Abstract
Background/Objectives: With technological development, ionizing radiation has found applications in numerous occupations. However, the determination and quantification of the damage resulting from exposure to it remains rather unclear, along with the damage to particular organs. The aim of this systematic review was to investigate the relationship between low-dose ionizing radiation (LDIR) in exposed workers and possible functional changes and cancer development in the thyroid gland. Methods: We included observational studies evidencing the correlation under study. Data extraction and analysis was conducted on all included studies. The research strategy included three electronic databases (PubMed, Scopus, and Web of Science). The systematic review followed PRISMA guidelines, and the research protocol was submitted to PROSPERO (CRD:42023425839). Results: The search initially yielded 166 articles and, once duplicates and irrelevant articles were removed, a total of 15 useful articles were reviewed. Qualitative analysis of the studies showed that the TSH value does not change following exposure, while a reduction in fT3 and an increase or reduction in fT4 can be observed. Furthermore, the correlation between thyroid cancer and occupational exposure to radiation was not shown with certainty, but there was some evidence of increased gland volume and nodule formation. Conclusions: Even at low doses, ionizing radiation adversely affects thyroid activity. In this regard, new studies should be carried out in order to further investigate and define this issue and, consequently, outline useful measures to ensure the protection of workers in contact with this particular physical agent.
1. Introduction
In the modern world, ionizing radiation (IR) plays an increasingly prominent role. Humans are constantly exposed to these rays through the environment, their occupation, medical use, or other sources [1]. Sensitivity to radiation varies from organ to organ. The bone marrow and thyroid are those most susceptible to radiation-induced transformation, which is why certain forms of leukemia and thyroid cancer are the neoplasms that occur more frequently and earlier in people exposed to IR [2]. Although the effects of high doses of ionizing radiation (HDIRs) are well known, the effects of low doses of ionizing radiation (LDIRs) on these organs, in particular on the thyroid, still remain ambiguous [3]. In order to unravel these contrasts, a number of studies and reports have been conducted on different populations, starting with the pediatric population [4,5]. In order to provide a large-scale international assessment related to mortality risks from prolonged exposures to low-dose and low-dose-rate ionizing radiation, the International Nuclear Workers Study (INWORKS) was undertaken [6]. The last update of this study shows an increase in the excess relative rate of solid cancer mortality with increasing cumulative exposure to ionizing radiation at the low dose rates typically encountered by French, UK, and US nuclear workers [7]. In light of this, it is clear that people professionally exposed to radiation must be reliably protected continuously from IR: for example, no solid evidence has been provided for an increase in thyroid cancer related to exposure to 131I in cohorts of patients with exposure to it therapeutically and diagnostically [8]. For the protection of workers from exposure to artificial and natural IR, in Italy, the Legislative Decree (D.Lgs.) 101/2020 is in effect, which amended Article 180 paragraph 3 of D.Lgs. 81/08. The decree divides exposed workers into two categories: A (a dose greater than 6 mSv per year up to 20 mSv) and B (workers who may receive a dose of between 1 mSv and 6 mSv per year). Two figures are identified within the decree, namely, the qualified expert, with the role of implementing the principles of radiation protection and physical surveillance, and the occupational physician, responsible for medical surveillance, with the task of taking any post-exposure measures [9]. Since the thyroid is an endocrine organ and highly sensitive to radiation, it is important to assess whether LDIR can cause alterations in its function. The aim of this systematic review, in fact, is to investigate the relationship between exposure to LDIR and possible functional changes and cancer development in the thyroid gland in exposed workers.
2. Materials and Methods
A systematic review was conducted to investigate the effects of LDIR exposure on thyroid functionality. This review was recorded in PROSPERO (the International Prospective Register Of Systematic Reviews), and the registration number is CRD42023425839. This study was conducted according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [10].
2.1. Search Strategy
The identification of studies relevant to this review was achieved by searching electronic databases of the published literature, including PubMed, Scopus, and Web of Science. The keywords used in the electronic databases were “thyroid AND low AND dose AND ionizing AND radiation AND (function OR cancer)”. The search was undertaken with no language or date of publication restrictions. The article search and data extraction were carried out during the period between 15 April 2023 and 31 May 2023.
