Abstract
The introduction of CT scans and the subsequent Agatston score in the 1990s drastically improved our ability to detect coronary artery calcification (CAC). This led to its incorporation into cardiovascular risk assessment guidelines set forth by organizations such as the American Heart Association (AHA) and the American College of Cardiology (ACC). Over time, these guidelines have evolved significantly, reflecting an increasing understanding of CAC. Physical activity has become a key factor in the management of cardiovascular disease. However, the relationship between physical activity and CAC remains complex. Although physical activity is generally beneficial for cardiovascular health, paradoxically, high levels of physical activity have been associated with elevated CAC scores. However, these higher CAC levels may indicate the presence of more stable, calcified plaques that provide protection against plaque rupture. These contradictory findings call for balanced interpretations that acknowledge the cardiovascular benefits of physical activity. This review examines the historical development of clinical guidelines for CAC, the paradoxical relationship between physical activity and CAC, and potential underlying mechanisms. It emphasizes the need for future research to utilize objective measures and consistent methodologies to better understand the relationship between physical activity and CAC.
1. Introduction
Coronary artery calcification (CAC) has long been recognized as a key marker in atherosclerosis research and is a strong predictor of cardiovascular events [1,2]. Quantifying CAC via computed tomography (CT) scans, typically reported as an Agatston score, offers a non-invasive measure of calcified plaque burden in coronary arteries [3]. Higher CAC scores are consistently linked to increased risks of adverse cardiovascular outcomes, underscoring their clinical utility in risk stratification. Additionally, arterial stiffness is associated with both arteriosclerosis [4] and diabetes [5], and is closely linked to CAC [6], further emphasizing its broader implications for cardiovascular health.
Physical activity (PA) is universally acknowledged as essential for cardiovascular health, offering a range of benefits, including enhanced cardiorespiratory fitness, improved lipid metabolism, better blood pressure regulation, optimized glucose homeostasis, and reduced systemic inflammation [7,8,9]. These physiological adaptations collectively contribute to PA’s protective effect against cardiovascular diseases (CVDs). As a result, authoritative organizations consistently recommend regular PA as a cornerstone of a healthy lifestyle [10].
The association between CAC and traditional cardiovascular risk factors, such as higher lipid values, hypertension, and arterial stiffness, has been well-documented [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. However, the relationship between PA and CAC is less clear. Emerging evidence suggests that high levels of PA, particularly vigorous-intensity PA, might be associated with increased CAC scores. This finding is counterintuitive given the well-established cardiovascular benefits of exercise. This paradox, where PA both protects against CVD and potentially increases CAC, calls for a deeper understanding of the underlying mechanisms and clinical implications.
This review aims to comprehensively examine the historical development of clinical guidelines for CAC, as well as the paradoxical relationship between PA and CAC. We will explore the interplay between PA and CAC, highlighting both supportive and contradictory evidence. By synthesizing current research findings, we aim to reconcile these seemingly contradictory observations and provide a nuanced understanding of the underlying mechanisms.
2. Historical Perspective and Evolution of Guidelines on Coronary Artery Calcification
Studies on calcification date back centuries, with evidence of calcified arteries even found in ancient mummies, indicating that atherosclerosis has affected humans for millennia [25]. Early autopsy studies identified calcified plaques within coronary arteries, establishing a clear link between calcification and atherosclerosis [26]. This foundational knowledge has driven further research and technological advancements.
The development of computed tomography (CT) scans revolutionized the detection and quantification of CAC. The introduction of the Agatston score in the 1990s provided a standardized measure of calcification, significantly enhancing cardiovascular risk assessment [27]. The development of non-invasive imaging technologies, such as electron beam CT and multi-detector CT, has enabled the precise visualization and quantification of coronary calcification, facilitating large-scale studies and improving risk stratification. As a result, major professional organizations, including the American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC), have incorporated CAC scoring into their cardiovascular risk assessment guidelines.
