Abstract
Background: Rigid bronchoscopy (RB) is the gold standard for managing central airway obstruction (CAO), a life-threatening condition caused by both malignant and benign etiologies. Anesthetic management is challenging as it requires balancing deep sedation with maintaining spontaneous breathing to avoid airway collapse. There is no consensus on the optimal anesthetic approach, with options including general anesthesia with neuromuscular blockers or spontaneous assisted ventilation (SAV). Methods: This case series presents our anesthetic protocol using remifentanil–propofol–ketamine total intravenous anesthesia (TIVA) with SAV in four patients with airway obstructions. Muscle relaxants were avoided in all cases. Results: Ketamine’s ability to preserve respiratory drive and airway reflexes, along with its bronchodilating properties, made it ideal for managing CAO. All procedures successfully restored airway patency without complications or drug-related side effects. Conclusions: Our findings suggest that remifentanil–propofol–ketamine TIVA combined with SAV is a viable anesthetic approach for therapeutic RB, offering effective sedation, maintaining airway patency, and minimizing perioperative complications.
1. Introduction
Rigid bronchoscopy (RB) is the gold standard for the management of central airway obstruction (CAO), a potentially life-threatening condition [1]. Malignant causes are more common, largely due to the increasing incidence of primary lung cancer [2]. Non-malignant CAO is most frequently caused by iatrogenic injury and connective tissue disorders [2]. Interventional techniques for CAO, such as laser ablation, argon plasma coagulation (APC), and airway stenting, are crucial in maintaining airway patency [3].
RB, despite its effectiveness, is a high-risk procedure, posing challenges for anesthetic and airway management [4]. There is no consensus on the optimal anesthetic approach for rigid bronchoscopy in adults with CAO, with options including general anesthesia with neuromuscular blockers (NMBs) or spontaneous assisted ventilation (SAV) [5]. The use of muscle relaxants results in loss of airway patency due to central airway collapse, complicating ventilation due to limited airway access for the anesthesiologist [4,6]. In contrast, SAV without NMB agents may reduce the risk of severe hypoxemia but requires continuous adjustments of anesthesia depth to ensure patient safety and comfort during therapeutic RB [1,5,7].
Remifentanil and propofol remain the mainstay drugs for TIVA due to their potent and short-acting properties [1]. Nevertheless, the combination of an opioid with propofol is associated with several side effects, such as respiratory depression, postoperative nausea and vomiting, propofol-induced hypotension, and opioid-induced bradycardia. On the other hand, there is growing interest regarding the use for ketamine for endobronchial procedures [8]. Ketamine is occasionally used as an adjunct to propofol in TIVA regimens due to its unique pharmacological properties [9]. It induces a dissociative state with analgesic, sedative, and amnesic effects, while maintaining respiratory drive and airway reflexes thus offering the advantage of avoiding respiratory or cardiovascular depression [7,10].
Although ketamine is commonly employed in airway surgery, evidence on bolus infusions of ketamine and propofol as adjuncts to remifentanil-based anesthesia for RB in adults remains limited.
We present our anesthetic protocol based on remifentanil–propofol–ketamine TIVA and SAV for RB-guided tracheobronchial stenting in four patients with CAO at a single institution.
2. Materials and Methods
We performed rigid bronchoscopic intervention in four patients with CAO. Three of them were affected by lung cancer and one patient suffered from benign post intubation tracheal stenosis. We describe our procedural and anesthesia-related outcomes. Patient characteristics and procedural details are listed in Table 1 and Table 2.

Table 1.
Demographics and clinical characteristics.

Table 2.
Procedural details, complications, and outcomes.
Written informed patient consent for the publication of this case series was obtained from all patients, and the manuscript adheres to Enhancing the Quality and Transparency of Research (EQUATOR) guidelines.
