widely among individuals, which may influence the risk of adverse outcomes. These mixed findings highlight the complexity of the relationship between psychedelics and suicidality, suggesting that further research is needed to clarify the potential risks and identify factors that may increase vulnerability to adverse outcomes. Factors such as prior mental health conditions, personal history of trauma, and the setting in which psychedelics are administered may all play a role in determining the effects on suicidality. As a result, personalized treatment approaches and careful risk assessment are essential for ensuring participant safety, particularly for those with pre-existing mental health concerns.
This systematic review added to the literature by synthesizing existing research on the effects of psychedelic therapies—specifically psilocybin and other serotonergic psychedelics—on suicidality in individuals with psychiatric disorders. By focusing on RCTs, observational studies, and other relevant data, this review evaluated the potential of psychedelics as a treatment for suicide risk. It highlighted the methodological gaps in current research, such as the exclusion of high-risk populations, inconsistent definitions of suicidality, and limited longitudinal data. Additionally, this review discussed the implications for clinical practice and future research directions, providing an evidence-based foundation for the safe integration of psychedelic therapies into psychiatric care, particularly for individuals at high risk for suicide. Given these findings, it is clear that psychedelic therapies hold promise for addressing suicidality in psychiatric patients, particularly when combined with psychotherapy. However, due to methodological limitations and a lack of data from high-risk populations, it is premature to implement these therapies as standard practice. Further research is required to better understand their long-term efficacy, safety, and optimal treatment protocols. Only through rigorous clinical trials that include high-risk individuals and consistent outcome measures can we determine the potential role of psychedelics in clinical settings.
Limitations
The limitations of this review include several factors that may affect the validity and generalizability of the results. First, the sample size is insufficient to detect significant effects, limiting the ability to generalize findings to broader populations. Selection bias is another concern, as participants may not be randomly selected or fully representative of the target population. Additionally, higher-risk patients are often excluded, which limits the study’s external validity and generalizability. Confounding variables, such as unmeasured factors influencing the outcomes, could also distort the findings. Moreover, the short duration of the study limits the ability to assess long-term effects or trends, which may be important in understanding sustained outcomes. If a study is cross-sectional, it can only establish associations, not causality, which is a significant limitation. Furthermore, the absence of subgroup analyses for specific psychedelics and populations limited the ability to assess the nuanced effects across different types of psychedelics and varied populations. The lack of data suitable for a meta-analysis prevented a more comprehensive synthesis of results across studies. Additionally, publication bias may lead to an overrepresentation of positive findings, as studies with negative or null results are less likely to be published, which may influence the conclusions drawn. These limitations should be carefully considered when interpreting the findings. Ethical considerations, accessibility, and scalability of psychedelic therapies are critical issues that must be addressed in future research. The potential for these therapies to be widely implemented in clinical settings depends on overcoming significant barriers related to their cost, regulatory approval, and the need for trained professionals to administer them safely. Additionally, the mechanisms by which psychedelics exert their therapeutic effects remain not fully understood, highlighting a need for further research into their pharmacodynamics and neurobiological mechanisms. Furthermore, although certain adverse effects have been identified, there is a clear gap in the systematic evaluation of potential long-term risks associated with psychedelic use, such as exacerbation of mental health symptoms or emergence of new psychological issues. These concerns must be addressed to ensure the safe and effective use of psychedelics in treating psychiatric disorders.
5. Future Directions
Future research should focus on well-designed longitudinal studies that consistently define and assess suicide-related outcomes, account for confounding factors, and evaluate long-term safety and efficacy. Expanding inclusion criteria to encompass high-risk populations will be critical in determining whether psychedelics can be safely and effectively used for suicide risk reduction. Standardizing adverse event reporting, including suicidality, is essential to clarify potential risks. Addressing these gaps will provide a clearer understanding of whether psychedelics have a meaningful role in managing suicidality in clinical and real-world settings.
