Abstract
Acute pulmonary embolism (PE) is a leading cause of mortality. Not only is PE associated with short-term mortality, but up to ~20% of patients might suffer from long-term consequences such as post-PE syndrome and chronic thromboembolic pulmonary hypertension. Current risk stratification tools poorly predict those who are at risk for short-term deterioration and those who develop long-term consequences. Traditionally, systemic thrombolysis has been considered the first-line therapy for patients with high-risk PE without contraindications; however, it comes with the risk of major bleeding (notably intracranial hemorrhage). The use of catheter-directed interventions (embolectomy or thrombolysis) has been increasing owing to their low bleeding risk; however, randomized trial data supporting their efficacy in improving clinical outcomes are limited. In this review, we highlight the evidence supporting the available advanced therapies for high- and intermediate-risk PE and summarize the ongoing trials which are evaluating these therapies.
1. Introduction
Acute pulmonary embolism (PE) is the third most common acute cardiovascular syndrome and a leading cause of death [1]. The annual PE incidence in the United States is 39–115 per 100,000 people [1], with an estimated 300,000 deaths annually [2]. The incidence and mortality rates related to PE have remained relatively stable between 1999 and 2018 [3]. Despite the widespread use of imaging modalities to diagnose PE such as CTPA, the current risk stratification tools are not accurate in predicting short-term mortality and long-term consequences. PE can be classified based on the presentations to: (i) low risk (hemodynamically stable patients without evidence of right ventricular dysfunction); (ii) intermediate-low risk (hemodynamically stable patients with either right ventricular dysfunction or elevated cardiac biomarkers), (iii) intermediate-high risk (hemodynamically stable patients with both right ventricular dysfunction and elevated biomarkers), and (iv) high-risk patients (hemodynamic instability) [4]. Traditionally, systemic thrombolysis has been considered first-line therapy for high-risk PE, but due to high rates of major bleeding, specifically intracranial hemorrhage, alternative therapies such as catheter-directed interventions are becoming more widely used. In this review, we discuss the risk stratification tools and emerging management options for intermediate- and high-risk PE.
2. Risk Factors for Pulmonary Embolism
Risk factors for PE are categorized as major transient, minor transient, non-malignant persistent, malignant, and underlying hypercoagulable states. Major transient risk factors include bed rest >3 days, and fractures; minor transient risk factors include central venous lines, long-haul travel, and oral contraceptive therapy; non-malignant persistent risk factors include inflammatory bowel disease and active autoimmune disease, whereas malignancy and hypercoagulable states are separate risk factors [4,5,6].
Venous thromboembolism (VTE) triggered by a major transient/reversible risk factor has an annual event rate of 3.3% (95% CI, 2.8%–3.9%) recurrence after therapy discontinuation [7]. Conversely, for VTEs caused by persistent and progressive risk factors (e.g., metastatic cancer), the risk of recurrence is 20.7% over 12 months (95% CI, 15.6%–25.8%) after discontinuing therapy [8]. Patients without significant transient/reversible risk factors for VTE (previously classified as unprovoked) face an intermediate risk of recurrence after completing anticoagulation therapy [9].
It is important to categorize patients who developed VTE according to their risk factors to determine the length of therapy and risk of recurrence (Table 1). Patients with hereditary thrombophilia, such as confirmed deficiencies of antithrombin, protein C, or protein S, are often candidates for indefinite anticoagulant treatment following an initial episode of PE without a major reversible risk factor [4].

Table 1.
Categorization of risk factors for VTE based on risk of recurrence [4].
3. Pathophysiology
PE, particularly saddle PE, leads to the sudden obstruction of the pulmonary arteries, primarily leading to right ventricular (RV) failure secondary to acute pressure overload [4]. When significant portions (30–50%) of the pulmonary bed are obstructed, there is a significant increase in pulmonary arterial pressure and elevated pulmonary vascular resistance [11]. The obstruction and resultant hypoxic vasoconstriction, combined with the release of neurohumoral factors such as serotonin, thromboxane A2, and histamine, escalate pulmonary vasculature resistance, imposing a substantial afterload on the RV [9].
The acute RV pressure overload induces RV dilation and hypokinesis, contributing to tricuspid regurgitation and reduced cardiac output. As the RV dilates, the interventricular septum shifts towards the left ventricle (LV), impairing LV diastolic filling and reducing systemic blood pressure [4]. The strain on the RV myocardium increases myocardial oxygen demand, with subsequent decreased coronary perfusion due to obstructive shock, leading to ischemia, decreased systemic perfusion, and potential RV infarction (Figure 1) [12].