2.2. Study Selection
The review process was carried out using a multi-stage approach. Five authors independently conducted duplicate screening and removal and, as computer software, used ZOTERO. Then, after title and abstract screening, full-text articles were assessed to determine whether they met the inclusion criteria. If an included publication was not available as the full text in English, the corresponding author was contacted to verify whether the eligibility criteria were met.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) studies involving workers exposed to LDIR; (2) the focus of the research was the effect of LDIR on thyroid function and cancer development. The exclusion criteria were as follows: (1) irrelevance to the research topic; (2) studies involving workers not exposed to LDIR; and (3) articles studying the effect of LDIR on other organs.
2.4. Data Extraction
Data extraction was conducted independently by five reviewers, extracting data from all included studies. In order to confirm the relevance of the studies and, consequently, extract their characteristics, the authors developed a data collection form in which the following information was included: name of the first author, title, country, year of publication, study design (cohort, case–control, cross-sectional, RCT), type of workplace, sample size (total), sample size (with problem), type of exposure (unit of measurement used, exposure intervals…), type of thyroid problem (cancer, nodules, hormone variations), main results, and assessment of the quality of the study. In cohort studies, the measure of association considered was the relative risk (RR), while in case–control studies, it was the odds ratio (OR). To ensure accurate data collection, the data extracted were compared independently by each reviewer. Discrepancies and disagreements were discussed and resolved through a consensus session with a third-party researcher.
2.5. Quality Assessment
A quality assessment of the observational studies was carried out using the Newcastle–Ottawa Scale (NOS). This is a validated, easy-to-use scale of 8 items in three domains, selection, comparability, and exposure/outcome, for case–control or cohort studies. Each item can be given one point, except comparability which has the potential to score up to two points. Studies are rated from 0 to 9, with studies rated 0–3 being poor-quality, 4–6 being fair-quality, and 7–9 being good/high-quality.
A Spearman correlation coefficient was calculated considering the association between quality assessment scores and the year of publication and sample size of the studies. Finally, a weighted (by sample size) regression analysis was carried out using the quality as the dependent variable and the year of publication as the independent variable. The result is described using the β coefficient (p).
3. Results
3.1. Search Results Summary
Research began in April 2023. The initial search across different electronic databases yielded 166 citations. First, a total of 44 duplicate papers were excluded, accompanied by the removal of 103 publications in the title/abstract screening. Among the nineteen full-text articles screened, three were not included. Finally, among the sixteen articles selected and evaluated for eligibility, three reports, upon further reading of the text, were excluded for the following reasons: two were narrative reviews, and the third had no useful correlation with the aim of our study. Through a search of literary citations, a further two useful articles were identified for review. At the end of the process, 15 studies remained for qualitative analysis (Figure 1).
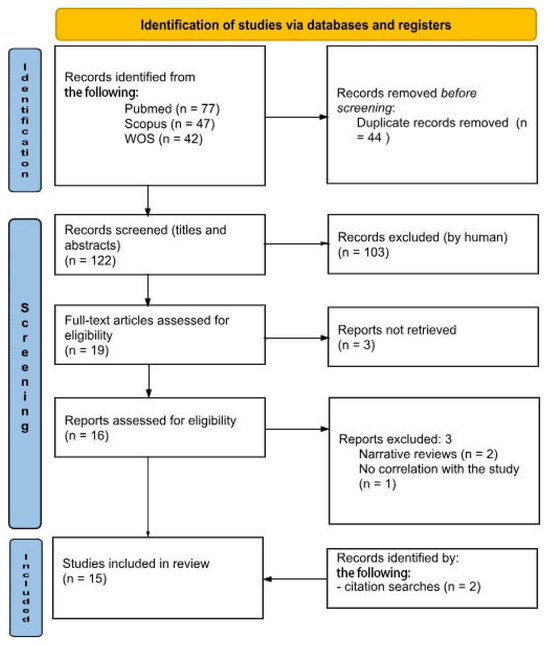
Figure 1.
PRISMA 2020 flow diagram.
