Abstract
Objective: This meta-analysis aims to quantitatively summarize current data on various potential risk factors of velamentous cord insertion (VCI). A better understanding of these risk factors could enhance prenatal identification both in settings with routine screening and in those where universal screening for cord insertion anomalies is not yet recommended. Methods: A systematic search was conducted in MEDLINE, Cochrane Library, and Scopus from their inception until 7 February 2024. Eligible studies included observational studies of singleton pregnancies with VCI, identified either prenatally or postnatally, compared with pregnancies with central or eccentric cord insertion. Analyses were performed using DerSimonian and Laird random-effects models, with outcomes reported as risk ratios (RR) or mean differences with 95% confidence intervals (CI). Results: In total, 14 cohort and 4 case-control studies were included, reporting on 952,163 singleton pregnancies. Based on the cohort studies, the overall prevalence of VCI among singleton pregnancies was calculated to be 1.54%. The risk of VCI was significantly higher among pregnancies conceived using assisted reproductive technology (RR, 2.32; 95% CI: 1.77–3.05), nulliparous women (RR, 1.21; 95% CI: 1.15–1.28), women who smoked (RR, 1.14; 95% CI: 1.08–1.19), and pregnancies diagnosed with placenta previa (RR, 3.60; 95% CI: 3.04–4.28). Conclusions: This meta-analysis identified assisted reproductive technology, nulliparity, smoking, and placenta previa as significant risk factors of VCI among singleton pregnancies. These findings could inform screening policies in settings where universal screening for cord insertion is not routinely performed, suggesting a targeted approach for women with these specific risk factors.
1. Introduction
In a velamentous cord insertion (VCI), the umbilical cord inserts into the fetal membranes (between the amnion and the chorion) away from the placental margin, and the vessels traverse between these membranes before reaching the placenta, as depicted in Figure 1 [1]. In cases of VCI, there may be an absence of the protective effect of Wharton’s jelly, normally present around the vessels [2]. Placentas with non-central insertions may be less effective in supporting fetal growth despite their normal or even increased size. This decreased placental efficiency may be explained by a relative reduction in the chorionic vascular density of the placenta, as the cord is displaced from the center [3]. The reported occurrence of VCI among singleton pregnancies is 1.4%, and it was associated with several adverse perinatal outcomes including stillbirth, pre-eclampsia, placental abruption, small-for-gestational-age neonates, preterm delivery, emergency cesarean section (CS), reduced Apgar score and higher admission rate to the neonatal intensive care unit [4].

Figure 1.
Ultrasound image depicting a velamentous cord insertion.
These findings, along with the feasibility of antenatal recognition of VCI, underscore the importance of a more systematic diagnostic approach; studies have shown that second-trimester sonographic identification of VCI is accurate, with an exceptionally high specificity of close to 100% but a lower sensitivity of about 70% [5]. Current recommendations vary regarding the need to identify a VCI antenatally. Thus, the American Institute of Ultrasound in Medicine recommends documenting abnormal cord insertions [6], whereas the International Society of Ultrasound in Obstetrics and Gynecology advises that an umbilical cord insertion assessment during mid-trimester scan is optional; however, an incidental finding of VCI should be documented [7].
A comprehensive understanding of risk factors for VCI may improve prenatal identification in settings with routine screening but also in settings where universal screening for cord insertion anomalies is not yet recommended and a targeted approach for women with specific risk factors should at least be considered. Published data have identified a variety of potential risk factors for VCI in singleton pregnancies, i.e., advanced maternal age, previous history of CS, Caucasian ethnicity, use of assisted reproductive technology (ART), smoking, placenta previa, nulliparity and chronic hypertension [8,9,10]. This meta-analysis aimed to conduct a rigorous evaluation and statistical analysis of the current evidence on possible risk factors for VCI.
2. Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [11] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [12] and was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with the protocol number: CRD42024512296. Given its nature of synthesizing data from previously published literature, the study was exempt from the need for ethical approval and patient consent.
2.1. Search Strategy
The research question guiding this systematic search was, “Which population characteristics could serve as indicators of increased risk for VCI in singleton pregnancies”. We crafted a search strategy employing keywords such as “umbilical cord insertion”, “cord insertion”, “insertion of the cord”, “placental cord insertion”, “velamentous”, “abnormal” and “aberrant”. The details of the search strategy can be found in the Supplementary Materials. We searched MEDLINE, Scopus and Cochrane databases from their inception until 7 February 2024. The identified records were managed using Rayyan (Rayyan Systems Inc., Cambridge, MA, USA), a web-based reference management tool. Further, we examined references from relevant articles and conducted manual searches online to identify additional studies. After removing duplicates, we screened titles and abstracts to exclude studies not pertinent to our question and then thoroughly reviewed the full texts of the remaining articles to determine their inclusion. This process was independently carried out by two reviewers (A.S. and A.G, both doctors) blind to each other’s selections, with disagreements resolved through discussion or, if necessary, by a third reviewer (I.T., biostatistician).