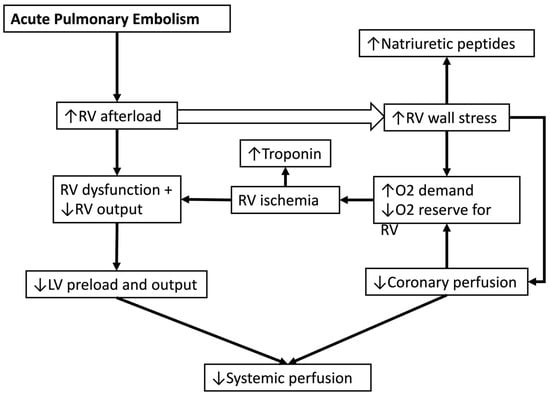
Figure 1.
Pathophysiology of shock in acute pulmonary embolism. RV: right ventricle, LV: left ventricle, O2: oxygen.
Additionally, the mismatch between ventilation and perfusion causes hypoxemia. In severe cases, the pressure gradient may reverse through a patent foramen ovale, resulting in right-to-left shunting and worsening hypoxemia. These pathophysiological changes rapidly progress towards obstructive shock if left untreated, necessitating prompt medical intervention to restore hemodynamic stability and prevent fatal outcomes [13].
4. Risk Stratification
The cornerstone of PE diagnosis remains imaging, namely with computed tomography pulmonary angiography (CTPA) [14]. Bedside transthoracic echocardiography (TTE) can visualize right ventricular dysfunction and suffice for the initiation of pulmonary reperfusion therapy in a hemodynamically unstable patient [14]. One meta-analysis including 1249 patients found that echocardiographic evidence of RV dysfunction was associated with 2.4-fold increased odds of all-cause mortality (OR 2.4, 95% CI, 1.3–4.3) [15]. A recent study found that patients with intermediate-risk PE presenting with McConnell’s sign had increased odds of normotensive shock (OR 8.4, 95% CI, 1.7–40.5) [16].
Earlier scoring systems such as the Pulmonary Embolism Severity Index (PESI) and simplified PESI (sPESI) have been used to classify patients as low risk or high risk. PESI utilizes 10 clinical markers, each with their own point value, to divide patients into classes I-V. These markers include the male sex (+10 points), chronic heart failure (+10 points), chronic pulmonary disease (+10 points), tachycardia >110 bpm (+20 points), tachypnea >30 breaths per min (+20 points), temperature >36 °C (+20 points), arterial oxyhemoglobin saturation <90% (+20 points), cancer (+30 points), systolic blood pressure <100 mmHg (+30 points), and acute encephalopathy (+60 points) [17]. The different classes are classified according to 30-day mortality risk: classes I–II (0–85 points) are considered low-risk, with a 30-day mortality up to 3.5%; class III (86–105 points) is moderate risk with a 3.2–7.1% 30-day mortality; class IV (106–125 points) carries a high 30-day mortality risk with a value up to 11.4%; and lastly class V (>125 points) is considered a very high mortality risk, with up to 24.5% 30-day mortality [4]. sPESI uses age >80, a history of chronic lung disease or heart failure, history of cancer, systolic blood pressure <100 mmHg, pulse rate >110 beats/min, and SaO2 < 90% for risk stratification, with each criteria giving 1 point to the patient [18]. Patients with a score of zero are classified as low risk while scores of ≥1 are considered high-risk PE [18]. The sPESI score performs well in identifying low-risk PE patients. One meta-analysis with over 50,000 patients showed that a low sPESI was associated with a 2.1% incidence of all-cause mortality [19]. Notably, both the PESI scores do not take into consideration the RV function, which is a strong predictor of potential hemodynamic compromise with worse outcomes [4]. Another limitation is the inclusion of several vitals and comorbidities which can be attributed to other clinical conditions like sepsis, heart failure, and chronic obstructive pulmonary disorder exacerbation.
The 2019 European Society of Cardiology (ESC) guidelines for the diagnosis and management of acute PE use markers of RV function (cardiac troponin, BNP, NT-pro-BNP) to risk stratify acute PE into low, intermediate-low, intermediate-high, and high-risk patients. The intermediate-low group is defined as sPESI ≥1 with or without RV function markers, the intermediate-high group as sPESI ≥1 with elevated troponins and positive RV dysfunction imaging findings, and the high-risk as sPESI ≥1 with elevated troponins, positive RV dysfunction imaging findings, and hemodynamic instability (Table 2) [4].

Table 2.
The classification of pulmonary embolism severity and the risk of early (in-hospital or 30-day) mortality [4].