3.2. Characteristics of Included Studies
Fifteen studies were selected for our systematic review (Table 1). Their publication dates ranged from 1997 to 2022. In terms of study design, six were cross-sectional, and nine were cohort studies, with 1,040,763 people included in our review. The total worker population in the cohort studies was 1,038,545 people, while in case–control studies, 2218 patients were considered. In particular, for case–control studies, hospital controls were used in the studies of Vázquez Rivas [11], El-Benhawy [12], and Cioffi [13], and community controls were used in the studies of Adibi [14], Gudzenko [15]. The studies were conducted mainly in Europe with seven studies, then four in Asia, three in the USA, and one in Africa. The workplaces considered by the studies were as follows: hospitals and healthcare with ten studies, nuclear power plants with six studies, universities and academia with two studies, industrial services with three studies, the war industry and barracks with three studies, and public services (which included drivers, workers in airports, stations, etc.) with one study. The thyroid disorders considered consisted of thyroid cancer, thyroid nodules, and changes in thyroid hormones (TSH, fT3, fT4). While in five studies [11,13,15,16,17,18,19] workers were divided into groups according to the exposure intervals they were subjected to, for the remaining studies, we have no clear data.

Table 1.
Characteristics of included studies.
3.3. Quality Assessment
The quality of each study was evaluated independently by two reviewers using the NOS scale: for case–control studies, the lowest rating was 7, the highest 8; for cohort studies, the lowest rating was 5, the highest 7. The average rating for all studies was 6.73.
The case–control studies (six) had a mean value of 7.17 (SD = 0.75), while the cohort studies (nine) had a mean value of 6.44 (SD = 0.73) (p = 0.210). Details of the ratings using the NOS scale can be found in Supplementary Materials S1. Interestingly, the year of publication is inversely correlated with the methodological quality of the studies, even if this is not statistically significant (r = −0.197; p = 0.481) (Figure 2). However, this correlation has a positive trend when the analysis is weighted by the sample size (β = 0.055; p < 0.001).
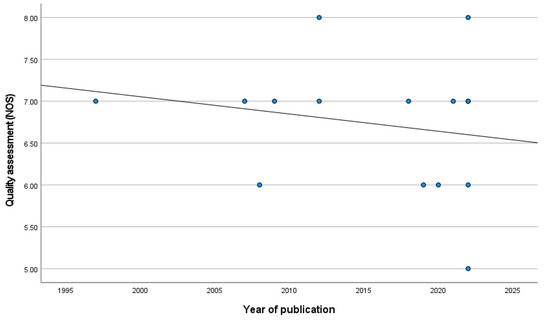
Figure 2.
Correlation between year of publication and methodological quality of the studies.
A positive correlation was found between the sample size and quality assessment (r = 0.111; p = 0.705) (Figure 3).
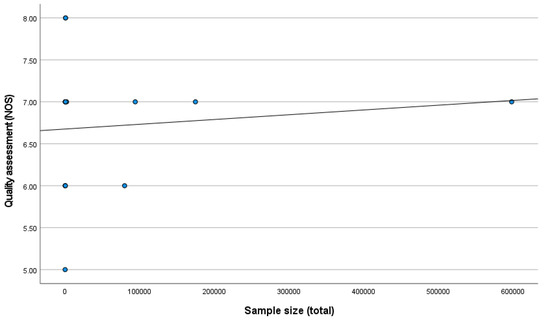
Figure 3.
Correlation between sample size and methodological quality of the studies.
3.4. Thyroid Cancer
Seven studies [15,16,18,19,20,23,24] evaluated the possible association between LDIR and thyroid cancer. In the first study, thyroid cancer was statistically significant in women working with radiation in medical and research institutions and in industry and in all nuclear energy workers, and there was a significant association with dose (ERR = 20.4 for Sv, 90% CI = 8–60, one-tailed p = 0.049). In the second study of nine healthcare workers with papillary thyroid carcinoma (PTC) exposed to LDIR, BRAF V600 genetic alterations were revealed in four cases, including one with RET/PTC1 fusion, and in one case, point mutations and fusions were found. In the third study, the risk of thyroid cancer was found to be increased for men and women compared with the general population: in particular, there appears to be an inversely proportional correlation according to cumulative badge dose categories (half of all cases have thyroid cancer for doses <1 mSv). In the fourth study, thyroid cancer risk was not associated with the cumulative, occupational IR dose; restricting to papillary thyroid cancer yielded nearly identical results. In the last study, a non-statistically significant SMR was found for thyroid cancer; however, there is weak evidence of thyroid cancer in two cases with a cumulative dose above 400 mSv. In the last two studies, a significant dose–response relationship was observed between the radiation dose to the thyroid among the highest dose categories, but there was no evidence about LDIR and thyroid cancer.