6. Conclusions
While clinical trials suggest that psychedelics, particularly psilocybin, may reduce suicide-related outcomes in individuals with psychiatric disorders, the evidence remains inconclusive. Reductions in suicidality appear closely linked to improvements in comorbid symptoms such as depression, PTSD, and anxiety, rather than a direct effect on suicidality itself. Methodological limitations, including inconsistent definitions of suicide-related outcomes, reliance on single-item measures, and variability in outcome assessment timing, weaken the strength of current findings. Additionally, observational and cross-sectional studies indicate more variable results, with some evidence suggesting increased suicidality in certain populations. The exclusion of high-risk individuals from clinical trials further limits the generalizability of these findings. At present, there is insufficient evidence to support the clinical use of psychedelics for suicide prevention, and further research is needed to determine their safety and efficacy in this context. Given these factors, it remains premature to conclude that psychedelics have a definitive role in mitigating suicidality.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14051416/s1.
Author Contributions
Conceptualization: S.M. and V.B.; methodology: S.M. and V.B.; formal analysis: S.M. and T.M.; investigation: S.M., T.M. and V.B.; writing—original draft preparation: S.M. and T.M.; writing—review and editing, all authors; supervision: V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Richard Zeifman received funding from the NYU Langone Psychedelic Medicine Research Training program (funded by MindMed) and the Canadian Institutes of Health Research (Grant Number: 202110MFE-472921-HTB-272687). Jennifer Swainson, Lisa Burback, Olga Winkler, and Yanbo Zhang are supported by the Academic Medicine and Health Services Program (AMHSP), a joint program funded by the University of Alberta and Alberta Health Services to ensure physicians affiliated with Alberta’s faculties of medicine are compensated for providing patient care along with their work related to research, innovation, education, administration, and leadership. Jennifer Swainson has received honoraria for speaking or advisory roles from Abbvie, Bausch Health, Biron, Eisai, Idorsia, Janssen, Lundbeck, Novo Nordisk, and Otsuka. Rakesh Jetly is the CMO of Mydecine Innovation Group. Muhammad I. Husain is supported by the Cameron Wilson Chair in Depression Studies from the University of Toronto. He is leading contracted research for Compass Pathways Ltd and has served as an advisor to Mindset Pharma, Psyched Therapeutics, and Wake Network. Venkat Bhat is supported by an Academic Scholar Award from the University of Toronto Department of Psychiatry and has received research funding from the Canadian Institutes of Health Research, Brain & Behavior Foundation, Ontario Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of National Defence (Government of Canada), New Frontiers in Research Fund, Associated Medical Services Inc. Healthcare, American Foundation for Suicide Prevention, Roche Canada, Novartis, and Eisai. David Erritzoe is acting as a paid scientific advisor for Aya Biosciences, Lophora Aps, Clerkenwell Health, Mindstate Design Lab. Manish Jha has received contract research grants from Acadia Pharmaceuticals, Neurocrine Bioscience, Navitor/Supernus and Janssen Research and Development; has received honorarium to serve as Section Editor of the Psychiatry and Behavioral Health Learning Network and as Guest Editor for Psychiatric Clinics of North America from Elsevier; has received consultant fees from Eleusis Therapeutics US, Janssen Global Services, Janssen Scientific Affairs, Boehringer Ingelheim and Guidepoint Global; has received fees to serve on Data Safety and Monitoring Board for Worldwide Clinical Trials (Eliem and Inversargo), Vicore Pharma and IQVIA (Click); and honoraria for educational presentations from North American Center for Continuing Medical Education, Medscape/WebMD, Clinical Care Options, H.C. Wainwright and Company and Global Medical Education.
References
- Masango, S.M.; Rataemane, S.T.; Motojesi, A.A. Suicide and suicide risk factors: A literature review. S. Afr. Fam. Pract. 2008, 50, 25–29. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention: WISQARS: Fatal Injury Reports, National, and Regional, 1999–2018. Available online: https://webappa.cdc.gov/sasweb/ncipc/mortrate.html (accessed on 20 February 2020).