2.2. Selection Criteria
Observational studies written in English, examining possible risk factors and population characteristics in singleton pregnancies identified with VCI either prenatally or following delivery, were considered eligible. The comparison group included pregnancies with central/eccentric cord insertion (CCI). In studies where the control groups included pregnancies with all types of non-velamentous umbilical cord insertions, adjustments were made, when possible, to ensure comparisons were exclusively with pregnancies having CCI. If such adjustments were not feasible, the studies were excluded. When the same database was utilized across two or more studies covering overlapping periods, we exclusively utilized data from the study encompassing the largest population. Raw data on perinatal outcomes were required. Abstracts and unpublished studies were not included.
2.3. Data Extraction
A standardized data collection template was prepared ahead of the study selection. This template captured study characteristics such as author, publication year, journal, study location, methodology, criteria for inclusion and exclusion, timing and definition of VCI diagnosis, study demographics and investigated risk factors. A second part of the template was dedicated to collecting data on predetermined outcomes, including raw data and, where available, adjusted odds ratios or adjusted risk ratios. Our protocol specified that outcomes reported in at least three studies would be considered for analysis, even if not initially outlined. We reached out to authors for missing data or clarifications and selected the most comprehensive report for studies with multiple publications on the same cohort.
2.4. Outcomes of Interest
Outcomes of interest included every risk factor reported by three or more studies, including ART, maternal age, prior CS, smoking, placenta previa, nulliparity, chronic hypertension and any other possible risk factor with adequate data as stipulated by our protocol.
2.5. Quality and Bias Assessment
The quality of the included studies was independently assessed by two researchers (A.S. and A.G) using the Newcastle−Ottawa scale [13], which evaluates the selection of the study groups, comparability of the groups, and ascertainment of the outcome/risk factor, employing a star system that assigns up to 9 points for high quality. Additionally, the Quality In Prognosis Studies (QUIPS) tool [14] was used to evaluate the risk of bias across six domains: study participation, attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis/reporting, with studies rated on a three-point scale (low, moderate, high). Discrepancies in the assessment of quality or bias were reviewed and resolved by a third reviewer (I.T.).
2.6. Statistical Analysis
In our primary analysis, the cumulative raw data were analyzed to calculate the various risk factors’ effect on the VCI prevalence. This data synthesis involved computing effect sizes and their 95% confidence intervals (CI) via Review Manager software, version 5.4.1. We determined risk ratios (RR) for binary outcomes using the Mantel–Haenszel technique and mean differences (MD) for continuous variables through the inverse variance method. Due to the significant variability in observational studies, we followed the Cochrane Handbook’s recommendation to employ the DerSimonian and Laird random-effects model as the default analytical method. To evaluate the heterogeneity of the included studies, we utilized two approaches. The I2 statistic was employed to quantify the proportion of the total variance in the observed effect sizes that was due to differences between studies rather than chance. I2 values of up to 40% might be unimportant, 30–60% moderate, 50–90% substantial and 75–100% considerable [15]. Additionally, we applied the Cochran Q test to examine the homogeneity of the effect sizes across studies, with a p-value threshold of 0.10 for statistical significance. In R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) [16], the package meta [17] was employed to generate the funnel plot and the package dmetar [18] to perform the Egger’s test. These methods were used to assess publication bias only for the outcome with the higher number of published studies.
2.7. Sensitivity Analysis
As per our established methodology, we carried out a sensitivity analysis that only included cases identified prenatally. This was based upon the premise that our end goal is a better and more focused prenatal diagnosis of VCI. Furthermore, we conducted another sensitivity review that considered only those studies deemed as having a low or moderate risk of bias, as per the QUIPS tool criteria. The objective here was to filter the data less likely to be affected by bias and verify if these refined results would align with our initial findings. Notably, a sensitivity analysis was only deemed feasible if data from three or more studies were available for evaluation.
3. Results
Initially, 1559 records were identified from the Medline, Scopus, and Cochrane databases, while other methods like Web search and citation searching contributed nine additional records. After removing 69 duplicates, we screened 1490 records and excluded 1311 for various reasons. Upon assessing for eligibility, we evaluated 175 full texts, and 18 studies met the eligibility criteria for the review. The other search methods resulted in nine relevant reports, none of which was eligible (Figure 2). The two reviewers achieved an excellent coefficient of agreement on article selection (Cohen’s kappa, 0.932), resulting in the retention of 14 cohort [8,9,10,19,20,21,22,23,24,25,26,27,28,29] and 4 case-control studies [30,31,32,33] for the final analysis. The characteristics of the included studies are presented in Table 1.
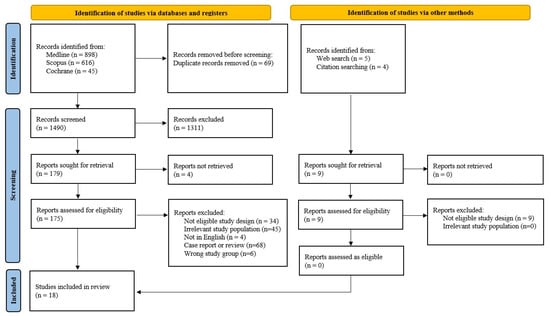
Figure 2.