3.5. Thyroid Nodules
Three studies [12,18,19] evaluate the association between thyroid nodules and LDIR exposure. In the first study, a significant association (p = 0.005) was reported between the presence of thyroid nodules in the exposed group compared with the control group, and in the second study, the prevalence of thyroid nodules in cases and controls was 22.6% and 24.6%, respectively (p > 0.05). It is noteworthy that both studies involved employees of healthcare facilities. In the last study, on the other hand, conducted on workers at the Chernobyl nuclear power plant following the nuclear accident, the presence of nodules was not significantly associated with the documented radiation dose from external sources.
3.6. Thyroid Hormones
There are five studies that have evaluated the association between hormonal changes and LDIR exposure [11,12,13,21,22]. In the first study, a significant increase in fT3 compared to the controls and anti-TPO is shown in subjects exposed to 131I; also reported was an increase in the mean thyroid volume in the exposed group. In the second study, there was no significant difference in hormonal changes between the exposed and unexposed groups, although higher levels of TSH were found in the category B and A exposed groups compared with the TSH levels in the control group; on the other hand, a statistically significant difference was found in the five-year dose of the personal dosimeters between the medium/high- and low-risk categories, being 0.1 ± 0.3 in the low-exposure zone and 0.9 ± 1.4 in the medium/high-exposure zone. In the third study, exposed workers presented significantly higher levels of TSH and significantly lower levels of fT3 and fT4 than non-exposed workers. In the fourth study, the data showed no variation in TSH levels related to occupational exposure between T1 and T2, an increase in fT3 hormone values, and a decrease in fT4. In the latest study, the authors observed declines in fT3 and fT4 over the study period but not in TSH: they also observed an increase in the TSH level after the ninth year of follow-up.
Finally, the study by Cardis [25] does not consider a specific thyroid-related problem but reports the deaths caused by thyroid problems of workers who were exposed to LDIR: all workers who died of thyroid problems were exposed to doses below 50 mSv. The Standardized Mortality Ratio (SMR) appeared to be maximum for a cumulative dose of 10 mSv (RR at 100 mSv: 0,91 [0.12, 2.84]).
4. Discussion
The purpose of this systematic review was to investigate the effects of LDIR exposure on thyroid functionality and cancer development in exposed workers. The health effect of LDIR has always been a source of heated debate. Although unequivocal evidence of the carcinogenic power of IR has been achieved, so much so that the IARC [26] has for many years placed it in class 1 of “carcinogenic agents for humans”, the results of the epidemiological studies conducted to date reveal a more complex, and in some areas controversial, picture.
And our review reflects this trend. We first looked for articles evaluating the possible association between LDIR and thyroid cancer, with mixed results. Two studies, both conducted in Korea, report a significant association between LDIR and cancer, while the other studies report no significant association. The study conducted by Duque also reports the presence of genetic alterations in some patients with papillary thyroid carcinoma. One part of the literature reports, in general, that the primary ill-health caused by low to moderate doses of IR is cancer, although the possibility of non-cancer effects (particularly cardiovascular disease) is of increasing concern [27]. In contrast, as far as thyroid cancer is concerned, it is still necessary to establish whether LDIR causes damage to the organ, as it is particularly radiosensitive, which is why further research on this issue is needed.
Regarding the correlation between the presence of nodules and exposure to LDIR, the literature provides few significant data; in particular, only two studies show an association between exposure and LDIR in a healthcare setting. Again, there are numerous studies in the literature demonstrating an association between HDIR and thyroid nodules [28,29], but very little has been analyzed in relation to the association with LDIR.