The role of CAC scoring in clinical guidelines has evolved significantly since its initial introduction. Initially proposed in the 2000 ACC/AHA guidelines as a potential tool for assessing CAD risk, it was not until 2007 that CAC scoring received a formal Class IIa recommendation. By 2010, the ACC/AHA guidelines upheld this recommendation, emphasizing the role of CAC scoring alongside traditional risk factors in intermediate-risk adults and older patients with diabetes, though its universal use was not advocated. However, in 2013, the ACC/AHA guidelines downgraded CAC scoring to a Class IIb recommendation due to concerns about radiation exposure, cost, and limited data. By 2018, CAC scoring was recommended for refining risk assessments, with a CAC score of 0 indicating no need for statin therapy unless other conditions were present, and a CAC score of ≥100 supporting statin initiation [28]. The 2024 ESC guidelines place greater emphasis on the importance of CAC [29]. For individuals with suspected chronic coronary syndrome and a low pre-test likelihood of obstructive CAD (5–15%), CAC scoring is now recommended to reclassify risk and identify those with a very low (≤5%) CACS-weighted clinical likelihood. In asymptomatic individuals with prior chest CT scans showing coronary artery calcification, these findings should be used to improve risk stratification and guide the management of modifiable risk factors.
Globally, guidelines are increasingly recognizing the value of CAC scoring in cardiovascular risk assessment [30]. The Australia and New Zealand guidelines (CSANZ) recommend CAC scoring for risk assessment and therapy decisions as follows: withholding statins when CAC = 0, favoring lifestyle changes when CAC = 1–100, recommending statins when CAC = 101–400 if above the 75th percentile, and necessitating statin therapy when CAC > 400 [31]. The Canadian Cardiovascular Society (CCS) guidelines primarily advocate for the use of CAC scoring for intermediate-risk patients (FRS 10–19.9%) to refine risk stratification and suggest repeating CAC scoring every five years if statins are withheld [32]. In East Asian countries, although CAC may be considered less critical than in Western nations, its importance is increasingly recognized. Japanese guidelines recommend CAC as a prognostic tool for intermediate- to high-risk individuals, though they call for additional studies due to Japan’s lower CAD morbidity and mortality rates compared to Western populations [33]. Chinese guidelines appreciate CAC scoring for aspirin allocation, although they primarily focus on traditional risk factors, such as blood pressure, cholesterol, smoking status, and age, for cardiovascular risk assessment [34].
3. Physical Activity and CAC
As CAC scoring becomes increasingly central in clinical guidelines, understanding its relationship with PA, especially concerning cardiovascular health, has garnered significant attention. Recent studies highlight a paradoxical association between high levels of PA and increased CAC scores. This section reviews key studies that have reported on the complex relationship between PA and CAC.
3.1. Physical Activity and Increased CAC Scores
Multiple studies have demonstrated an association between high PA levels and elevated CAC scores. In a 25-year follow-up study of 3175 participants from the CARDIA study, Laddu et al. found that individuals with PA levels exceeding recommended guidelines had a higher likelihood of having a CAC score above zero [35]. This association was particularly pronounced in White males who engaged in high levels of PA, with increased odds of developing detectable CAC (AOR 1.86, 95% CI: 1.16–2.98). These findings suggest that high PA levels may increase CAC risk in certain demographic groups.
Supporting this, Sung et al. conducted a large prospective study of 25,485 healthy adults and found that individuals classified as health-enhancing physically active (HEPA) had higher baseline CAC scores (mean 12.04, 95% CI: 10.81–13.26) and greater CAC progression over five years (mean 23.03, 95% CI: 20.11–19.89) compared to inactive individuals (mean: 9.45, 95% CI: 8.76–10.14 and mean: 14.87, 95% CI: 13.18–16.55, respectively) [36] (Table 1). The authors propose that this increase in CAC might represent a stabilizing adaptation rather than elevated atherosclerotic risk. An editorial by Gulsin and Moss supports this perspective, suggesting that increased CAC in physically active individuals may indicate a protective response to chronic inflammation, promoting plaque stability [37] (Figure 1). They highlight that calcified plaques, as opposed to non-calcified plaques, are associated with a lower risk of rupture and subsequent cardiovascular events. This phenomenon, termed the “calcium paradox”, underscores the complexity of interpreting CAC scores. Gulsin and Moss have argued that, while CAC is a marker of coronary atherosclerosis, increased scores in the context of high PA should not be viewed solely as a negative outcome but may instead signify a shift toward a more stable plaque phenotype, emphasizing the overall cardiovascular benefits of PA.

Table 1.
Longitudinal studies on the impact of PA on CAC.