However, this classification does not predict clinical deterioration in the intermediate-high-risk group who might further deteriorate and develop hemodynamic compromise. Some authors have classified this group of patients as “normotensive shock” (i.e., systolic blood pressure ≥ 90 mmHg with a cardiac index ≤2.2 L/min/m2). In one study of 384 patients with intermediate risk PE who underwent mechanical thrombectomy, almost a third had normotensive shock. A composite shock score including elevated cardiac troponins, elevated natriuretic peptides, RV dysfunction, saddle PE, concomitant DVT, and tachycardia was developed. The prevalence of normotensive shock increased with a larger number of components of the composite shock score: a score of 0 had a 0% prevalence of normotensive shock, whereas a score of 6 had a normotensive shock prevalence of 58.3% (OR 5.8, 95% CI, 2.0–17.0) [20]. This composite shock score was also found to predict worse in-hospital outcomes among patients with intermediate-risk PE in one retrospective single-center study [21]. Future prospective multicenter studies are needed to validate this composite shock score in a larger population of intermediate-risk PE patients.
5. Long-Term Complications
Long PE syndrome is a common entity comprising persistent dyspnea or poor physical performance over several months to years following acute PE. These patients may develop chronic thromboembolic disease or chronic thromboembolic pulmonary hypertension. The majority of PE survivors restore their pulmonary artery bed patency within the first few months following the acute insult [22]. However, cohort studies have revealed that these symptoms frequently persist for 6 months to 3 years [4]. One meta-analysis of 26 studies with 3671 patients showed that the prevalence of persistent RV dysfunction after 3 months was 18.1% and the prevalence of at least mild functional impairment (NYHA II-IV) was 33.2%. There was no difference in the incidence of these functional impairments based on the initial modality of therapy (thrombolysis versus anticoagulation alone) [23]. A recent cohort study in Canada followed 100 PE survivors over one year; 46.5% of patients had reduced maximal aerobic capacity, defined as a peak oxygen consumption <80% on cardiopulmonary exercise testing [24]. Of note, these patients had pulmonary function tests and echocardiographic findings largely within normal limits at follow-up. Some factors have been demonstrated to be associated with reduced functional exercise capacity and quality of life such as obesity, prior lung disease, the female sex, higher pulmonary artery systolic pressures on a day-10 echocardiogram, and higher main pulmonary artery diameter on the baseline CTPA [25]. The development of long PE syndrome appears unrelated to the degree of hemodynamic compromise at the time of the initial event, and can include chronic thromboembolic pulmonary hypertension and chronic thromboembolic disease without pulmonary hypertension. In one study of 20 survivors with intermediate-high and high-risk PE, there was no association between exercise impairment and persistent RV dysfunction [26]. It remains unclear whether advanced therapies besides systemic anticoagulation would reduce the risk of developing long PE syndrome.
6. Management Strategies
Systemic anticoagulation is the cornerstone of PE therapy, with advanced therapies including thrombolytics and embolectomy reserved for the high-risk and intermediate-risk PE. Certain cases may require adjunctive therapy with extracorporeal mechanical circulatory support (ECMO), which provides immediate support for acute circulatory collapse but does not treat the PE.
6.1. Systemic Thrombolysis
The benefits of this treatment modality include availability and applicability without additional training. Unfortunately, PE patients often have absolute or relative contraindications to systemic thrombolysis. A meta-analysis of 16 trials of 2115 patients (of which 8 trials enrolled intermediate-risk PE patients) showed that systemic thrombolysis reduced all-cause mortality (OR 0.5, 95% CI, 0.3–0.9, number needed to treat = 59), but with the expense of excess intracranial hemorrhage (OR 4.6, 95% CI, 1.8–12.0, number needed to harm = 78), especially in older patients (i.e., >65 years) (OR 3.1, 95% CI, 2.1–5.6, number needed to harm = 11) [27]. The PEITHO trial compared tenecteplase versus a placebo in 1005 intermediate-high-risk PE patients, which showed a decreased incidence of death or hemodynamic decompensation in the tenecteplase group (OR 0.4, 95% CI, 0.2–0.9, p = 0.02) but this was associated with elevated risk of extracranial bleeding (6.3% vs. 1.2%, p = 0.001) and stroke (2.4% vs. 0.1%, p = 0.003) [28]. Based on the findings of this trial, systemic thrombolysis is not recommended among patients with intermediate PE (i.e., without hemodynamic instability).