Finally, regarding the association between LDIR and thyroid hormones, the current literature about the fT3 trend is very conflicting, as it increased in two studies and decreased in another two, and it is concordant in the fT4 trend, as it increased in three studies, and the TSH trend, as it increased in three studies and remained unchanged in one. A recent study conducted in China on a large sample of healthcare workers has clarified the relationship between LDIR and the thyroid gland; in particular, it was seen that long-term exposure to LDIR had an effect on thyroid abnormalities in medical radiation workers. Among them, females, physicians, and those working in the department of diagnostic radiology were at a higher risk of abnormal thyroid function; being female, having increased years of radiation work, and radiation exposure onset at age ≥ 30 years were associated with a higher risk of reporting abnormal thyroid morphology [30].
Ours is the first review analyzing the association between LDIR exposure and thyroid function. Among the strengths, we mention the large sample size and cohort design of most of the studies, some of which were based on data obtained from official national registers. Furthermore, numerous aspects relating to the thyroid (cancer, nodules, hormones) were taken into consideration in order to have the most complete view possible regarding the correlation with LDIR. Another strength of our study is the quality of the studies considered, which is moderate. However, there are limitations. First of all, there are no studies from Oceania and South America, as they are not present in the literature. The settings analyzed by the studies are very different and vary from the controlled and standardized hospital environment to nuclear power plants and industries in which the ways of collecting worker data could be different. Moreover, there were no data regarding previously treated thyroid diseases. Finally, all included studies were cross-sectional or cohort studies, so although it is possible to find associations, it is not possible to establish what the underlying pathophysiological mechanisms are.
5. Conclusions
Studies present different results regarding the correlation between exposure to LDIR and thyroid dysfunction. From our review, it emerges that in the literature there are few studies relating to the correlation between LDIR and thyroid function, and these studies present data that are sometimes conflicting with each other. It is clear, however, that even at low doses, it negatively affects the activities of the thyroid. In this regard, it is necessary to collect more documentation, carry out new studies in order to delve deeper and define this topic, and, consequently, outline useful measures with the aim of ensuring the protection of workers in contact with this particular physical agent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020588/s1.
Author Contributions
Conceptualization, G.L.T. and C.C.; methodology, G.L.T. and C.C.; software, G.L.T.; validation, G.L.T. and C.C.; formal analysis, G.L.T. and C.C.; investigation, C.C., F.L., I.R., G.M. and S.A.; data curation, C.C.; writing—original draft preparation, C.C., F.L., I.R., G.M. and S.A.; writing—review and editing, C.C. and F.L.; visualization, C.C.; supervision, G.L.T.; project administration, G.L.T. and C.C.; funding acquisition, none for this project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, Y.; Rohde, L.H.; Emami, K.; Hammond, D.; Casey, R.; Mehta, S.K.; Jeevarajan, A.S.; Pierson, D.L.; Wu, H. Suppressed expression of non-DSB repair genes inhibits gammaradiation-induced cytogenetic repair and cell cycle arrest. DNA Repair 2008, 7, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- AIRC-Radiazioni Ionizzanti e Cancro. Available online: https://www.airc.it/cancro/informazioni-tumori/cose-il-cancro/radiazioni-ionizzanti-cancro#:~:text=Le%20radiazioni%20ionizzanti%20possono%20danneggiare,allo%20sviluppo%20di%20un%20tumore.&text=Le%20radiazioni%20ionizzanti%20sono%20un,per%20l'insorgenza%20del%20cancro (accessed on 13 July 2024).