- Mann, J.J. A current perspective of suicide and attempted suicide. Ann. Intern. Med. 2002, 136, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J.; Apter, A.; Bertolote, J.; Beautrais, A.; Currier, D.; Haas, A.; Hegerl, U.; Lonnqvist, J.; Malone, K.; Marusic, A.; et al. Suicide prevention strategies: A systematic review. JAMA 2005, 294, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Tondo, L. Suicidal risks in 12 DSM-5 psychiatric disorders. J. Affect. Disord. 2020, 271, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Panagioti, M.; Gooding, P.A.; Triantafyllou, K.; Tarrier, N. Suicidality and posttraumatic stress disorder (PTSD) in adolescents: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Stack, S. Contributing factors to suicide: Political, social, cultural and economic. Prev. Med. 2021, 152, 106498. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.A.; Bschor, T. Antidepressants and suicidality: A re-analysis of the re-analysis. J. Affect. Disord. 2020, 266, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, L.; Qian, Y.; Jin, X.H.; Yu, H.R.; Du, L.; Fu, X.L.; Zhu, B.; Chen, H.L. The significance of cognitive-behavioral therapy on suicide: An umbrella review. J. Affect. Disord. 2022, 317, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Peltzman, T.; Shiner, B.; Watts, B.V. Effects of electroconvulsive therapy on short-term suicide mortality in a risk-matched patient population. J. ECT 2020, 36, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Müller-Oerlinghausen, B.; Lewitzka, U. The contributions of lithium and clozapine for the prophylaxis and treatment of suicidal behavior. In Biological Aspects of Suicidal Behavior; Karger Publishers: Basel, Switzerland, 2016; Volume 30, pp. 145–160. [Google Scholar]
- Chen, C.C.; Zhou, N.; Hu, N.; Feng, J.G.; Wang, X.B. Acute Effects of Intravenous Sub-Anesthetic Doses of Ketamine and Intranasal Inhaled Esketamine on Suicidal Ideation: A Systematic Review and Meta-Analysis. Neuropsychiatr. Dis. Treat. 2023, 19, 587–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegel, A.N.; Di Vincenzo, J.D.; Brietzke, E.; Gill, H.; Rodrigues, N.B.; Lui, L.M.W.; Teopiz, K.M.; Ng, J.; Ho, R.; McIntyre, R.S.; et al. Antisuicidal and antidepressant effects of ketamine and esketamine in patients with baseline suicidality: A systematic review. J. Psychiatr. Res. 2021, 137, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.R.; dos Santos Costa, C.; Wiltenburg, V.D.; Morales-Lima, G.; Fernandes, J.A.; Filev, R. Classic and non-classic psychedelics for substance use disorder: A review of their historic, past and current research. Addict. Neurosci. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Richards, W.A.; McCann, U.; Jesse, R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 2006, 187, 268–283. [Google Scholar] [CrossRef]
- Heifets, B.D.; Olson, D.E. Therapeutic mechanisms of psychedelics and entactogens. Neuropsychopharmacology 2024, 49, 104–118. [Google Scholar] [CrossRef]
- Wardle, M.C.; de Wit, H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology 2014, 231, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Meshkat, S.; Doyle, Z.; Kaczmarek, E.; Brudner, R.M.; Kratiuk, K.; Mansur, R.B.; Schulz-Quach, C.; Sethi, R.; Abate, A.; et al. Psilocybin-assisted psychotherapy for treatment resistant depression: A randomized clinical trial evaluating repeated doses of psilocybin. Med 2024, 5, 190–200. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’Donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: A randomized clinical trial. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaczmarek, E.; Doyle, Z.; Brudner, R.M.; Gomes, F.A.; Blainey, M.G.; Weiglein, G.; McIntyre, R.S.; Mansur, R.B.; Rosenblat, J.D. Psilocybin-Assisted Psychotherapy for Treatment-Resistant Depression in Bipolar II Disorder. Psychedelic Med. 2024, 5, 190–200.e5. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora, G.M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedo, S.; Balliett, B.; et al. MDMA-assisted therapy for moderate to severe PTSD: A randomized, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef]