6.2. Catheter-Directed Thrombolysis
This is a minimally invasive procedure that involves delivering lower doses of thrombolytic therapy locally into the pulmonary vasculature [29]. This can be carried out using ultrasound-assisted catheter-directed thrombolysis (UA-CDT), where ultrasonic pressure waves are emitted along the catheter which improve the delivery of the thrombolytic agent, in vitro or using multi-hole catheters such as Unifuse and Cragg-McNamara (Table 3). This allows for the delivery of a smaller dose over a shorter period. The ULTIMA trial, which enrolled 59 patients with intermediate-risk PE, showed that UA-CDT was superior in reversing the RV/LV ratio at 24 h (mean decrease of 0.3 ± 0.0 vs. 0.0 ± 0.2, p < 0.001) without increasing bleeding complications, compared with systemic anticoagulation alone [30]. The SEATTLE 2 trial was a prospective single-arm multicenter trial of 150 patients that also showed US-CDT decreasing RV dilatation (mean difference, −0.4; p < 0.0001) and reduced pulmonary artery systolic pressure (51.4 mmHg vs. 36.9 mmHg, p < 0.0001) at 48 h post-procedure [31]. No patients developed ICH in the UA-CDT arm in the ULTIMA or SEATTLE 2 trials.

Table 3.
Treatment options for intermediate- and high-risk pulmonary embolus [32].
In the OPTALYSE trial, the investigators attempted to find the optimal dosing of tissue plasminogen activator (tPA) and the delivery duration during UA-CDT. They enrolled 101 patients with intermediate-risk PE and administered four different dose duration variations ranging from 4 mg per lung over two hours to 12 mg per lung over six hours. One patient developed ICH, and thus the trial concluded that shorter durations with smaller tPA doses were associated with improved RV function and a reduced clot burden [33].
The SUNSET sPE trial examined 81 patients with intermediate-risk PE and randomized them to US CDT or standard CDT. This trial found that patients in both treatment arms had similar reductions in thrombus reduction (p = 0.76) [34]. In one large retrospective study of 39,430 patients from the National Inpatient Sample between 2016 and 2020, there was no difference in the incidence of in-hospital mortality (OR 0.75, 95% CI, 0.5–1.1, p = 0.1) and major bleeding between US-CDT and standard CDT [35].
A large meta-analysis of 45 studies, including 18 randomized control trials (RCT) and 28 observational studies including 81,705 patients with intermediate- and high-risk PE (of which 20 studies exclusively included intermediate-risk PE patients), found that compared with anticoagulation, CDT was associated with lower mortality but higher bleeding risk (OR 0.6, 95% CI, 0.4–0.8 and OR 1.8, 95% CI, 1.1–3.1, respectively). CDT was found to have a better safety profile when compared with systemic therapy [36]. The findings were mostly consistent in the subgroup analysis of the intermediate-risk PE patients.
6.3. Catheter-Directed Embolectomy
This is another minimally invasive procedure using larger-bore catheters to extract the thrombus instead of delivering thrombolytic therapy. Catheter-directed embolectomy (CDE) is performed either through suction/aspiration or extraction/disruption. Currently, there are no RCTs comparing CDE to anticoagulation or CDT.
The FLARE study was a single-arm, multicenter, prospective trial analyzing the effectiveness of percutaneous mechanical thrombectomy in treating patients with intermediate-risk PE. The study enrolled 106 patients across 18 U.S. sites and treated patients with the FlowTriever system. The results of the trial revealed that mechanical thrombectomy improved the RV/LV ratio, with minimal major bleeding (mean RV/LV ratio reduction of 0.4, p < 0.0001) at 48 h. Furthermore, the advantages of CDE include immediate thrombus removal, the absence of fibrinolytic complications, and a reduced need for post-procedural critical care [37].
The FLASH registry analyzed 800 PE patients (77% with intermediate-risk PE) from a U.S. national registry to evaluate the safety and effectiveness of mechanical thrombectomy for intermediate- and high-risk PE in a real-world population. A total of 14 major adverse events (1.8%) were observed at 48 h with a 0.8% 30-day all-cause mortality and only three (0.4%) intraprocedural major adverse events. Immediately following thrombectomy, the cardiac index improved by a mean of 0.3 L/min/m2 (mean change of 19.0%, p < 0.0001) and the RV/LV ratio decreased (1.2 ± 0.4–1.0 ± 0.3, p < 0.0001) at 48 h [38].
Indigo is a smaller caliber CDE catheter using high-velocity suction to perform thrombectomy. This device was studied in a single-arm prospective, multicenter study named EXTRACT-PE, which included 119 patients. This study showed a mean RV/LV ratio reduction at 48 h from a baseline of 0.4 (95% 0.4–0.5, p < 0.0001), with a low adverse event rate. The trial did not include patients in shock and thrombolytics were avoided in 98.3% of patients [23].