- Feinendegen, L.E.; Brooks, A.L.; Morgan, W.F. Biological consequences and health risks of low-level exposure to ionizing radiation: Commentary on the workshop. Health Phys. 2011, 100, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.D. Excerpts of UNSCEAR white paper on “evaluation of data on thyroid cancer in regions affected by the Chernobyl accident”. Radiat. Prot. Environ. 2018, 41, 160. [Google Scholar] [CrossRef]
- Lubin, J.H.; Adams, M.J.; Shore, R.; Holmberg, E.; Schneider, A.B.; Hawkins, M.M.; Robison, L.L.; Inskip, P.D.; Lundell, M.; Johansson, R.; et al. Thyroid Cancer Following Childhood Low-Dose Radiation Exposure: A Pooled Analysis of Nine Cohorts. J. Clin. Endocrinol. Metab. 2017, 102, 2575–2583. [Google Scholar] [CrossRef]
- Hamra, G.B.; Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Haylock, R.; Laurier, D.; Leuraud, K.; Moissonnier, M.; et al. Cohort Profile: The International Nuclear Workers Study (INWORKS). Int. J. Epidemiol. 2016, 45, 693–699. [Google Scholar] [CrossRef]
- Richardson, D.B.; Leuraud, K.; Laurier, D.; Gillies, M.; Haylock, R.; Kelly-Reif, K.; Bertke, S.; Daniels, R.D.; Thierry-Chef, I.; Moissonnier, M.; et al. Cancer mortality after low dose exposure to ionising radiation in workers in France, the United Kingdom, and the United States (INWORKS): Cohort study. Br. Med. J. 2023, 382, e074520. [Google Scholar] [CrossRef]
- National Research Council. Committee on the Biological Effects of Ionizing Radiation. In Health Risks from Exposure to Low Levels of Ionizing Radiation. BEIR: VII Report, Phase 2; The National Academic Press: Washington, DC, USA, 2006. [Google Scholar]
- Dipartimento Della Protezione Civile, Presidenza del Consiglio dei Ministri-Decreto Legislativo n.101 del 31 Luglio 2020. Available online: https://www.protezionecivile.gov.it/it/normativa/decreto-legislativo-n101-del-31-luglio-2020-0/ (accessed on 13 July 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar]
- Vázquez Rivas, F.; Mahillo, I.; Valverde, J.; Garayoa, J.; Teresa Del Campo, M. Radiaciones ionizantes en trabajadores sanitarios: Función tiroidea y niveles de riesgo de exposición laboral [Ionizing radiation in healthcare workers: Thyroid function and risk levels of occupational exposure]. Rev. Asoc. Esp. Espec. Med. Trab. 2022, 31, 29–40. [Google Scholar]
- El-Benhawy, S.A.; Fahmy, E.I.; Mahdy, S.M.; Khedr, G.H.; Sarhan, A.S.; Nafady, M.H.; Yousef Selim, Y.A.; Salem, T.M.; Abu-Samra, N.; El Khadry, H.A. Assessment of thyroid gland hormones and ultrasonographic abnormalities in medical staff occupationally exposed to ionizing radiation. BMC Endocr. Disord. 2022, 22, 287. [Google Scholar] [CrossRef]
- Cioffi, D.L.; Fontana, L.; Leso, V.; Dolce, P.; Vitale, R.; Vetrani, I.; Galdi, A.; Iavicoli, I. Low dose ionizing radiation exposure and risk of thyroid functional alterations in healthcare workers. Eur. J. Radiol. 2022, 132, 109279. [Google Scholar] [CrossRef]
- Adibi, A.; Rezazade, A.; Hovsepian, S.; Koohi, R.; Hosseini, M. The relationship between occupational radiation exposure and thyroid nodules. J. Res. Med. Sci. 2012, 17, 434–438. [Google Scholar] [PubMed]
- Gudzenko, N.; Mabuchi, K.; Brenner, A.V.; Little, M.P.; Hatch, M.; Drozdovitch, V.; Vij, V.; Chumak, V.; Bakhanova, E.; Trotsyuk, N.; et al. Risk of thyroid cancer in Ukrainian cleanup workers following the Chornobyl accident. Eur. J. Epidemiol. 2022, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.S.; Park, R.M.; Koh, D.H. Cancer admission and mortality in workers exposed to ionizing radiation in Korea. J. Occup. Environ. Med. 2008, 50, 791–803. [Google Scholar] [CrossRef]