- Thiessen, M.S.; Walsh, Z.; Bird, B.M.; Lafrance, A. Psychedelic use and intimate partner violence: The role of emotion regulation. J. Psychopharmacol. 2018, 32, 749–755. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Graziosi, M.; Nayak, S.M.; Yaden, D.B. Adverse events in studies of classic psychedelics: A systematic review and meta-analysis. JAMA Psychiatry 2024, 81, 1225–1235. [Google Scholar] [CrossRef]
- Jones, G.M.; Nock, M.K. MDMA/ecstasy use and psilocybin use are associated with lowered odds of psychological distress and suicidal thoughts in a sample of US adults. J. Psychopharmacol. 2022, 36, 46–56. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Porritt, K.; Gomersall, J.; Lockwood, C. JBI’s systematic reviews: Study selection and critical appraisal. Am. J. Nurs. 2014, 114, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Peck, S.K.; Shao, S.; Gruen, T.; Yang, K.; Babakanian, A.; Trim, J.; Finn, D.M.; Kaye, W.H. Psilocybin therapy for females with anorexia nervosa: A phase 1, open-label feasibility study. Nat. Med. 2023, 29, 1947–1953. [Google Scholar] [CrossRef]
- von Rotz, R.; Schindowski, E.M.; Jungwirth, J.; Schuldt, A.; Rieser, N.M.; Zahoranszky, K.; Seifritz, E.; Nowak, A.; Nowak, P.; Jäncke, L.; et al. Single-dose psilocybin-assisted therapy in major depressive disorder: A placebo-controlled, double-blind, randomised clinical trial. EClinicalMedicine 2022, 56, 101809. [Google Scholar] [CrossRef]
- Raison, C.L.; Sanacora, G.; Woolley, J.; Heinzerling, K.; Dunlop, B.W.; Brown, R.T.; Kakar, R.; Hassman, M.; Trivedi, R.P.; Robison, R.; et al. Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial. JAMA 2023, 330, 843–853. [Google Scholar] [CrossRef]
- Ross, S.; Agin-Liebes, G.; Lo, S.; Zeifman, R.J.; Ghazal, L.; Benville, J.; Franco Corso, S.; Bjerre Real, C.; Guss, J.; Bossis, A.; et al. Acute and Sustained Reductions in Loss of Meaning and Suicidal Ideation Following Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Life-Threatening Cancer. ACS Pharmacol. Transl. Sci. 2021, 4, 553–562. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.T.; Danforth, A.; Daroff, P.R.; Stauffer, C.; Ekman, E.; Agin-Liebes, G.; Trope, A.; Boden, M.T.; Dilley, P.J.; Mitchell, J.; et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine 2020, 27, 100538. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.T.; van der Vaart, A.; Miller, T.; LaPratt, J.; Swartz, K.; Shoultz, A.; Lauterbach, M.; Sackeim, H.A.; Suppes, T. Single-Dose Synthetic Psilocybin With Psychotherapy for Treatment-Resistant Bipolar Type II Major Depressive Episodes: A Nonrandomized Open-Label Trial. JAMA Psychiatry 2024, 81, 555–562. [Google Scholar] [CrossRef]
- Ellis, S.; Bostian, C.; Feng, W.; Fischer, E.; Schwartz, G.; Eisen, K.; Lean, M.; Conlan, E.; Ostacher, M.; Aaronson, S.; et al. Single-dose psilocybin for U.S. military Veterans with severe treatment-resistant depression—A first-in-kind open-label pilot study. J. Affect. Disord. 2025, 369, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Ot’alora, G.M.; Grigsby, J.; Poulter, B.; Van Derveer, J.W., 3rd; Giron, S.G.; Jerome, L.; Feduccia, A.A.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. J. Psychopharmacol. 2018, 32, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Xin, Y.; Sepeda, N.; Averill, L.A. Open-label study of consecutive ibogaine and 5-MeO-DMT assisted-therapy for trauma-exposed male Special Operations Forces Veterans: Prospective data from a clinical program in Mexico. Am. J. Drug Alcohol. Abuse. 2023, 49, 587–596. [Google Scholar] [CrossRef]
- Davis, A.K.; Averill, L.A.; Sepeda, N.D.; Barsuglia, J.P.; Amoroso, T. Psychedelic Treatment for Trauma-Related Psychological and Cognitive Impairment Among US Special Operations Forces Veterans. Chronic Stress 2020, 4, 2470547020939564. [Google Scholar] [CrossRef]