Study selection flow diagram.

Table 1.
Characteristics of the included studies.
3.1. Quality and Risk of Bias Assessment of the Studies
The quality of the studies assessed using the Newcastle–Ottawa Scale varied, with six achieving the top score of nine stars [8,9,19,20,24,26], indicating excellent methodology. Five studies earned eight stars [23,25,27,29,32] and five received seven stars [10,21,22,28,33], reflecting solid research approaches, while two studies scored six stars [30,31], suggesting some methodological concerns. The commonest weakness of the studies was the comparability category. No other significant deficits were noted (Table 2). A risk of bias visualization according to QUIPS was constructed for each study next to every forest plot. The QUIPS tool’s domains were each assigned a corresponding letter, ranging from A for study participation, B for study attrition, C for the measurement of prognostic factors, D for outcome measurement, E for study confound, and F for statistical analysis and reporting. This systematic approach facilitated a comprehensive evaluation of each study’s methodological quality and potential biases.

Table 2.
Quality assessment of the included studies according to the Newcastle.
3.2. Raw Data Analysis
We included 14 relevant cohort studies and, based on their data (951,343 singleton pregnancies), the prevalence of VCI was calculated to be 1.54% (95% CI 1.52% to 1.57%).
3.3. Risk Factors’ Analyses
In a composite analysis of eight cohort [9,10,20,24,25,26,27,29] and three case-control [30,32,33] studies, 692 cases of VCI were reported among the ART group, accounting for approximately 3.48% of the pregnancies, while the control group had 13,800 VCI cases, representing approximately 1.52% of the pregnancies. The occurrence of VCI in pregnancies with ART was significantly higher than those in the control group, with an RR of 2.32 (95% CI 1.77 to 3.05). Substantial heterogeneity was observed across the studies (p = 0.007; I2 = 59%) (Figure 3).
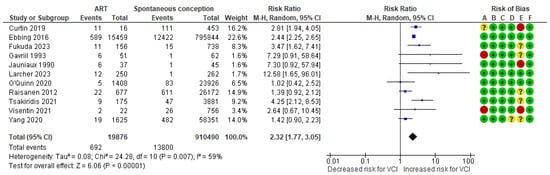
Figure 3.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to the use of ART. Abbreviations: ART, assisted reproductive technology; CI, confidence interval; M−H, Mantel−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of six cohort [9,10,21,22,25,26] and two case-control [30,31] studies, the mean maternal age was assessed. The mean difference in maternal age between the VCI and CCI groups was +0.40 (95% CI −0.09 to 0.90)—not significantly different. There was low-to-moderate heterogeneity observed across the studies (p = 0.18; I2 = 31%) (Figure 4).

Figure 4.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to mean maternal age. Abbreviations: CCI, central/eccentric cord insertion; CI, confidence interval; IV, weighted mean difference; SD, standard deviation; VCI, velamentous cord insertion.
In a composite analysis of six cohort [9,10,20,24,25,26] and two case-control [30,31] studies, 6682 cases of VCI were reported among the pregnancies of nulliparous women, accounting for approximately 1.73% of the pregnancies, while the control group had 7818 cases, representing approximately 1.44% of the pregnancies. The occurrence of VCI among nulliparous women was significantly higher compared to those in the multiparous group, with an RR of 1.21 (95% CI 1.15 to 1.28). There was low heterogeneity observed across the studies (p = 0.39; I2 = 6%) (Figure 5).
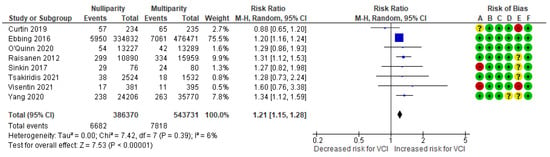
Figure 5.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to parity. Abbreviations: CI, confidence interval; M−H, Mantel−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of six cohort [8,9,23,24,25,26] and one case-control [30] study, 1941 cases of VCI were reported among the group of smokers, accounting for approximately 1.87% of the pregnancies, while the control group had 7878 cases, representing approximately 1.57% of the pregnancies. The occurrence of VCI among women who smoked was significantly different from those in the non-smoking group, with an RR of 1.14 (95% CI 1.08 to 1.19). No heterogeneity was observed across the studies (p = 0.50; I2 = 0%) (Figure 6).
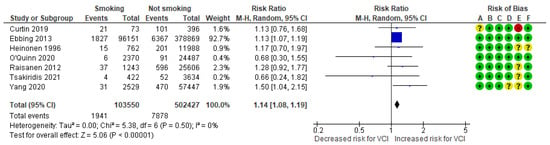
Figure 6.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to smoking. Abbreviations: CI, confidence interval; M−H, Mantel−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of four cohort [19,23,24,26] and one case-control study [31], 2635 cases with VCI were reported among the group with prior CS, accounting for approximately 2.39% of the pregnancies, while the control group had 10,175 cases, representing approximately 1.51% of the pregnancies. The occurrence of VCI in pregnancies with prior CS was not significantly different from those in the control group, with an RR of 0.92 (95% CI 0.58 to 1.47). There was considerable heterogeneity observed across the studies (p < 0.001; I2 = 92%) (Figure 7).