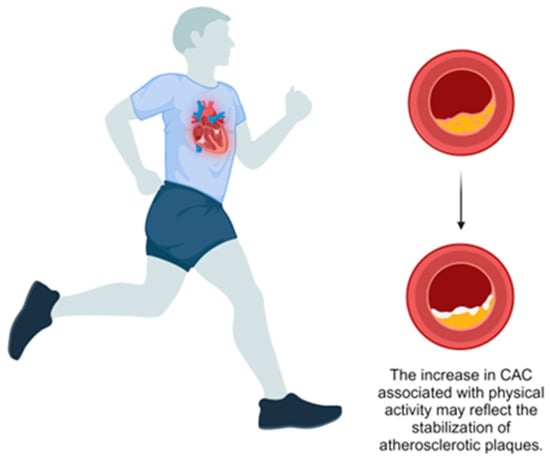
Figure 1.
Effect of physical activity on coronary artery calcification.
3.2. Effects of High-Intensity and Prolonged Physical Activity on CAC Levels
Increased CAC scores are notably more common among individuals engaging in high-intensity or prolonged PA. Pavlovic et al. found that longer weekly PA duration was linked to higher CAC scores [46] (Table 2), while Kermott et al. observed increased CAC in individuals with the highest cardiorespiratory fitness levels [47] (Table 2). Long-term follow-up studies further support these findings. Aengevaeren et al. demonstrated that very vigorous exercise could accelerate CAC progression, with exercise intensity playing a larger role than volume [38] (Table 1). Additionally, Bhatia et al. also noted that higher PA is associated with increased CAC density [39] (Table 2). These findings highlight that, while regular PA is beneficial, particularly intense or prolonged PA may be associated with higher CAC scores. However, the stability and composition of these calcified plaques, rather than their mere presence, appear to be key to cardiovascular risk assessment.

Table 2.
Cross-sectional studies on the impact of PA on CAC.
3.3. Stabilizing Effects of Physical Activity on Plaque Composition
Research increasingly supports that calcified plaques in physically active individuals may exhibit greater stability than those in sedentary individuals, suggesting a potential stabilizing effect. Merghani et al. conducted a cross-sectional study of 152 masters athletes and age-matched controls, finding that male athletes had a higher prevalence of atherosclerotic plaques (44.3% vs. 22.2%). However, these plaques were predominantly calcified (72.7%), indicating a stable morphology [48] (Table 2). Similarly, Aengevaeren et al. studied lifelong exercise histories in 284 middle-aged men and observed that high lifetime PA volumes were associated with increased CAC. Those engaging in over 2000 MET-minutes per week showed significantly higher CAC scores (9.4 vs. 0.6–0.9) and prevalence of CAC (68%) [49] (Table 2). Nonetheless, these highly active individuals tended to exhibit plaques with a more benign composition, suggesting that, while high PA levels may increase CAC, they also enhance plaque stability, potentially reducing cardiovascular risks associated with softer, rupture-prone plaques.
3.4. Cardiovascular Benefits of Physical Activity Despite Increased CAC
Building on findings that high PA levels can elevate CAC, it is important to consider the broader cardiovascular benefits emphasized by both the ACC/AHA and ESC guidelines [10,54]. Regular PA is essential for cardiovascular health, as it reduces risks associated with high blood pressure, dyslipidemia, and insulin resistance. Exercise improves endothelial function, lowers systemic inflammation, and aids in weight management, all contributing to lower CVD incidence and mortality. The guidelines recommend a minimum of 150 min of moderate-intensity or 75 min of vigorous-intensity activity per week, with even greater benefits observed at higher levels of PA.
Supporting this perspective, the Cooper Center Longitudinal Study (CCLS) found that individuals with high PA levels are associated with prevalent CAC compared to less active individuals. However, at any given CAC level, higher PA was associated with lower mortality risk, suggesting a protective effect of exercise even with a large coronary calcification burden [55]. These results are consistent with the notion that, while higher levels of PA may be accompanied by greater CAC, they are also associated with lower cardiovascular risk. This evidence supports the safety and cardiovascular benefits of regular intensive PA, reinforcing its role in promoting heart health even in the presence of elevated CAC scores.