Topical anesthetics reduced the need for sedatives in RB, including 0.5% ropivacaine (25 mg), dexamethasone (4 mg), and 2% nebulized lidocaine 2% + 10 mcg dexmedetomidine (DXM) via an intranasal atomizer [11]. All patients also received 20 mg IV dexamethasone and 0.5 mg atropine to prevent edema and reduce secretions. Thereafter, analgosedation with midazolam 20–40 mcg/kg and fentanyl 1 mg/kg was administered as premedication to alleviate patient discomfort.
Bronchoscopy was performed in the emergency operating room. The operative report and anesthetic record from each procedure was reviewed and details of the procedure, anesthetic approach, intraoperative hemodynamic and respiratory parameters, and postoperative outcomes were collected. All patients were monitored with electrocardiograph (EKG), pulse oxygen saturation (SpO2) and Bispectral Index (BIS). Noninvasive blood pressure (NIBP) was measured in 5 min intervals during RB. The respiratory circuit of the anesthesia machine (Dräger Perseus® A500, Lübeck, Germany) remained continuously connected to the lateral ventilation port of the bronchoscope, allowing patients to breathe through it (Figure 1). Set to manual/spontaneous mode, the machine had the adjustable pressure-limiting valve fully open, fresh gas flow at maximum (15 L/min), and an O2 concentration of 50–80%. This setup enabled spontaneous breathing support, with options for manual or mechanical assistance, as well as continuous positive airway pressure (CPAP) if needed. High fresh gas flow and leaks from the open proximal end of the bronchoscope compromised accurate measurements of respiratory rate (RR) and end-tidal (ET) CO2, resulting in unreliable data; thus, these parameters were not reported

Figure 1.
Ventilation through the external side port of the rigid bronchoscope.
Case 1
The first patient was a 75-year-old male with squamous cell carcinoma (SCC) involving the right lung and causing complete obstruction of the right stem bronchus. After preoxygenation for 5 min with 100% oxygen, TIVA was started with remifentanil target-controlled infusion (TCI) 0.5–3 ng/mL and ketamine (0.75–1 ng/kg). For the latter, an initial dosage of 50 mg was administered, followed by additional doses of ketamine (20 mg) and propofol (TCI 0.5–1.5 ng/mL) every 20–40 min until the end of the procedure. After intubation with the rigid bronchoscope, spontaneous breathing was maintained throughout the procedure. The patient remained hemodynamically stable throughout the procedure. An episode of oxygen saturation below 90% occurred; ventilation was assisted manually and oxygen levels promptly restored. APC allowed debulking of the tumor to achieve the desired patency. Thereafter, successful placement of a NOVATECH® GSSTM (La Ciotat, France) bronchial silicon stent was performed to re-establish airway patency (Figure 2).
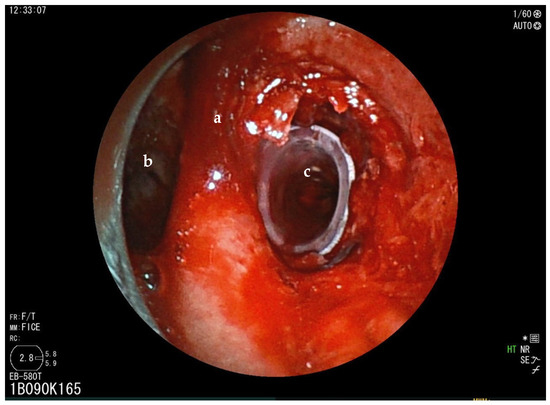
Figure 2.
Rigid bronchoscopy of case 1 (a = carina; b = left main bronchus). Bronchoscopic view immediately after dilation and then stenting with bronchial silicon stent. Lumen of right stem bronchus is now completely open (c).
The procedure concluded in 120 min and the patient was then transferred to the intensive care unit (ICU) for observation. The patient was discharged from the hospital after 5 days and he was able to maintain a satisfactory life without dyspnea for 3 months thereafter.