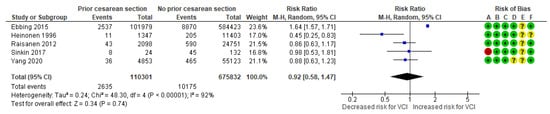
Figure 7.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to having a prior cesarean section. Abbreviations: CI, confidence interval; M−H, Mantel−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of five cohort studies [8,10,23,24,26], 125 cases of VCI were reported among the pregnancies complicated by placenta previa, accounting for approximately 5.59% of the pregnancies, while the control group had 10,753 cases of VCI, representing approximately 1.60% of the pregnancies. The occurrence of VCI in pregnancies with placenta previa was significantly different from those in the control group, with an RR of 3.60 (95% CI 3.04 to 4.28). No heterogeneity was observed across the studies (p = 0.54; I2 = 0%) (Figure 8).
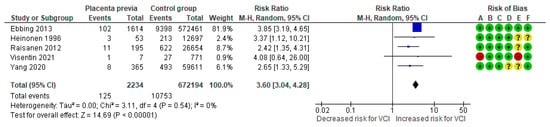
Figure 8.
Forest plot of risk for VCI in singleton pregnancies relative to placenta previa. Abbreviations: CI, confidence interval; M−H, Mantel−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of three cohort [8,25,28] and one case-control study [31], 78 cases of VCI were reported among the women diagnosed with chronic hypertension, accounting for approximately 2.23% of the pregnancies, while the control group had 9594 cases, representing approximately 1.59% of the pregnancies. The occurrence of VCI in pregnancies with chronic hypertension was not significantly different from those in the control group, with an RR of 1.488 (95% CI 0.998 to 2.219). There was low heterogeneity observed across the studies (p = 0.29; I2 = 20%) (Figure 9).
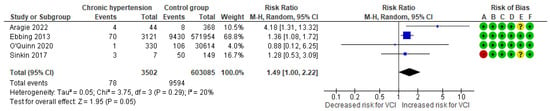
Figure 9.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to chronic hypertension. Abbreviations: CI, confidence interval; M−H, Mante−Haenszel method; VCI, velamentous cord insertion.
In a composite analysis of three cohort studies [8,24,25], 91 cases of VCI were reported among pregnancies with pre-existing diabetes mellitus, accounting for approximately 2.03% of the pregnancies, while the control group had 10,137 cases, representing approximately 1.61% of the pregnancies. The occurrence of VCI in pregnancies of women with a diagnosis of diabetes was not significantly different from those in the control group, with an RR of 1.23 (95% CI 0.84 to 1.79). There was low heterogeneity observed across the studies (p = 0.31; I2 = 14%) (Figure 10).
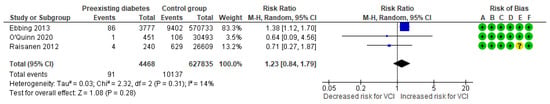
Figure 10.
Forest plot demonstrating the risk for VCI in singleton pregnancies relative to pre-existing diabetes. Abbreviations: CI, confidence interval; M−H, Mante−Haenszel method; VCI, velamentous cord insertion.
The cumulative results of our primary analysis are compiled and displayed in Table 3.

Table 3.
Results of the meta-analysis regarding risk factors of velamentous cord insertion in singleton pregnancies.
3.4. Sensitivity Analyses Regarding Prenatal Diagnosis and Risk of Bias
Limiting our analysis to studies that provided data on prenatal diagnosis of umbilical cord insertion, we examined 18 studies, with 4 involving prenatal identification of VCI, and we could sufficiently assess three risk factors: ART, mean maternal age and nulliparity. The updated findings were consistent with the main results (Table 4 and Supplementary Figures S1–S3).

Table 4.
Confidence intervals from all the analyses performed.
Subsequently, the studies identified as a high risk of bias were removed. Within the scope of ten analyses, seven were affected by the presence of high-risk bias studies. Following their exclusion, six out of seven analyses maintained comparable results to the initial findings. The updated findings were consistent with the main results (Table 4 and Supplementary Figures S4–S10).
3.5. Publication Bias
The risk factor with the most included studies was ART, which was tested for publication bias. Neither the funnel plot nor the Egger’s test demonstrated any indication of publication bias (Figure 11).

Figure 11.
Funnel plot and Egger’s test regarding our most investigated outcome—assisted reproductive technology.
4. Discussion
4.1. Principal Findings
Our analysis found that first, the reported prevalence of VCI in singleton pregnancies is 1.54%, and second, the factors associated with an increased risk of VCI include ART, nulliparity, smoking and placenta previa.