4. Potential Mechanisms of PA-Induced CAC
Although the mechanisms underlying increased CAC with PA remain under investigation, several pathways beyond plaque stabilization have been proposed. High-intensity PA can cause significant hemodynamic changes, increasing shear stress on arterial walls, especially in regions of disturbed flow, like at arterial bifurcations. This shear stress may exacerbate endothelial injury, accelerating atherosclerosis in individuals with pre-existing plaques or endothelial dysfunction [56].
Another potential mechanism is oxidative stress. High-intensity PA increases reactive oxygen species (ROS) production in endothelial cells, which can contribute to vascular damage and the formation of atherosclerotic plaques through the oxidation of low-density lipoproteins (LDLs). While regular PA enhances antioxidant capacity by modulating the activity of pro- and antioxidant enzymes, transient ROS elevation during intense PA could contribute to increases in CAC [57].
Parathyroid hormone (PTH), a key regulator of calcium homeostasis, is also implicated. Patients with hyperparathyroidism demonstrate higher rates of CVD and vascular calcifications. Exercise-induced increases in PTH levels suggest a potential mechanism for exercise-related atherosclerosis, with repeated exposure to elevated PTH post-exercise potentially accelerating coronary atherosclerosis [58].
Inflammatory processes also play a key role in the development and progression of atherosclerotic plaques. Oxidized LDL can recruit immune cells and release proinflammatory cytokines, promoting plaque growth [59]. While regular exercise has an anti-inflammatory effect, acute high-intensity exercise can provoke a transient inflammatory response that may accelerate atherosclerosis in individuals engaging in frequent, prolonged, intense exercise regimens.
5. Contradictory Findings and Protective Effects of Exercise
Despite evidence suggesting that high PA levels can increase CAC, other studies have found an inverse or non-significant relationship between PA and CAC progression. Shuval et al. investigated the relationship between high-volume leisure-time aerobic PA and CAC progression in 8771 men and women and found a cross-sectional association between higher PA and higher CAC in men, although there was no significant longitudinal association between high PA with CAC progression [42] (Table 1). However, their study had methodological concerns, such as violations of the proportional hazards assumption in their Cox regression model, which requires constant hazard ratios for covariates over time. High initial levels of CAC among individuals who exercised extensively did not further increase with continued exercise, indicating a time-varying effect of PA on CAC progression. This could lead to potentially inaccurate hazard ratios. Additionally, transitioning from electron beam tomography to a 64-slice scanner during their study might have introduced variability in CAC measurements, influencing their results. These issues suggest that their conclusions may not fully capture the temporal dynamics of the relationship between PA and CAC.
Several additional studies suggest that moderate to vigorous physical activity may reduce CAC. Kamimura et al. reported that ideal PA levels are associated with lower high-sensitivity C-reactive protein levels, a lower prevalence of CAC, and reduced coronary heart disease (CHD) incidence [51] (Table 2). Jae et al. found that higher fitness levels correlate with a lower prevalence of subclinical atherosclerosis, including CAC, especially in men with cardiometabolic syndrome [52] (Table 2). Similarly, Kwaśniewska et al. demonstrated that maintaining high levels of PA in middle and older age may protect against atherosclerosis as measured by CAC, intima–media thickness (IMT), and reactive hyperemia index (RHI) [44] (Table 1). Gabriel et al. highlighted that higher levels of moderate- to vigorous-intensity physical activity (MVPA) are associated with lower subclinical disease markers in older women [45] (Table 1). Furthermore, a long-term follow-up study by Chedid et al. found that higher METs are linked to lower CAC scores [43] (Table 1). These studies illustrate that the relationship between PA and CAC is complex and influenced by factors such as population demographics, measurement methods, and types and intensities of PA. While high levels of PA may increase CAC in some individuals, the protective cardiovascular effects of exercise—such as reduced inflammation, improved endothelial function, and the control of traditional risk factors—should not be overlooked.
6. Future Research Directions
The inconsistent and even conflicting results pertaining to the association between PA and CAC underscore the need for further research to provide more conclusive insights. Future studies should incorporate objective measures of PA, such as wearable fitness trackers, to yield more accurate data. Wearable devices can collect real-time information on the frequency, intensity, and duration of PA, which may provide a more comprehensive and accurate measurement of activity levels than that provided by self-reports. This approach addresses the limitations of self-reported data, which are often affected by recall bias and inaccuracies.