- Ragnhildstveit, A.; Khan, R.; Seli, P.; Bass, L.C.; August, R.J.; Kaiyo, M.; Barr, N.; Jackson, L.K.; Gaffrey, M.S.; Barsuglia, J.P.; et al. 5-MeO-DMT for post-traumatic stress disorder: A real-world longitudinal case study. Front. Psychiatry. 2023, 14, 1271152. [Google Scholar] [CrossRef]
- Zeifman, R.J.; Palhano-Fontes, F.; Hallak, J.; Arcoverde, E.; Maia-Oliveira, J.P.; Araujo, D.B. The Impact of Ayahuasca on Suicidality: Results From a Randomized Controlled Trial. Front. Pharmacol. 2019, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Singhal, N.; Dos Santos, R.G.; Sanches, R.F.; de Lima Osório, F.; Hallak, J.E.C.; Weissman, C.R. Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: Results from an open-label trial. Psychopharmacology 2021, 238, 453–459. [Google Scholar] [CrossRef]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar] [CrossRef]
- Céline, B.; François, B.; Stanislas, S. Physical and psychosocial factors associated with psychostimulant use in a nationally representative sample of French adolescents: Specificities of cocaine, amphetamine, and ecstasy use. Addict. Behav. 2019, 92, 208–224. [Google Scholar] [CrossRef]
- Kim, J.; Fan, B.; Liu, X.; Kerner, N.; Wu, P. Ecstasy use and suicidal behavior among adolescents: Findings from a national survey. Suicide Life Threat. Behav. 2011, 41, 435–444. [Google Scholar] [CrossRef]
- Han, B.; Blanco, C.; Einstein, E.B.; Compton, W.M. Mental health conditions and receipt of mental health care by illicit lysergic acid diethylamide (LSD) use status among young adults in the United States. Addiction 2022, 117, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Yockey, R.A.; King, K.A.; Vidourek, R.A. “Go ask Alice, when she’s 10-feet tall”: Psychosocial correlates to lifetime LSD use among a national sample of US adults. J. Psychedelic Stud. 2019, 3, 308–314. [Google Scholar] [CrossRef]
- Wong, S.S.; Zhou, B.; Goebert, D.; Hishinuma, E.S. The risk of adolescent suicide across patterns of drug use: A nationally representative study of high school students in the United States from 1999 to 2009. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.S.; Thorne, C.B.; Clark, C.B.; Coombs, D.W.; Johnson, M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 2015, 29, 280–288. [Google Scholar] [CrossRef]
- Argento, E.; Strathdee, S.A.; Tupper, K.; Braschel, M.; Wood, E.; Shannon, K. Does psychedelic drug use reduce risk of suicidality? Evidence from a longitudinal community-based cohort of marginalised women in a Canadian setting. BMJ Open 2017, 7, e016025. [Google Scholar] [CrossRef]
- Argento, E.; Braschel, M.; Walsh, Z.; Socias, M.E.; Shannon, K. The moderating effect of psychedelics on the prospective relationship between prescription opioid use and suicide risk among marginalized women. J. Psychopharmacol. 2018, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.D.; Crawford, M.S.; Sweat, N.W.; Varley, A.; Green, E.E.; Hendricks, P.S. Prevalence and epidemiological associates of novel psychedelic use in the United States adult population. J. Psychopharmacol. 2019, 33, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.D.; Nichols, C.D.; Hendricks, P.S. Population Survey Data Informing the Therapeutic Potential of Classic and Novel Phenethylamine, Tryptamine, and Lysergamide Psychedelics. Front. Psychiatry 2020, 10, 896. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Wagner, A.C.; Watts, R.; Kettner, H.; Mertens, L.J.; Carhart-Harris, R.L. Post-Psychedelic Reductions in Experiential Avoidance Are Associated With Decreases in Depression Severity and Suicidal Ideation. Front. Psychiatry 2020, 11, 782. [Google Scholar] [CrossRef]
- Desai, S.; Jain, V.; Xavier, S.; Du, W. Hopelessness, Suicidality, and Co-Occurring Substance Use among Adolescent Hallucinogen Users-A National Survey Study. Children 2022, 9, 1906. [Google Scholar] [CrossRef]
- Jones, G.; Arias, D.; Nock, M. Associations between MDMA/ecstasy, classic psychedelics, and suicidal thoughts and behaviors in a sample of U.S. adolescents. Sci. Rep. 2022, 12, 21927. [Google Scholar] [CrossRef]