4.2. Interpretation of the Findings
The largest investigated population was in the ART-VCI analysis, which incorporated 11 studies. The analysis demonstrated that ART is associated with a two-fold higher risk for VCI among singleton pregnancies and this association persisted in cases prenatally diagnosed with VCI and after excluding studies at high risk of bias. Our findings are consistent with those of a recent meta-analysis, which reported an OR of 2.14; however, this study included twin gestations in the analysis and the control group was broadly defined [34]. Our results support earlier epidemiological findings that pregnancies conceived via ART are associated with a higher incidence of umbilico-placental abnormalities [35,36,37]. The mechanism that ART could disrupt placentation has not been established; nevertheless, interventions such as controlled ovarian hyperstimulation, intrauterine insemination, gamete or embryo freezing, in-vitro fertilization, embryo culture, cell biopsy and blastocyst or embryo transfer may exercise oxidative, thermal, and mechanical stresses, and changes in DNA methylation that could alter the natural biological processes of reproduction [38,39]. Finally, related surgical procedures, such as septum excision, myomectomy, and other surgical treatments of uterine anomalies, may also contribute to the elevated risk of VCI, underscoring the need for further investigation in this area.
The analysis of maternal age in relation to the risk of VCI encompassed eight studies and revealed no significant increase in risk; only one study that categorized maternal age found that women aged over 35 years exhibited a higher risk of developing VCI (RR, 1.61) [23]. Moreover, two additional studies that were excluded from our meta-analysis due to the inclusion of cases with marginal cord insertion in the control group similarly identified maternal age above 35 years as a risk factor for VCI [40,41]. These findings suggest that the relationship between maternal age and VCI may be more nuanced, with significant risks manifesting particularly after the age of 35. This association could be partially attributed to the increased utilization of ART among this age group.
Nulliparity was identified as a significant risk factor for VCI, even when focusing on prenatally diagnosed pregnancies and studies with low risk of bias. While most small studies did not report a statistically significant association between nulliparity and VCI [9,10,25,30,31], the three larger studies [20,24,26], which made up over 90% of the pooled data, showed a strong association. No relevant studies explaining the pathophysiological cause were found, but we hypothesize that the lack of physiological adaptations that occur in the uterus and placenta during subsequent pregnancies may be a plausible explanation.
Regarding smoking, although most individual studies did not establish a significant relationship with VCI, the aggregated analysis indicated a 14% increased risk of VCI among smokers. Maternal smoking has been documented to adversely impact both the local immune response and microcirculation within decidual tissues [42]. It is conceivable that these alterations in the decidual environment during the implantation and the embryogenesis phase may play a role in the formation of VCI. A study of 83,708 women utilizing multiple regression models observed that exposure to fine particulate matter was positively associated with VCI and described two possible mechanisms: ischemia of the endometrium and intrauterine inflammation [43].
Prior CS was not associated with VCI in our meta-analysis. It seems that contrary to the low placental implantation, which is strongly associated with prior CS, abnormal cord insertion is not associated with them. However, placenta previa was identified as a significant risk factor for VCI, increasing its incidence fourfold [44].
Finally, no association was detected with chronic hypertension or pre-existing diabetes and VCI. However, it is noteworthy that chronic hypertension had a high RR of 1.49, indicating a potential 50% increase in the risk of VCI. The marginal lack of statistical significance is likely due to the sample size and the use of random effect models. Therefore, additional data are required to make a definitive conclusion about this matter.
4.3. Clinical and Research Implications
Currently, there is no consensus on the usefulness of universal screening for cord insertion anomalies. Therefore, our findings on the risk factors for VCI may inform the development of a more targeted screening approach for women exhibiting these risk factors. Given that isolated VCI is a primary risk factor for various adverse perinatal outcomes, including stillbirth [4], while also being the main risk factor for vasa previa [45]—a condition associated with significant perinatal mortality if prenatally undiagnosed [46] but preventable if diagnosed [47]—the importance of identifying VCI cannot be overstated [1,48]. Furthermore, recent studies have linked VCI with a twofold increased risk of cerebral palsy, suggesting that early detection of VCI could be crucial in identifying fetuses at a higher risk for this condition [49].
4.4. Strengths and Limitations
Our study’s main strength lies in its comprehensive design, which allowed us to include numerous studies and explore a wide array of possible risk factors. We maintained strict selection criteria, which led to more reliable estimates of effects and potentially reduced variability among the included studies. Our focus was to provide additional information on the prenatal diagnosis of VCI. To this end, our sensitivity analysis specifically targeting pregnancies with prenatal diagnosis of VCI may enhance the broader applicability of our findings.
The primary limitation of our meta-analysis stems from the nature of the included studies, all of which were observational, including case-control designs. Additionally, most of these studies focused primarily on the perinatal outcomes of VCI rather than investigating risk factors, making them susceptible to selection and recall biases. Furthermore, we were unable to examine the association between prenatally diagnosed VCI and all its risk factors, as not every study provided the necessary data. A further limitation is that in the studies with prenatally diagnosed cases, there was no systematic postnatal confirmation. Finally, none of the studies reported adjusted effect measures, preventing us from accounting for significant confounding variables.