Additionally, assessing calcium volume and density, rather than relying exclusively on the Agatston score, will provide a clearer understanding of calcification and its relationship with exercise. The Agatston score measures the amount of calcification without providing information on specific types of calcified plaques. By measuring calcium volume and density, researchers can distinguish between stable, dense calcifications and less stable, softer plaques. This differentiation is critical, as stable calcified plaques are less prone to rupture and subsequent cardiovascular events. Thus, a detailed analysis of calcium characteristics can provide a clearer perspective on how increased CAC in physically active individuals impacts plaque stability and progression.
Longitudinal studies are essential for establishing causality and clarifying the long-term effects of various types and intensities of PA on CAC progression. Population-specific research is needed to account for differences in age, gender, race, and genetic background, which can guide personalized exercise recommendations. Future investigations should also compare the effects of different exercise intensities and durations to identify the optimal balance that maximizes health benefits while minimizing adverse effects. The use of a consistent methodology across studies—including standardized measurement techniques, study designs, and data analysis methods—is essential to reconcile conflicting findings. Addressing these research priorities will clarify the relationship between exercise and CAC and facilitate the optimization of cardiovascular benefits.
7. Conclusions
This review underscored the paradoxical relationship between PA and CAC. Although PA is broadly recognized for its cardiovascular benefits, emerging evidence suggests that high levels of PA, especially vigorous exercise, might be associated with elevated CAC scores. Key studies have observed that individuals with high levels of PA often have higher CAC scores than those with lower activity levels. This increase in CAC score in highly active individuals necessitates a balanced interpretation. Elevated CAC scores do not inherently indicate a greater risk of cardiovascular events if calcified plaques are stable. Clinicians should consider the overall cardiovascular health benefits of PA when interpreting CAC scores. While continued PA should be promoted, it should be accompanied by personalized clinical assessments to consider individual risk factors and health conditions.
Author Contributions
K.-C.S.: conceptualization, methodology, supervision, and project administration; D.-E.S.: conceptualization, methodology, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Created in BioRender. sung, D. (2024) https://BioRender.com/h16v981 (accessed on 27 October 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.; Rader, D.J.; Rubin, G.D.; et al. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.S. Coronary artery calcium scanning: Past, present, and future. JACC Cardiovasc. Imaging 2015, 8, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Mortensen, M.B.; Kianoush, S.; Tota-Maharaj, R.; Cainzos-Achirica, M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Avolio, A. Arteriosclerosis and Atherosclerosis Assessment in Clinical Practice: Methods and Significance. Pulse 2023, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C. Arterial Stiffness and Incident Diabetes. Pulse 2024, 12, 12–18. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, S.; Lee, S.H.; Kim, B.J.; Kim, B.S.; Kang, J.H.; Cheong, E.S.; Kim, J.Y.; Park, J.B.; Sung, K.C. Association between brachial-ankle pulse wave velocity and progression of coronary artery calcium: A prospective cohort study. Cardiovasc. Diabetol. 2015, 14, 147. [Google Scholar] [CrossRef]
- Radford, N.B.; DeFina, L.F.; Leonard, D.; Barlow, C.E.; Willis, B.L.; Gibbons, L.W.; Gilchrist, S.C.; Khera, A.; Levine, B.D. Cardiorespiratory Fitness, Coronary Artery Calcium, and Cardiovascular Disease Events in a Cohort of Generally Healthy Middle-Age Men: Results From the Cooper Center Longitudinal Study. Circulation 2018, 137, 1888–1895. [Google Scholar] [CrossRef]