Case 2
Case 2 is a 24-year-old obese (BMI 34.89) female patient who developed a fibrotic mid-tracheal complex stenosis after urgent 4-day intubation due to a seizure episode. After a month of extubation, the patient exhibited severe stridor, dyspnea, and tirage. Anesthesia was provided using the identical drug and dose regimen as in case 1. Even incomplete, the mechanical dilation of the stenosis with the rigid bronchoscope was immediate (Figure 3). Given the complex stenosis, a NOVATECH® GSSTM BD standard tracheal silicone stent was placed through the rigid bronchoscope to achieve the desired patency and to prevent disease recurrence. HR (80–90 bpm) and BP (110/70 mmHg) were stable and arterial oxygen saturation (SaO2) was maintained around 88–95% during the procedure. The patient tolerated the procedure without complications and noted immediate improvement in her respiratory status. She was discharged from the hospital after 4 days and required no further airway procedures.
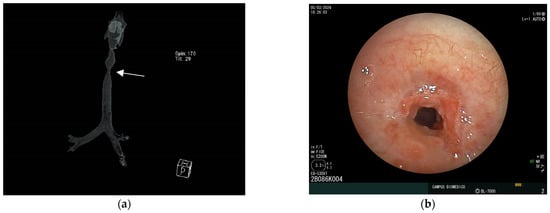
Figure 3.
Benign subglottic stenosis secondary to traumatic intubation treated by sequential rigid dilations. (a) Reconstruction of CT scan of trachea showing mid-tracheal stenosis (arrow pointing at narrowest caliber); (b) bronchoscopic images showing post intubation scar with stenosis of tracheal lumen.
Case 3
Case 3 is a 77-year-old male with advanced lung adenocarcinoma presented with dyspnea and hypoxia caused by complete obstruction of the right main bronchus. The anesthesia drug regimen included IV remifentanil and ketamine (identical dosage as in case 1). In this case, instead of propofol, a continuous infusion of DXM 0.7–1.4 μg/kg/h was administered to maintain intraoperative depth of anesthesia. RB started after the patients had no obvious physical activity. The rigid bronchoscope was advanced gently into the stenosis and APC was used as a method to desiccate and coagulate the tumor. Despite mechanical debulking of the endobronchial lesion, there was still 50% obstruction of the trachea. The decision was made to place a NOVATECH® GSSTM BD bronchial silicon stent to re-establish airway patency. Despite DXM infusion, the patient remained hemodynamically stable, probably thanks to the ketamine infusion. SaO2 was maintained at 92–100% during the procedure. The intervention was tolerated without further distress or complications. The patient’s main symptoms were relieved; however, he died two weeks later of disease progression.
Case 4
Case 4 is a 74-year-old female with lung SCC who presented with a two-week history of dyspnea, cough, solid food dysphagia, and weight loss. The patient had a subcarinal tracheal mass invading into the carina and lower portion of the trachea causing approximately 60% obstruction. Anesthesia was induced and maintained with the same regimen as case 1 with IV propofol, remifentanil, and ketamine. With the patient still breathing spontaneously, rigid dilation and tumor debulking with APC were performed. After dilation to desired patency, the bronchoscope was positioned at the estimated distal end of the stent. The scope was then retracted to the desired proximal section of the stent by grasping the end of the rigid barrel. A customized Merit Medical Systems AERO® (South Salt Lake, UT, USA) 40 mm metallic stent was placed in the trachea and main stem bronchi. The patient had good hemodynamics (HR: 70–80 bpm; BP: 110/75 mmHg) and oxygen saturation (89–100%) during the procedure. The patient’s respiratory condition strongly improved immediately after the procedure. However, she died at the hospital one month later of disease progression.