5. Conclusions
This meta-analysis identified ART, nulliparity, smoking and placenta previa as significant risk factors for VCI. These findings may assist the screening policy in settings where cord insertion is not universally offered. Additionally, this may enhance the antenatal detection of vasa previa, a condition that poses significant risks to pregnancies, as VCI is the primary risk factor for its development. Furthermore, the findings may also induce further high-quality research that addresses potential confounding variables to substantiate these associations. The exploration into the pathophysiological mechanisms underlying these relationships is imperative to enhance our understanding and further guide obstetric policies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13185551/s1, Figure S1: Forest plot demonstrating the risk for prenatally diagnosed VCI in singleton pregnancies relative to the use of ART; Figure S2: Forest plot demonstrating the risk for prenatally diagnosed VCI in singleton pregnancies relative to mean maternal age; Figure S3: Forest plot demonstrating the risk for prenatally diagnosed VCI in singleton pregnancies relative to parity; Figure S4: Risk of bias sensitivity analysis regarding ART—VCI association in singleton pregnancies; Figure S5: Risk of bias sensitivity analysis regarding mean maternal age—VCI association in singleton pregnancies; Figure S6: Risk of bias sensitivity analysis regarding nulliparity—VCI association in singleton pregnancies; Figure S7: Risk of bias sensitivity analysis regarding smoking—VCI association in singleton pregnancies; Figure S8: Risk of bias sensitivity analysis regarding prior cesarean section—VCI association in singleton pregnancies; Figure S9: Risk of bias sensitivity analysis regarding placenta previa—VCI association in singleton pregnancies; Figure S10: Risk of bias sensitivity analysis regarding chronic hypertension—VCI association in singleton pregnancies.
Author Contributions
Conceptualization, I.T. and T.D.; methodology, A.S., I.T. and M.M.G.; data collection, A.S., A.G. and I.T.; software, A.S.; meta-analysis, A.S. and A.G.; writing—original draft preparation, A.S. and I.T.; writing—review and editing, C.D.P.M., M.M.G., P.C. and T.D.; supervision, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Given its nature of synthesizing data from previously published literature, the study was exempt from the need for ethical approval and patient consent.
Informed Consent Statement
Given its nature of synthesizing data from previously published literature, the study was exempt from the need for ethical approval and patient consent.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ART | assisted reproductive technology |
| CCI | central/eccentric cord insertion |
| CI | confidence interval |
| CS | cesarean section |
| MD | mean difference |
| QUIPS | Quality In Prognosis Studies |
| RR | risk ratio |
| VCI | velamentous cord insertion |
References
- Jauniaux, E.; Ebbing, C.; Oyelese, Y.; Maymon, R.; Prefumo, F.; Bhide, A. European association of perinatal medicine (EAPM) position statement: Screening, diagnosis and management of congenital anomalies of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 298, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sherer, D.M.; Al-Haddad, S.; Cheng, R.; Dalloul, M. Current Perspectives of Prenatal Sonography of Umbilical Cord Morphology. Int. J. Women’s Health 2021, 13, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Yampolsky, M.; Salafia, C.M.; Shlakhter, O.; Haas, D.; Eucker, B.; Thorp, J. Centrality of the umbilical cord insertion in a human placenta influences the placental efficiency. Placenta 2009, 30, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Siargkas, A.; Tsakiridis, I.; Pachi, C.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Impact of velamentous cord insertion on perinatal outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100812. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Hughes, A.; Bobrowska, A.; Visintin, C.; Attilakos, G.; Marshall, J. Velamentous cord insertion: Results from a rapid review of incidence, risk factors, adverse outcomes and screening. Syst. Rev. 2020, 9, 147. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine. AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J. Ultrasound Med. 2013, 32, 1083–1101. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Sotiriadis, A.; Odibo, A.; Khalil, A.; D’Antonio, F.; Feltovich, H.; Salomon, L.J.; Sheehan, P.; Napolitano, R.; Berghella, V.; et al. ISUOG Practice Guidelines: Role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 60, 435–456. [Google Scholar] [CrossRef]
- Ebbing, C.; Kiserud, T.; Johnsen, S.L.; Albrechtsen, S.; Rasmussen, S. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: A population-based study of 634,741 pregnancies. PLoS ONE 2013, 8, e70380. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Dagklis, T.; Athanasiadis, A.; Dinas, K.; Sotiriadis, A. Impact of Marginal and Velamentous Cord Insertion on Uterine Artery Doppler Indices, Fetal Growth, and Preeclampsia. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2022, 41, 2011–2018. [Google Scholar] [CrossRef]
- Visentin, S.; Londero, A.P.; Santoro, L.; Pizzi, S.; Andolfatto, M.; Venturini, M.; Saraggi, D.; Coati, I.; Sacchi, D.; Rugge, M.; et al. Abnormal umbilical cord insertions in singleton deliveries: Placental histology and neonatal outcomes. J. Clin. Pathol. 2022, 75, 751–758. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. [Google Scholar]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Cochrane: Chichester, UK, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 28 May 2024).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. -Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. dmetar: Companion R Package for the Guide ‘Doing Meta-Analysis in R’. R Package Version 0.0.9000. 2019. Available online: http://dmetar.protectlab.org/ (accessed on 28 May 2024).