- Lavie, C.J.; Wisloff, U.; Blumenthal, R.S. Extreme Physical Activity and Coronary Artery Calcification-Running Heavily and Safely With “Hearts of Stone”. JAMA Cardiol. 2019, 4, 182–183. [Google Scholar] [CrossRef]
- Arnson, Y.; Rozanski, A.; Gransar, H.; Hayes, S.W.; Friedman, J.D.; Thomson, L.E.J.; Berman, D.S. Impact of Exercise on the Relationship Between CAC Scores and All-Cause Mortality. JACC Cardiovasc. Imaging 2017, 10, 1461–1468. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Sung, K.C.; Lim, Y.H.; Park, S.; Kang, S.M.; Park, J.B.; Kim, B.J.; Shin, J.H. Arterial stiffness, fatty liver and the presence of coronary artery calcium in a large population cohort. Cardiovasc. Diabetol. 2013, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chang, Y.; Kim, S.; Sung, K.C.; Shin, H.; Ryu, S. Increased burden of coronary artery calcium from elevated blood pressure in low-risk young adults. Atherosclerosis 2019, 282, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kang, J.G.; Lee, S.H.; Lee, J.Y.; Sung, K.C.; Kim, B.S.; Kang, J.H. Relationship of Echocardiographic Epicardial Fat Thickness and Epicardial Fat Volume by Computed Tomography with Coronary Artery Calcification: Data from the CAESAR Study. Arch. Med. Res. 2017, 48, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Kang, J.G.; Lee, H.J.; Kim, N.H.; Sung, J.W.; Cheong, E.; Sung, K.C. Absence of association between gallstone and coronary artery calcification. Atherosclerosis 2017, 258, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; So, M.S.; Kim, B.J.; Kang, J.G.; Sung, K.C.; Kim, B.S.; Kang, J.H. The association between epicardial fat thickness and coronary artery calcification according to blood pressure status in nonhypertensive individuals: From the CAESAR study. J. Clin. Lipidol. 2015, 9, 305–312. [Google Scholar] [CrossRef]
- Rhee, E.J.; Ryu, S.; Lee, J.Y.; Lee, S.H.; Cheong, E.; Park, S.E.; Park, C.Y.; Won, Y.S.; Kim, J.M.; Cho, D.S.; et al. The association between dietary cholesterol intake and subclinical atherosclerosis in Korean adults: The Kangbuk Samsung Health Study. J. Clin. Lipidol. 2017, 11, 432–441.e3. [Google Scholar] [CrossRef]
- Sung, K.C.; Chang, Y.; Ryu, S.; Chung, H.K. High levels of serum vitamin D are associated with a decreased risk of metabolic diseases in both men and women, but an increased risk for coronary artery calcification in Korean men. Cardiovasc. Diabetol. 2016, 15, 112. [Google Scholar] [CrossRef]
- Sung, K.C.; Cho, E.J.; Lim, Y.H.; Shin, J.; Pyun, W.B.; Kang, S.M.; Rosenson, R.S. HDL-C levels modify the association between C-reactive protein and coronary artery calcium score. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1240–1245. [Google Scholar] [CrossRef]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Lee, S.H.; Cheong, E.S.; Wild, S.H.; Byrne, C.D. Fatty Liver, Insulin Resistance, and Obesity: Relationships with Increase in Coronary Artery Calcium over Time. Clin. Cardiol. 2016, 39, 321–328. [Google Scholar] [CrossRef]
- Sung, K.C.; Wild, S.H.; Byrne, C.D. Controlling for apolipoprotein A-I concentrations changes the inverse direction of the relationship between high HDL-C concentration and a measure of pre-clinical atherosclerosis. Atherosclerosis 2013, 231, 181–186. [Google Scholar] [CrossRef]
- Sung, K.C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty liver, insulin resistance, and features of metabolic syndrome: Relationships with coronary artery calcium in 10,153 people. Diabetes Care 2012, 35, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Yoo, T.K.; Lee, M.Y.; Byrne, C.D.; Zheng, M.H.; Targher, G. Comparative Associations of Nonalcoholic Fatty Liver Disease and Metabolic Dysfunction-Associated Fatty Liver Disease with Coronary Artery Calcification: A Cross-Sectional and Longitudinal Cohort Study. Arter. Thromb. Vasc. Biol. 2023, 43, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, M.Y.; Sung, K.C. The Risk of Coronary Artery Calcification according to Different Lipid Parameters and Average Lipid Parameters. J. Atheroscler. Thromb. 