3. Discussion
RB is the gold standard for managing CAO, a potentially life-threatening condition that can arise from both malignant and benign causes [12]. Currently, airway stenting through RB typically requires deep sedation or even general anesthesia [12]. Neuromuscular blockade is not an absolute requirement and there are some debates whether the use of paralytic agents is safe and indispensable in this procedure [4]. The use of NMBs abolishes muscle tone, potentially leading to complete obstruction and loss of airway patency [1,6]. In this process, multiple ventilation strategies through an open circuit can be considered including apneic oxygenation, SAV, controlled ventilation, and jet ventilation [4,6]. SAV is a ventilation technique in which sedation levels are closely titrated during the procedure to maintain spontaneous breathing by the patient [6]. Specifically, SAV may have a lower rate of post-procedural reintubation due to the avoidance of NMB agents [6].
As described in patient 1, in the case of oxygen desaturation, assisted or controlled ventilation can be achieved by removing instruments from the bronchoscope, attaching the ventilator circuit adapter to its distal end, and using the rigid scope as an endotracheal tube [2]. This system allows the delivery of positive pressure breaths and positive end-expiratory pressure (PEEP), improving ventilation and oxygenation [2]. In the same case, we applied a moderate pressure support of 10 cm H2O to assist spontaneous ventilation. Mouth packing was not required as air leakage during SAV did not affect oxygenation, and the brief period of pressure support ventilation did not compromise gas exchange. As stated above, data on ET-CO2 and RR were not reported because they were unreliable due to an unsealed circuit; however, continuous monitoring of respiratory movements, chest expansion, and pulse oximetry allowed us to detect any respiratory changes.
Since SAV proved sufficient to effectively maintain gas exchange, we were able to avoid the use of jet ventilation in all cases. This helped prevent potential complications such as hypercapnia, barotrauma with air trapping, tension pneumothorax, and subcutaneous emphysema [6]. On the other hand, using SAV without NMB requires the anesthesiologist to tailor the administration of analgesic and hypnotic agents [6]. Murgo et al. showed that RB can be safely conducted using a combination of TIVA and SAV [5]. Accordingly, we employed a remifentanil–propofol–ketamine-based TIVA with SAV, avoiding NMBs in all cases. Generally a combination of propofol and short-acting opioids is administered simultaneously [2]. Propofol and remifentanil-based TIVA is preferred over inhalational anesthesia to prevent environmental leakage from the unsealed respiratory circuit [7].
The pharmacological properties and clinical application of ketamine, especially for sedation for flexible bronchoscopic procedures, were already investigated [6,13]. This medication has bronchodilating properties and it does not cause respiratory or cardiovascular depression [9]. In our center, similar procedures without ketamine have traditionally been managed with general anesthesia, using propofol and remifentanil for sedation and analgesia, along with muscle relaxation.
Despite ketamine use, the remifentanil TCI rate had to be gradually adjusted to standard levels (1.5–3 ng/mL) to control hemodynamic responses. Without ketamine, higher remifentanil doses may have been required, potentially suppressing respiratory drive and leading to respiratory depression or apnea. Therefore, adding ketamine to TIVA regimens has a synergistic pharmacodynamics impact that lowers the need for opioids [14]. Additionally, increasing the risk of postoperative hyperalgesia was prevented by intraoperative ketamine infusion, suggesting a role for the N-methyl-D-aspartate pain-facilitation process [15].
On the other hand, ketamine sedation has some side effects. While BIS was used, its accuracy may be compromised due to ketamine’s known effects on readings. Although controversial, BIS monitoring remains useful in minimizing the risk of respiratory depression from remifentanil overdose and reducing reflex movement [1,5]. Moreover, ketamine increases arterial BP, HR, and cardiac output [1]. Indeed, its hypertensive effects may elevate the risk of bleeding during the debulking process through APC. Both propofol and midazolam mitigate ketamine’s sympathomimetic effects through CNS inhibition, achieving a balanced sedative state during procedures [10]. Additionally, ketamine should not be administered alone because it can lead to significant dissociative effects and hallucinations, especially in the elderly. [16]. In a surgical setting, ketamine is typically combined with benzodiazepines to mitigate these adverse psychological symptoms [16]. Finally, ketamine has the potential to increase postoperative nausea and vomiting (PONV), but no episodes were observed among our cases [9]. In our experience, considering the life-saving nature of the procedure, we determined that maintaining hemodynamic stability clearly outweighed these potential complications.