In an analysis of the Nationwide Readmission Database between the years 2016 and 2019 and including 3216 patients, there was no difference in the incidence of all-cause mortality (OR 1.3, 95% CI, 1.0–1.7), ICH (OR 1.6, 95% CI, 0.8–3.3), and non-ICH major bleeding (OR 1.2, 95% CI, 0.9–1.6) between CDT and CDE [39]. In another analysis of the same database, there was an inverse relationship between a larger procedure volume with CDT or CDE (>12 procedures annually) and in-hospital mortality, length of stay, and cost [40]. The REAL-PE study included >4000 patients treated with US-CDT or mechanical thrombectomy, comparing the risk of bleeding through direct laboratory analysis and transfusion administration documentation which showed an increased risk of bleeding with mechanical thrombectomy (17.3% vs. 12.4%, p = 0.002) [41]. However, the findings should be interpreted in the context of a retrospective observational study, and the reason for the allocation of one therapy versus the other could not be ascertained.
6.4. Surgical Thrombectomy
Surgical thrombectomy is not commonly performed, and mainly reserved for high-risk PE patients who have contraindications to thrombolysis or for unstable patients with systemic thrombolysis despite being in highly experienced centers. Surgical thrombectomy has been linked with high in-hospital mortality in several single-center series [42,43,44]. One meta-analysis consisting of eight observational studies with 1403 patients compared CDT to surgical- and catheter-directed thrombectomy and showed that CDT had lower-in hospital mortality (RR 0.6, 95% CI, 0.4–0.9, p = 0.01), with similar rates of major bleeding (p = 0.6), stroke (p = 0.4), and atrial fibrillation (p = 0.7) [45]. These findings should be considered in light of retrospective observational studies, which are limited by selection and ascertainment biases.
6.5. Mechanical Circulatory Support
Critically ill patients with PE occasionally experience circulatory collapse requiring mechanical circulatory support (MCS). This can be achieved with veno-arterial extracorporeal membrane oxygenation (VA-ECMO). The VA-ECMO oxygenates blood drawn from the right atrium through an external oxygenation membrane and returns it into an artery. This allows complete the bypass of the cardiopulmonary system as a bridge to more permanent therapy. The use of VA-ECMO as a “bridge to recovery” by only treating the patient with anticoagulation is not supported by randomized clinical trial data [4], and is based on single-center experiences. Because patients who received VA-ECMO in these studies were high-risk patients, the mortality was considerably high [46,47]. MCS can be utilized as a bridge to another advanced therapy modality such as percutaneous embolectomy, or surgical embolectomy [48].
7. Selection of Therapy
Given the paucity of high-quality RCT data supporting the use of most of these advanced therapies, there are controversies in the level of recommendation for these therapies between the guidelines (Table 4). The choice of therapy often is related to the institutional expertise and availability of advanced therapies. Pulmonary Embolism Response Teams (PERTs) are multidisciplinary teams of healthcare professionals with expertise related to the management of PE. There have been a number of single-center experiences which have showed that the implementation of PERT is linked with the increased use of advanced therapies (particularly catheter-based interventions), and some of these studies showed improved outcomes after the implementation of PERT [49,50,51,52].

Table 4.
Major recommendations for high-risk PE from 2019 European Society of Cardiology (ESC) [4], 2021 American Heart Association (AHA) [53], and 2018 Pulmonary Embolism Response Team (PERT) [54] guidelines.
8. Future Directions
Future research on the management of PE (particularly intermediate-risk PE) should focus on identifying better risk stratification models, integrating biomarkers, and improved imaging techniques. Articifical intellignece could potentially be trained to improve imaging findings. Since prior trials testing catheter-based interventions have only evaluated surrogate outcomes; there are a number of ongoing trials evaluating the clinical outcomes with these catheter-directed therapies for patients with intermediate- and high-risk PE (Table 5). One trial (Hi-PEITHO) is specifically investigating whether CDT will improve long-term functional outcomes among patients with intermediate-risk PE. Additionally, exploring the implementation of comprehensive care pathways that involve multidisciplinary teams can optimize patient management. Conducting long-term studies on the chronic effects of PE and the development of rehabilitation programs is essential to improve patient quality of life and recovery. Emphasizing patient education and adherence to treatment plans will also play a vital role in reducing recurrence rates and enhancing overall outcomes. Investing in healthcare infrastructure to support these innovations is necessary to ensure broad accessibility and effective implementation.

Table 5.
Ongoing trials studying advanced therapies for PE.