- Inskip, P.D.; Hartshorne, M.F.; Tekkel, M.; Rahu, M.; Veidebaum, T.; Auvinen, A.; Crooks, L.A.; Littlefield, L.G.; McFee, A.F.; Salomaa, S.; et al. Thyroid nodularity and cancer among Chernobyl cleanup workers from Estonia. Radiat. Res. 1997, 147, 225–235. [Google Scholar] [CrossRef]
- Muirhead, C.R.; O’Hagan, J.A.; Haylock, R.G.E.; Phillipson, M.A.; Willcock, T.; Berridge, G.L.C.; Zhang, W. Mortality and cancer incidence following occupational radiation exposure: Third analysis of the National Registry for Radiation Workers. Br. J. Cancer 2009, 100, 206–212. [Google Scholar] [CrossRef]
- Kesminiene, A.; Evrard, A.S.; Ivanov, V.K.; Malakhova, I.V.; Kurtinaitise, J.; Stengrevics, A.; Tekkel, M.; Chekin, S.; Drozdovitch, V.; Gavrilin, Y.; et al. Risk of thyroid cancer among chernobyl liquidators. Radiat. Res. 2012, 178, 425–436. [Google Scholar] [CrossRef]
- Duque, C.S.; Vélez, A.; Cuartas, J.; Jaimes, F.; Dueñas, J.P.; Agudelo, M.; Nikiforova, M.N.; Nikiforov, Y.E.; Condello, V. Molecular profiling of papillary thyroid carcinomas in healthcare workers exposed to low dose radiation at the workplace. Endocrine 2022, 76, 95–100. [Google Scholar] [CrossRef]
- Sernia, S.; Bongiovanni, A.; De Giorgi, A.; De Sio, S.; Cafolla, A.; La Torre, G. Thyroid parameters variations in healthcare workers and students exposed to low-dose ionizing radiations. G. Ital. Med. Lav. Ergon. 2022, 44, 338–346. [Google Scholar]
- Wong, Y.S.; Cheng, Y.Y.; Cheng, T.J.; Huang, C.C.; Yeh, J.J.; Guo, H.R. The Relationship Between Occupational Exposure to Low-dose Ionizing Radiation and Changes in Thyroid Hormones in Hospital Workers. Epidemiology 2019, 30, S32–S38. [Google Scholar] [CrossRef]
- Lee, W.J.; Ko, S.; Bang, Y.J.; Choe, S.A.; Choi, Y.; Preston, D.L. Occupational radiation exposure and cancer incidence in a cohort of diagnostic medical radiation workers in South Korea. Occup. Environ. Med. 2021, 78, 876–883. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Preston, D.L.; Neta, G.; Little, M.P.; Doody, M.M.; Simon, S.L.; Sigurdson, A.J.; Alexander, B.H.; Linet, M.S. Occupational radiation exposure and thyroid cancer incidence in a cohort of U.S. radiologic technologists, 1983–2013. Int. J. Cancer 2018, 143, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: Estimates of radiation-related cancer risks. Radiat. Res. 2007, 167, 396–416. [Google Scholar] [CrossRef] [PubMed]
- Modan, B. Low-dose radiation carcinogenesis. Eur. J. Cancer 1992, 28 Pt A, 1010–1012. [Google Scholar] [CrossRef]
- McLean, A.R.; Adlen, E.K.; Cardis, E.; Elliott, A.; Goodhead, D.T.; Harms-Ringdahl, M.; Hendry, J.H.; Hoskin, P.; Jeggo, P.A.; Mackay, D.J.C.; et al. A restatement of the natural science evidence base concerning the health effects of low-level ionizing radiation. Proc. Biol. Sci. 2017, 284, 20171070. [Google Scholar] [CrossRef] [PubMed]
- Krátký, J.; Vítková, H.; Bartáková, J.; Telička, Z.; Antošová, M.; Límanová, Z.; Jiskra, J. Thyroid nodules: Pathophysiological insight on oncogenesis and novel diagnostic techniques. Physiol. Res. 2014, 63 (Suppl. S2), S263–S275. [Google Scholar] [CrossRef]
- Rabinovich, E.I.; Povolotskaya, S.V.; Degteva, M.O.; Tolstykh, E.I. Relation between the thyroid diseases prevalence and doses of radiation exposure in the individuals relocated from radioactively contaminated areas in South Urals. Radiatsionnaya Gygiena 2022, 15, 36–46. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Guo, W.; Li, M.; Mai, W.; Zhang, L.; Jia, Y.; Yang, Y.; Chen, H.; Huang, W. Thyroid abnormalities and influencing factors in medical radiology workers in Guangdong Province. J. Environ. Occup. Med. 2023, 40, 323–330. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).