- Ebbing, C.; Kiserud, T.; Johnsen, S.L.; Albrechtsen, S.; Rasmussen, S. Third stage of labor risks in velamentous and marginal cord insertion: A population-based study. Acta Obstet. Et Gynecol. Scand. 2015, 94, 878–883. [Google Scholar] [CrossRef]
- Ebbing, C.; Johnsen, S.L.; Albrechtsen, S.; Sunde, I.D.; Vekseth, C.; Rasmussen, S. Velamentous or marginal cord insertion and the risk of spontaneous preterm birth, prelabor rupture of the membranes, and anomalous cord length, a population-based study. Acta Obstet. Et Gynecol. Scand. 2017, 96, 78–85. [Google Scholar] [CrossRef]
- Hasegawa, J.; Matsuoka, R.; Ichizuka, K.; Kotani, M.; Nakamura, M.; Mikoshiba, T.; Sekizawa, A.; Okai, T. Atypical variable deceleration in the first stage of labor is a characteristic fetal heart-rate pattern for velamentous cord insertion and hypercoiled cord. J. Obstet. Gynaecol. Res. 2009, 35, 35–39. [Google Scholar] [CrossRef]
- Hasegawa, J.; Matsuoka, R.; Ichizuka, K.; Sekizawa, A.; Farina, A.; Okai, T. Velamentous cord insertion into the lower third of the uterus is associated with intrapartum fetal heart rate abnormalities. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2006, 27, 425–429. [Google Scholar] [CrossRef]
- Heinonen, S.; Ryynänen, M.; Kirkinen, P.; Saarikoski, S. Perinatal diagnostic evaluation of velamentous umbilical cord insertion: Clinical, Doppler, and ultrasonic findings. Obstet. Gynecol. 1996, 87, 112–117. [Google Scholar] [CrossRef]
- Räisänen, S.; Georgiadis, L.; Harju, M.; Keski-Nisula, L.; Heinonen, S. Risk factors and adverse pregnancy outcomes among births affected by velamentous umbilical cord insertion: A retrospective population-based register study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 231–234. [Google Scholar] [CrossRef]
- O’Quinn, C.; Cooper, S.; Tang, S.; Wood, S. Antenatal Diagnosis of Marginal and Velamentous Placental Cord Insertion and Pregnancy Outcomes. Obs. Gynecol 2020, 135, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, Y.; Li, M.; Li, W.; Li, X.; Zhang, X.; Wang, R.; Zhang, J.; Zhou, F.; Yang, Q.; et al. Clinical features of velamentous umbilical cord insertion and vasa previa: A retrospective analysis based on 501 cases. Medicine 2020, 99, e23166. [Google Scholar] [CrossRef] [PubMed]
- Larcher, L.; Jauniaux, E.; Lenzi, J.; Ragnedda, R.; Morano, D.; Valeriani, M.; Michelli, G.; Farina, A.; Contro, E. Ultrasound diagnosis of placental and umbilical cord anomalies in singleton pregnancies resulting from in-vitro fertilization. Placenta 2023, 131, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Aragie, H.; Kibret, A.A.; Teshager, N.W.; Adugna, D.G. Velamentous cord insertion at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Clin. Epidemiol. Glob. Health 2022, 18, 101180. [Google Scholar] [CrossRef]
- Fukuda, E.; Hamuro, A.; Kitada, K.; Kurihara, Y.; Tahara, M.; Misugi, T.; Nakano, A.; Tamaue, M.; Shinomiya, S.; Yoshida, H.; et al. The Impact of Assisted Reproductive Technology on Umbilical Cord Insertion: Increased Risk of Velamentous Cord Insertion in Singleton Pregnancies Conceived through ICSI. Medicina 2023, 59, 1715. [Google Scholar] [CrossRef]
- Curtin, W.M.; Hill, J.M.; Millington, K.A.; Hamidi, O.P.; Rasiah, S.S.; Ural, S.H. Accuracy of fetal anatomy survey in the diagnosis of velamentous cord insertion: A case-control study. Int. J. Women’s Health 2019, 11, 169–176. [Google Scholar] [CrossRef]
- Sinkin, J.A.; Craig, W.Y.; Jones, M.; Pinette, M.G.; Wax, J.R. Perinatal Outcomes Associated With Isolated Velamentous Cord Insertion in Singleton and Twin Pregnancies. J. Ultrasound Med. 2018, 37, 471–478. [Google Scholar] [CrossRef]
- Jauniaux, E.; Englert, Y.; Vanesse, M.; Hiden, M.; Wilkin, P. Pathologic features of placentas from singleton pregnancies obtained by in vitro fertilization and embryo transfer. Obstet. Gynecol. 1990, 76, 61–64. [Google Scholar]
- Gavriil, P.; Jauniaux, E.; Leroy, F. Pathologic examination of placentas from singleton and twin pregnancies obtained after in vitro fertilization and embryo transfer. Pediatr. Pathol. 1993, 13, 453–462. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Ueda, Y.; Matsuzaki, S.; Nagase, Y.; Kakuda, M.; Lee, M.; Maeda, M.; Kurahashi, H.; Hayashida, H.; Hisa, T.; et al. Assisted Reproductive Technique and Abnormal Cord Insertion: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1722. [Google Scholar] [CrossRef]