9. Conclusions
PE remains a leading cause of death. Our current risk stratification tools do not reliably predict clinical deterioration especially among intermediate risk patients. The currently available risk stratification tools also poorly predict who may benefit from advanced therapies. Traditionally, systemic thrombolysis has been considered the first-line therapy for high-risk PE based on older RCTs but with the expense of an elevated risk of ICH. Catheter-based interventions have shown promising results in improving surrogate outcomes, and there are several ongoing trials to test their efficacy in improving clinical outcomes.
Funding
This correspondence received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This research received no external funding.
Acknowledgments
This work was supported by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities. We thank Christopher Bray, Katy Robinson, and Vaughan James for their expertise and assistance throughout all aspects of our study and for their help in completing this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.J.; Giri, J.; Duffy, Á.; Jaber, W.A.; Khandhar, S.; Ouriel, K.; Toma, C.; Tu, T.; Horowitz, J.M. Incidence of Mortality and Complications in High-Risk Pulmonary Embolism: A Systematic Review and Meta-Analysis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100548. [Google Scholar] [CrossRef]
- Martin, K.A.; Molsberry, R.; Cuttica, M.J.; Desai, K.R.; Schimmel, D.R.; Khan, S.S. Time Trends in Pulmonary Embolism Mortality Rates in the United States, 1999 to 2018. J. Am. Heart Assoc. 2020, 9, e016784. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Levine, D.A.; Blumberg, N.; Flanders, S.A.; Chopra, V.; Langa, K.M. Triggers of hospitalization for venous thromboembolism. Circulation 2012, 125, 2092–2099. [Google Scholar] [CrossRef]
- Iorio, A.; Kearon, C.; Filippucci, E.; Marcucci, M.; Macura, A.; Pengo, V.; Siragusa, S.; Palareti, G. Risk of Recurrence After a First Episode of Symptomatic Venous Thromboembolism Provoked by a Transient Risk Factor: A Systematic Review. Arch. Intern. Med. 2010, 170, 1710–1716. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.A.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; O’Fallon, W.M.; Petterson, T.M.; Lohse, C.M.; Silverstein, M.D.; Mohr, D.N.; Melton, L.J., III. Relative Impact of Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism: A Population-Based Study. Arch. Intern. Med. 2002, 162, 1245–1248. [Google Scholar] [CrossRef]
- Marques, M.A.; Panico, M.D.B.; Porto, C.L.L.; Milhomens, A.L.M.; Vieira, J.M. Venous thromboembolism prophylaxis on flights. J. Vasc. Bras. 2018, 17, 215–219. [Google Scholar] [CrossRef]
- Stein, P.D.; Beemath, A.; Matta, F.; Weg, J.G.; Yusen, R.D.; Hales, C.A.; Hull, R.D.; Leeper, K.V.; Sostman, H.D.; Tapson, V.F.; et al. Clinical Characteristics of Patients with Acute Pulmonary Embolism. Am. J. Med. 2007, 120, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Svensson, P.J.; Dahlbäck, B.; Lind-Hallden, C.; Hallden, C.; Elf, J. Genetic risk factors for venous thromboembolism. Expert Rev. Hematol. 2020, 13, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W.H.; Heit, J.A.; Clagett, G.P.; Pineo, G.F.; Colwell, C.W.; Anderson, F.A.; Wheeler, H.B. Prevention of venous thromboembolism. Chest 2001, 119, 132S–175S. [Google Scholar] [CrossRef] [PubMed]
- Chopard, R.; Behr, J.; Vidoni, C.; Ecarnot, F.; Meneveau, N. An Update on the Management of Acute High-Risk Pulmonary Embolism. J. Clin. Med. 2022, 11, 4807. [Google Scholar] [CrossRef] [PubMed]