In patient 3, as an alternative to propofol, we administered DXM to maintain patient compliance. DXM, as an alpha2 receptor agonist, carries bradycardia and hypotension as common side effects. The combination of DXM and ketamine seems to provide adequate sedation mitigating hemodynamic changes related to both drugs administered singularly [7,12,17]. Beside sedation and ventilation, topical anesthesia has a major role in this kind of procedure. In the described cases, we adapted the recent findings on local anesthetic mixtures and adjuncts and used a ropivacaine–dexamethasone–DXM mixture, which ensured effective analgesia and minimized coughing, with no additional pain medication needed in the first 24 h [18].
The insertion of fixed-diameter metallic and silicone stents through RB provides immediate palliation of symptoms in patients with CAO and severe breathing difficulties [17]. Our stents, including the NOVATECH® silicone stents and AERO® 40 mm fully covered metallic stents, provided immediate relief of symptoms, even in complex and advanced disease scenarios. Complications related to RB may be classified as pre-operative, anesthesia-associated, or due to the procedure itself. Careful planning and coordination by experienced surgical and anesthetic teams is essential [6,19].
4. Conclusions
Our series showed that remifentanil–propofol–ketamine-based TIVA is a feasible and beneficial approach for therapeutic RB. Nevertheless, the limited sample size restricts the generalizability of our results, necessitating cautious interpretation. Randomized studies are needed to validate the safety, optimal dosages, and efficacy of this approach in high-risk procedures.
Author Contributions
Conceptualization, L.F., F.C. and A.S. (Antonio Sarubbi); methodology, L.F.; software, A.S. (Alessandro Strumia); validation, F.C., L.F., F.L. and D.S.; formal analysis, A.S. (Antonio Sarubbi) and A.S. (Alessandro Strumia); investigation, F.C. and A.S. (Antonio Sarubbi); resources, F.L.; data curation, L.F. and A.S. (Antonio Sarubbi); writing—original draft preparation, A.S. (Antonio Sarubbi); writing—review and editing, F.C., A.S. (Antonio Sarubbi), A.S. (Alessandro Strumia) and L.F.; visualization, F.L. and V.M.; supervision, L.F., F.C. and P.C.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This article is a revised and expanded version of a paper [20], which was presented at [XXII National Congress of the Società Italiana di Endoscopia Toracica (SIET) held in Florence on 18 October 2024].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bakan, M.; Topuz, U.; Umutoglu, T.; Gundogdu, G.; Ilce, Z.; Elicevik, M.; Kaya, G. Remifentanil-based total intravenous anesthesia for pediatric rigid bronchoscopy: Comparison of adjuvant propofol and ketamine. Clinics 2014, 69, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Semaan, R.; Yarmus, L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J. Thorac. Dis. 2015, 7 (Suppl. S4), S352–S362. [Google Scholar] [CrossRef] [PubMed]
- Barnwell, N.; Lenihan, M. Anaesthesia for airway stenting. BJA Educ. 2022, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, N.; Ma, W.J.; Bao, M.X.; Chen, Z.Y.; Ding, Z.N. Safety application of muscle relaxants and the traditional low-frequency ventilation during the flexible or rigid bronchoscopy in patients with central airway obstruction: A retrospective observational study. BMC Anesthesiol. 2021, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Murgu, S.; Laxmanan, B.; Stoy, S.; Egressy, K.; Chaddha, U.; Farooqui, F.; Brunner, R.; Hogarth, K.; Chaney, M. Evaluation of Safety and Short-term Outcomes of Therapeutic Rigid Bronchoscopy Using Total Intravenous Anesthesia and Spontaneous Assisted Ventilation. Respiration 2020, 99, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Welsby, I.; Mahmood, K.; Wahidi, M.; MacIntyre, N.; Shofer, S. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann. Am. Thorac. Soc. 2014, 11, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Thakore, S.; Gupta, N.; Madan, K.; Bhatnagar, S. Near ideal anesthetic technique for tracheal stenting in central airway obstruction with dexmedetomidine-ketamine infusion: A case report. Braz. J. Anesthesiol. 2021, 71, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; McCormack, S. Ketamine for Sedation During Bronchoscopy Procedures: A Review of Clinical Effectiveness, Safety, and Guidelines. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565475/ (accessed on 6 October 2024).