2024, 31, 1194–1214. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, S.H.; Rhim, H.C.; Lee, M.Y.; Cheong, E.S.; Seo, M.H.; Sung, K.C. Association of Cardiovascular Mortality with Concurrent Coronary Artery Calcification and Physical Activity: A Cohort Study. Medicina 2023, 59, 522. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Allam, A.H.; Lombardi, G.P.; Wann, L.S.; Sutherland, M.L.; Sutherland, J.D.; Soliman, M.A.; Frohlich, B.; Mininberg, D.T.; Monge, J.M.; et al. Atherosclerosis across 4000 years of human history: The Horus study of four ancient populations. Lancet 2013, 381, 1211–1222. [Google Scholar] [CrossRef]
- Eggen, D.A.; Strong, J.P.; McGill, H.C., Jr. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 1965, 32, 948–955. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Golub, I.S.; Termeie, O.G.; Kristo, S.; Schroeder, L.P.; Lakshmanan, S.; Shafter, A.M.; Hussein, L.; Verghese, D.; Aldana-Bitar, J.; Manubolu, V.S.; et al. Major Global Coronary Artery Calcium Guidelines. JACC Cardiovasc. Imaging 2023, 16, 98–117. [Google Scholar] [CrossRef]
- Liew, G.; Chow, C.; van Pelt, N.; Younger, J.; Jelinek, M.; Chan, J.; Hamilton-Craig, C. Cardiac Society of Australia and New Zealand Position Statement: Coronary Artery Calcium Scoring. Heart Lung Circ. 2017, 26, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kitagawa, T.; Kihara, Y. Clinical implications of the coronary artery calcium score in Japanese patients. J. Atheroscler. Thromb. 2014, 21, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chinese Medical Association Cardiovascular Disease Branch; Chinese Association of Rehabilitation Medicine Cardiac Prevention and Rehabilitation Committee; Chinese Society of Gerontology and Geriatrics Cardiology Committeee; Thrombosis Prevention and Treatment Committee of Cardiovascular Medicine Branch of Chinese Medical Doctor Association. Chinese guideline on the primary prevention of cardiovascular diseases. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 1000–1038. [Google Scholar] [CrossRef]
- Laddu, D.R.; Rana, J.S.; Murillo, R.; Sorel, M.E.; Quesenberry, C.P., Jr.; Allen, N.B.; Gabriel, K.P.; Carnethon, M.R.; Liu, K.; Reis, J.P.; et al. 25-Year Physical Activity Trajectories and Development of Subclinical Coronary Artery Disease as Measured by Coronary Artery Calcium: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Mayo Clin. Proc. 2017, 92, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Hong, Y.S.; Lee, J.Y.; Lee, S.J.; Chang, Y.; Ryu, S.; Zhao, D.; Cho, J.; Guallar, E.; Lima, J.A.C. Physical activity and the progression of coronary artery calcification. Heart 2021, 107, 1710–1716. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Moss, A.J. Coronary artery calcium paradox and physical activity. Heart 2021, 107, 1686–1687. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Bakker, E.A.; Braber, T.L.; Nathoe, H.M.; Sharma, S.; Thompson, P.D.; Velthuis, B.K.; Eijsvogels, T.M.H. Exercise Volume Versus Intensity and the Progression of Coronary Atherosclerosis in Middle-Aged and Older Athletes: Findings From the MARC-2 Study. Circulation 2023, 147, 993–1003. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Lin, F.; Thomas, I.C.; Denenberg, J.; Kandula, N.R.; Budoff, M.J.; Criqui, M.H.; Kanaya, A.M. Coronary artery calcium incidence and changes using direct plaque measurements: The MASALA study. Atherosclerosis 2022, 353, 41–46. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Colangelo, L.A.; Reis, J.; Venkatesh, B.A.; Sharma, R.; Abbasi, S.A.; Goff, D.C., Jr.; Carr, J.J.; Rana, J.S.; et al. Association of Fitness in Young Adulthood with Survival and Cardiovascular Risk: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern. Med. 2016, 176, 87–95. [Google Scholar] [CrossRef]