- Coutance, G.; Cauderlier, E.; Ehtisham, J.; Hamon, M.; Hamon, M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: A meta-analysis. Crit. Care 2011, 15, R103. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Rhee, A.R.; Grossman, K.G.; Singh, A.S.A.; Nayar, A.N.; Elbaum, L.E.; Greco, A.G.; Postelnicu, R.P.; Alviar, C.A.; Bangalore, S.B. McConnell’s sign predicts normotensive shock in patients with acute pulmonary embolism. Eur. Heart J. Acute Cardiovasc. Care, 2024; in press. [Google Scholar] [CrossRef]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.M.; Fine, M.J. Derivation and validation of a prognostic model for pulmonary embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 10411046. [Google Scholar] [CrossRef]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D.; et al. Simplification of the Pulmonary Embolism Severity Index for Prognostication in Patients With Acute Symptomatic Pulmonary Embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Ben, S.-Q.; Chen, H.-L.; Ni, S.-S. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: A meta-analysis. Respir. Res. 2012, 13, 111. [Google Scholar] [CrossRef]
- Bangalore, S.; Horowitz, J.M.; Beam, D.; Jaber, W.A.; Khandhar, S.; Toma, C.; Weinberg, M.D.; Mina, B. Prevalence and Predictors of Cardiogenic Shock in Intermediate-Risk Pulmonary Embolism. JACC Cardiovasc. Interv. 2023, 16, 958–972. [Google Scholar] [CrossRef]
- Zhang, R.S.; Yuriditsky, E.; Zhang, P.; Maqsood, M.H.; Amoroso, N.E.; Maldonado, T.S.; Xia, Y.; Horowitz, J.M.; Bangalore, S. Composite Pulmonary Embolism Shock Score and Risk of Adverse Outcomes in Patients with Pulmonary Embolism. Circ. Cardiovasc. Interv. 2024, 17, e014088. [Google Scholar] [CrossRef] [PubMed]
- den Exter, P.L.; van Es, J.; Kroft, L.J.M.; Erkens, P.M.G.; Douma, R.A.; Mos, I.C.M.; Jonkers, G.; Hovens, M.M.C.; Durian, M.F.; ten Cate, H.; et al. Thromboembolic resolution assessed by CT pulmonary angiography after treatment for acute pulmonary embolism. Thromb. Haemost. 2015, 114, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sista, A.K.; Horowitz, J.M.; Tapson, V.F.; Rosenberg, M.; Elder, M.D.; Schiro, B.J.; Dohad, S.; Amoroso, N.E.; Dexter, D.J.; Loh, C.T.; et al. Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc. Interv. 2021, 14, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Hirsch, A.M.; Akaberi, A.; Hernandez, P.; Anderson, D.R.; Wells, P.S.; Rodger, M.A.; Solymoss, S.; Kovacs, M.J.; Rudski, L.; et al. Functional and Exercise Limitations After a First Episode of Pulmonary Embolism: Results of the ELOPE Prospective Cohort Study. Chest 2017, 151, 1058–1068. [Google Scholar] [CrossRef]
- Kahn, S.R.; Akaberi, A.; Granton, J.T.; Anderson, D.R.; Wells, P.S.; Rodger, M.A.; Solymoss, S.; Kovacs, M.J.; Rudski, L.; Shimony, A.; et al. Quality of Life, Dyspnea, and Functional Exercise Capacity Following a First Episode of Pulmonary Embolism: Results of the ELOPE Cohort Study. Am. J. Med. 2017, 130, 990.e9–990.e21. [Google Scholar] [CrossRef]
- Albaghdadi, M.S.; Dudzinski, D.M.; Giordano, N.; Kabrhel, C.; Ghoshhajra, B.; Jaff, M.R.; Weinberg, I.; Baggish, A. Cardiopulmonary Exercise Testing in Patients Following Massive and Submassive Pulmonary Embolism. J. Am. Heart Assoc. 2018, 7, e006841. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: A meta-analysis. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.N.; Devarapally, S.R.; Lee, L.S.; Gupta, N. Catheter-Directed Thrombolysis of Pulmonary Embolism. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK536918/ (accessed on 26 April 2024).
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Management of PE [Internet]. Am. Coll. Cardiol. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2020/01/27/07/42/Management-of-PE (accessed on 25 June 2024).