- Huang, X.; Ai, P.; Wei, C.; Sun, Y.; Wu, A. Comparison of the Effects of Esketamine/Propofol and Sufentanil/Propofol on the Incidence of Intraoperative Hypoxemia during Bronchoscopy: Protocol for a Randomized, Prospective, Parallel-Group Trial. J. Clin. Med. 2022, 11, 4587. [Google Scholar] [CrossRef] [PubMed]
- Fruchter, O.; Manevich, Y.; Carmi, U.; Rozengarten, D.; Kramer, M.R. Prospective Randomized Trial Evaluating Ketamine for Adult Bronchoscopy. J. Bronchol. Interv. Pulmonol. 2017, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Xu, M.; Lu, H.; Huang, Q.; Wu, J. Nebulized dexmedetomidine-lidocaine inhalation as a premedication for flexible bronchoscopy: A randomized trial. J. Thorac. Dis. 2019, 11, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.; Begdeda, H.; Grande, B.; Caviezel, C.; Zalunardo, M.P.; Franzen, D.P. Rigid bronchoscopy in malignant airway fistula using dexmedetomidine. J. Thorac. Dis. 2020, 12, 6120–6124. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jeon, Y.; Park, H.P.; Lim, Y.J.; Oh, Y.S. Comparison of alfetanil and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol. Scand. 2005, 49, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Cavus, Z.; Moralar, D.G.; Gok, A.K.; Gunaydin, A.S. The Effects of Ketamine-Propofol and Remifentanil-Propofol Combinations on Integrated Pulmonary Index During Sedation in Gastrointestinal System Endoscopy. Med. Bull. Sisli Etfal Hosp. 2024, 58, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Joly, V.; Richebe, P.; Guignard, B.; Fletcher, D.; Maurette, P.; Sessler, D.I.; Chauvin, M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology 2005, 103, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.B.; Gupta, V.; Patel, P.; Palacios, J.L. Ketamine. [Updated 30 January 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK470357/ (accessed on 6 October 2024).
- Okada, T.; Yoshida, M.; Matsushita, T.; Ishida, Y.; Furukawa, K.; Murozono, M. Anesthetic management of airway stent placement by rigid bronchoscopy with superior laryngeal nerve block while preserving spontaneous breathing: A case report. Clin. Case Rep. 2023, 11, e8232. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, M.; Zhang, X.R.; Wang, X.R.; Wang, Z.H.; Feng, X.Y.; Lei, Y.J.; Zhang, J.W. Ropivacaine with Dexmedetomidine or Dexamethasone in a Thoracic Paravertebral Nerve Block Combined with an Erector Spinae Plane Block for Thoracoscopic Lobectomy Analgesia: A Randomized Controlled Trial. Drug Des. Dev. Ther. 2022, 16, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Sarubbi, D.; Sarubbi, A.; Strumia, A.; Pascarella, G.; Crucitti, P.; Longo, F.; Frasca, L.; Martuscelli, M.; Tomaselli, E.; Carassiti, M.; et al. I-gel® as airways management in a challenging adult tracheobronchial foreign body aspiration. Indian J. Thorac. Cardiovasc. Surg. 2024, 40, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Sarubbi, A.; Frasca, L.; Lomgo, F.; Tacchi, G. Safety and Feasibility of Ketamine for Rigid Bronchoscopic Interventions: A Single-Center Experience. In Proceedings of the XXII National Congress of the Società Italiana di Endoscopia Toracica (SIET), Florence, Italy, 18 October 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).