- Hamer, M.; Venuraju, S.M.; Lahiri, A.; Rossi, A.; Steptoe, A. Objectively assessed physical activity, sedentary time, and coronary artery calcification in healthy older adults. Arter. Thromb. Vasc. Biol. 2012, 32, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Shuval, K.; Leonard, D.; DeFina, L.F.; Barlow, C.E.; Berry, J.D.; Turlington, W.M.; Pavlovic, A.; Radford, N.B.; Gabriel, K.P.; Khera, A.; et al. Physical Activity and Progression of Coronary Artery Calcification in Men and Women. JAMA Cardiol. 2024, 9, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chedid, G.; Malik, A.; Daher, R.; Welty, F.K. Higher exercise capacity, but not omega-3 fatty acid consumption, predicts lower coronary artery calcium scores in women and men with coronary artery disease. Atherosclerosis 2023, 384, 117168. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, M.; Jegier, A.; Kostka, T.; Dziankowska-Zaborszczyk, E.; Rębowska, E.; Kozińska, J.; Drygas, W. Long-term effect of different physical activity levels on subclinical atherosclerosis in middle-aged men: A 25-year prospective study. PLoS ONE 2014, 9, e85209. [Google Scholar] [CrossRef]
- Gabriel, K.P.; Matthews, K.A.; Pérez, A.; Edmundowicz, D.; Kohl, H.W., 3rd; Hawkins, M.S.; Janak, J.C.; Kriska, A.M.; Kuller, L.H. Self-reported and accelerometer-derived physical activity levels and coronary artery calcification progression in older women: Results from the Healthy Women Study. Menopause 2013, 20, 152–161. [Google Scholar] [CrossRef][Green Version]
- Pavlovic, A.; DeFina, L.F.; Leonard, D.; Radford, N.B.; Farrell, S.W.; Barlow, C.E.; Shuval, K.; Berry, J.D.; Levine, B.D. Coronary Artery Calcification and High-Volume Physical Activity: Role of Lower Intensity versus Longer Duration of Exercise. Eur. J. Prev. Cardiol. 2024, 31, 1526–1534. [Google Scholar] [CrossRef]
- Kermott, C.A.; Schroeder, D.R.; Kopecky, S.L.; Behrenbeck, T.R. Cardiorespiratory Fitness and Coronary Artery Calcification in a Primary Prevention Population. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 122–130. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes with a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Braber, T.L.; Prakken, N.H.J.; Doevendans, P.A.; Grobbee, D.E.; Thompson, P.D.; Eijsvogels, T.M.H.; Velthuis, B.K. Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 2017, 136, 138–148. [Google Scholar] [CrossRef]
- DeFina, L.; Radford, N.; Leonard, D.; Gibbons, L.; Khera, A. Cardiorespiratory fitness and coronary artery calcification in women. Atherosclerosis 2014, 233, 648–653. [Google Scholar] [CrossRef]
- Kamimura, D.; Cain-Shields, L.R.; Clark, D.; Oshunbade, A.A.; Ashley, K.E.; Guild, C.S.; Loprinzi, P.D.; Newton, R.; Blaha, M.J.; Suzuki, T.; et al. Physical Activity, Inflammation, Coronary Artery Calcification, and Incident Coronary Heart Disease in African Americans: Insights from the Jackson Heart Study. Mayo Clin. Proc. 2021, 96, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Jae, S.Y.; Franklin, B.A.; Schmidt-Trucksass, A.; Kim, D.K.; Choi, Y.H.; Park, J.B. Relation of Cardiorespiratory Fitness to Risk of Subclinical Atherosclerosis in Men with Cardiometabolic Syndrome. Am. J. Cardiol. 2016, 118, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Storti, K.L.; Pettee Gabriel, K.K.; Underwood, D.A.; Kuller, L.H.; Kriska, A.M. Physical activity and coronary artery calcification in two cohorts of women representing early and late postmenopause. Menopause 2010, 17, 1146–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. 2022, 75, 429. [Google Scholar] [CrossRef]
- DeFina, L.F.; Radford, N.B.; Barlow, C.E.; Willis, B.L.; Leonard, D.; Haskell, W.L.; Farrell, S.W.; Pavlovic, A.; Abel, K.; Berry, J.D.; et al. Association of All-Cause and Cardiovascular Mortality with High Levels of Physical Activity and Concurrent Coronary Artery Calcification. JAMA Cardiol. 2019, 4, 174–181. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, H.B.; Li, P.S.; Yuan, W.X.; Liu, B.; Liu, S.T.; Qin, K.R. ROS and NO Dynamics in Endothelial Cells Exposed to Exercise-Induced Wall Shear Stress. Cell. Mol. Bioeng. 2019, 12, 107–120. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Bouassida, A.; Latiri, I.; Bouassida, S.; Zalleg, D.; Zaouali, M.; Feki, Y.; Gharbi, N.; Zbidi, A.; Tabka, Z. Parathyroid hormone and physical exercise: A brief review. J. Sports Sci. Med. 2006, 5, 367–374. [Google Scholar]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).