- Yanaihara, A.; Hatakeyama, S.; Ohgi, S.; Motomura, K.; Taniguchi, R.; Hirano, A.; Takenaka, S.; Yanaihara, T. Difference in the size of the placenta and umbilical cord between women with natural pregnancy and those with IVF pregnancy. J. Assist. Reprod. Genet. 2018, 35, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, L.; Kok, N.; Limpens, J.; Derks, J.B.; de Graaf, I.M.; Mol, B.W.; Pajkrt, E. Systematic review of accuracy of ultrasound in the diagnosis of vasa previa. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Konishi, K.; Wada, K.; Tamura, T.; Goto, Y.; Koda, S.; Mizuta, F.; Iwasa, S. Maternal Acrylamide Intake during Pregnancy and Sex Hormone Levels in Maternal and Umbilical Cord Blood and Birth Size of Offspring. Nutr. Cancer 2019, 71, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Vrooman, L.A.; Xin, F.; Bartolomei, M.S. Morphologic and molecular changes in the placenta: What we can learn from environmental exposures. Fertil. Steril. 2016, 106, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Kubonoya, K.; Yamaguchi, T. Incidence and risk factors for velamentous umbilical cord insertion in singleton pregnancies after assisted reproductive technology. J. Obstet. Gynaecol. Res. 2021, 47, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Esakoff, T.F.; Cheng, Y.W.; Snowden, J.M.; Tran, S.H.; Shaffer, B.L.; Caughey, A.B. Velamentous cord insertion: Is it associated with adverse perinatal outcomes? J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2015, 28, 409–412. [Google Scholar] [CrossRef]
- Eddleman, K.A.; Lockwood, C.J.; Berkowitz, G.S.; Lapinski, R.H.; Berkowitz, R.L. Clinical significance and sonographic diagnosis of velamentous umbilical cord insertion. Am. J. Perinatol. 1992, 9, 123–126. [Google Scholar] [CrossRef]
- Prins, J.R.; Hylkema, M.N.; Erwich, J.J.; Huitema, S.; Dekkema, G.J.; Dijkstra, F.E.; Faas, M.M.; Melgert, B.N. Smoking during pregnancy influences the maternal immune response in mice and humans. Am. J. Obstet. Gynecol. 2012, 207, 76.e1–76.e14. [Google Scholar] [CrossRef]
- Michikawa, T.; Morokuma, S.; Takeda, Y.; Yamazaki, S.; Nakahara, K.; Takami, A.; Yoshino, A.; Sugata, S.; Saito, S.; Hoshi, J.; et al. Maternal exposure to fine particulate matter over the first trimester and umbilical cord insertion abnormalities. Int. J. Epidemiol. 2022, 51, 191–201. [Google Scholar] [CrossRef]
- Santana, E.F.M.; Castello, R.G.; Rizzo, G.; Grisolia, G.; Araujo Júnior, E.; Werner, H.; Lituania, M.; Tonni, G. Placental and Umbilical Cord Anomalies Diagnosed by Two- and Three-Dimensional Ultrasound. Diagnostics 2022, 12, 2810. [Google Scholar] [CrossRef]
- Gross, A.; Markota Ajd, B.; Specht, C.; Scheier, M. Systematic screening for vasa previa at the 20-week anomaly scan. Acta Obstet. Et Gynecol. Scand. 2021, 100, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Geris, S.; Al-Emara, N.; Ramadan, G.; Sotiriadis, A.; Akolekar, R. Perinatal outcome of pregnancies with prenatal diagnosis of vasa previa: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 57, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Conyers, S.; Oyelese, Y.; Javinani, A.; Jamali, M.; Zargarzadeh, N.; Akolekar, R.; Hasegawa, J.; Melcer, Y.; Maymon, R.; Bronsteen, R.; et al. Incidence and causes of perinatal death in prenatally diagnosed vasa previa: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2024, 230, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y.; Javinani, A.; Gudanowski, B.; Krispin, E.; Rebarber, A.; Akolekar, R.; Catanzarite, V.; D’Souza, R.; Bronsteen, R.; Odibo, A.; et al. Vasa previa in singleton pregnancies: Diagnosis and clinical management based on an international expert consensus. Am. J. Obstet. Gynecol. 2024, in press. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kessler, J.; Moster, D. Association of placental and umbilical cord characteristics with cerebral palsy: National cohort study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 61, 224–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).