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Maholic, R.L.; Ross, C.B.; Natarajan, K.; Fong, P.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Jaber, W.; Lacomis, J.; Markel, K.; McDaniel, M.; Rivera-Lebron, B.N.; Ross, C.B.; Sechrist, J.; Toma, C.; Chaer, R.; et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism. JACC Cardiovasc. Interv. 2021, 14, 1364–1373. [Google Scholar] [CrossRef]
- Shatla, I.; Iskandarani, M.E.; Khan, M.Z.; Elkaryoni, A.; Elbadawi, A.; Goel, S.S.; Saad, M.; Balla, S.; Darki, A.; Elgendy, I.Y. Ultrasound-Assisted Versus Standard Catheter-Directed Thrombolysis for Acute Pulmonary Embolism: Insights From National Inpatient Sample. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 35, 101360. [Google Scholar] [CrossRef]
- Zhang, R.S.; Maqsood, M.H.; Sharp, A.S.P.; Postelnicu, R.; Sethi, S.S.; Greco, A.; Alviar, C.; Bangalore, S. Efficacy and Safety of Anticoagulation, Catheter-Directed Thrombolysis, or Systemic Thrombolysis in Acute Pulmonary Embolism. JACC Cardiovasc. Interv. 2023, 16, 2644–2651. [Google Scholar] [CrossRef]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef]
- Toma, C.; Jaber, W.A.; Weinberg, M.D.; Bunte, M.C.; Khandhar, S.; Stegman, B.; Gondi, S.; Chambers, J.; Amin, R.; Leung, D.A.; et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 18, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, R.; Elbadawi, A.; Megaly, M.; Athar, A.; Bharadwaj, A.S.; Prasad, V.; Cameron, S.J.; Weinberg, I.; Mamas, M.A.; Messerli, A.W.; et al. Outcomes with catheter-directed thrombolysis vs. catheter-directed embolectomy among patients with high-risk pulmonary embolism: A nationwide analysis. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, R.; Elbadawi, A.; Megaly, M.; Jaber, W.A.; Cameron, S.J.; Weinberg, I.; Mamas, M.A.; Elgendy, I.Y. Hospital procedural volume and outcomes with catheter-directed intervention for pulmonary embolism: A nationwide analysis. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Ahern, R.; Banerjee, S.; Desai, K.R.; Kadian-Dodov, D.; Webber, E.; Omidvar, S.; Troy, P.; Parikh, S.A. Modern Treatment of Pulmonary Embolism (USCDT vs MT): Results from a Real-World, Big Data Analysis (REAL-PE). J. Soc. Cardiovasc. Angiogr. Interv. 2023, 3, 101192. [Google Scholar] [CrossRef]
- Stulz, P.; Schläpfer, R.; Feer, R.; Habicht, J.; Grädel, E. Decision making in the surgical treatment of massive pulmonary embolism. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 1994, 8, 188–193. [Google Scholar] [CrossRef]
- Aklog, L.; Williams, C.S.; Byrne, J.G.; Goldhaber, S.Z. Acute pulmonary embolectomy: A contemporary approach. Circulation 2002, 105, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Osborne, Z.J.; Rossi, P.; Aucar, J.; Dharamsy, S.; Cook, S.; Wheatley, B. Surgical pulmonary embolectomy in a community hospital. Am. J. Surg. 2014, 207, 337–341. [Google Scholar] [CrossRef]
- Ismayl, M.; Ismayl, A.; Hamadi, D.; Aboeata, A.; Goldsweig, A.M. Catheter-directed thrombolysis versus thrombectomy for submassive and massive pulmonary embolism: A systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2024, 60, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, R.; Rosenfield, K.; Borges, J.; Young, M.N.; Albaghdadi, M.; Rosovsky, R.; Kabrhel, C. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A case series and review of the literature. Perfusion 2019, 34, 22–28. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Parazino, M.; Omar, H.R.; Davis, G.; Guglin, M.; Gurley, J.; Smyth, S. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation 2018, 122, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, D.; Dyk, W.; Wróbel, K.; Biederman, A. Surgical pulmonary embolectomy: State of the art. Kardiochirurgia Torakochirurgia Pol./Pol. J. Cardio-Thorac. Surg. 2023, 20, 111–117. [Google Scholar] [CrossRef]
- Pandya, V.; Chandra, A.A.; Scotti, A.; Assafin, M.; Schenone, A.L.; Latib, A.; Slipczuk, L.; Khaliq, A. Evolution of Pulmonary Embolism Response Teams in the United States: A Review of the Literature. J. Clin. Med. 2024, 13, 3984. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, P.; Gadre, S.K.; Schneider, E.; Renapurkar, R.D.; Gomes, M.; Haddadin, I.; Heresi, G.A.; Tong, M.Z.; Bartholomew, J.R. Impact of Multidisciplinary Pulmonary Embolism Response Team Availability on Management and Outcomes. Am. J. Cardiol. 2019, 124, 1465–1469. [Google Scholar] [CrossRef]
- Wright, C.; Goldenberg, I.; Schleede, S.; McNitt, S.; Gosev, I.; Elbadawi, A.; Pietropaoli, A.; Barrus, B.; Chen, Y.L.; Mazzillo, J.; et al. Effect of a Multidisciplinary Pulmonary Embolism Response Team on Patient Mortality. Am. J. Cardiol. 2021, 161, 102–107. [Google Scholar] [CrossRef]
- Rosovsky, R.; Chang, Y.; Rosenfield, K.; Channick, R.; Jaff, M.R.; Weinberg, I.; Sundt, T.; Witkin, A.; Rodriguez-Lopez, J.; Parry, B.A.; et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J. Thromb. Thrombolysis 2019, 47, 31–40. [Google Scholar] [CrossRef]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lebron, B.; McDaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin. Appl. Thromb. 2019, 25, 1076029619853037. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).