- Yang, K.H.; Han, B.H.; Palamar, J.J. Past-year hallucinogen use in relation to psychological distress, depression, and suicidality among US adults. Addict. Behav. 2022, 132, 107343. [Google Scholar] [CrossRef] [PubMed]
- Giugovaz, A.; Grassi, M.; Marchetti, I. Substance addictions and suicidal thoughts and behaviors: Evidence from a multi-wave epidemiological study. Psychiatry Res. 2024, 334, 115821. [Google Scholar] [CrossRef] [PubMed]
- Soboka, M.; Stewart, S.H.; Tibbo, P.; Wang, J. Substance use and risk of suicide among adults who sought mental health and addiction specialty services through a centralised intake process in Nova Scotia: A cross-sectional study. BMJ Open 2024, 14, e086487. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.E.; Barron, D.A.; Sudol, K.; Zisook, S.; Oquendo, M.A. Suicidal behavior across a broad range of psychiatric disorders. Mol. Psychiatry 2023, 28, 2764–2810. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Polychroniou, P.E.; Rakofsky, J.J.; Nemeroff, C.B.; Craighead, W.E.; Mayberg, H.S. Suicidal ideation and other persisting symptoms after CBT or antidepressant medication treatment for major depressive disorder. Psychol. Med. 2019, 49, 1869–1878. [Google Scholar] [CrossRef]
- Hochschild, A.; Grunebaum, M.F.; Mann, J.J. The rapid anti-suicidal ideation effect of ketamine: A systematic review. Prev. Med. 2021, 152, 106524. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.H.; Fink, M.; Knapp, R.; Petrides, G.; Husain, M.; Rummans, T.; Mueller, M.; Bernstein, H.; Rasmussen, K.; O’Connor, K.; et al. Relief of expressed suicidal intent by ECT: A consortium for research in ECT study. Am. J. Psychiatry 2005, 162, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; Savarino, J.; Operskalski, B.; Wang, P.S. Suicide risk during antidepressant treatment. Am. J. Psychiatry 2006, 163, 41–47. [Google Scholar] [CrossRef]
- De Vos, C.M.; Mason, N.L.; Kuypers, K.P. Psychedelics and neuroplasticity: A systematic review unraveling the biological underpinnings of psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.D.; Arango, V. Evidence for neurodegeneration and neuroplasticity as part of the neurobiology of suicide. Biol. Psychiatry 2011, 70, 306–307. [Google Scholar] [CrossRef]
- Lam, R.W.; Kennedy, S.H.; Adams, C.; Bahji, A.; Beaulieu, S.; Bhat, V.; Blier, P.; Blumberger, D.M.; Brietzke, E.; Chakrabarty, T.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023: Mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can. J. Psychiatry 2024, 69, 07067437241245384. [Google Scholar]
- Büscher, R.; Torok, M.; Terhorst, Y.; Sander, L. Internet-based cognitive behavioral therapy to reduce suicidal ideation: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e203933. [Google Scholar] [CrossRef]
- Schimmers, N.; Breeksema, J.J.; Smith-Apeldoorn, S.Y.; Veraart, J.; van den Brink, W.; Schoevers, R.A. Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: A systematic review. Psychopharmacology 2022, 239, 15–33. [Google Scholar] [CrossRef]
- Fauvel, B.; Strika-Bruneau, L.; Piolino, P. Changes in self-rumination and self-compassion mediate the effect of psychedelic experiences on decreases in depression, anxiety, and stress. Psychol. Conscious. Theory Res. Pract. 2023, 10, 88. [Google Scholar] [CrossRef]
- Smigielski, L.; Scheidegger, M.; Kometer, M.; Vollenweider, F.X. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. NeuroImage 2019, 196, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, R.J.; Wagner, A.C.; Monson, C.M.; Carhart-Harris, R.L. How does psilocybin therapy work? An exploration of experiential avoidance as a putative mechanism of change. J. Affect. Disord. 2023, 334, 100–112. [Google Scholar] [CrossRef]
- Lindegaard, T. Do psychedelics facilitate emergence of unconscious psychological processes? Psychodyn. Psychiatry 2023, 51, 270–286. [Google Scholar] [CrossRef]
- Szigeti, B.; Heifets, B. Expectancy effects in psychedelic trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Stackman, R.W., Jr. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).