Abstract
(1) Background: The clinical management of anticoagulated patients treated with direct oral anticoagulants (DOAC) or Vitamin K antagonists (VKA) needing emergency surgery is challenging. (2) Methods: The prospective German RADOA registry investigated treatment strategies in DOAC- or VKA-treated patients needing emergency surgery within 24 h after admission. Effectiveness was analysed by clinical endpoints including major bleeding. Primary observation endpoint was in hospital mortality until 30 days after admission. (3) Results: A total of 78 patients were included (DOAC: 44; VKA: 34). Median age was 76 years. Overall, 43% of the DOAC patients and 79% of the VKA patients were treated with prothrombin complex concentrates (PCC) (p = 0.002). Out of the DOAC patients, 30% received no hemostatic treatment compared to 3% (1/34) of the VKA patients (p = 0.002), and 7% of the DOAC patients and 21% of the VKA patients developed major or clinically relevant non-major bleeding at the surgical site (p = 0.093). In-hospital mortality was 13% with no significant difference between the two treatment groups (DOAC: 11%, VKA: 15%; p > 0.20). (4) Conclusions: The 30-day in-hospital mortality rate was comparable between both patient groups. VKA patients required significantly more hemostatic agents than DOAC patients in the peri- and postoperative surgery period.
1. Introduction
Patients with nonvalvular atrial fibrillation, deep vein thrombosis or pulmonary embolism require anticoagulation with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOACs) [1,2,3,4,5]. With the demographical change of a worldwide increase in aging patients, the prevalence of anticoagulated patients rises. In international guidelines, DOACs are recommended preferentially to VKA as first choice anticoagulant for these indications [6,7,8] but both VKA- and DOAC-treatments require dedicated hemostatic management in case of emergencies. Each year, about 0.5–1% of anticoagulated patients need emergency surgery [9]. However, clinical management of these patients is highly diverse.
Perioperative management may include factor concentrates such as prothrombin complex concentrates (PCC), fresh frozen plasma (FFP), transfusion of red blood cells (RBC) and/or platelet concentrates, and other hemostatic agents such as vitamin K or tranexamic acid. Furthermore, the reversal agent idarucizumab is now available for dabigatran, although it is rather expensive. [10,11]. Andexanet alfa, the specific reversal agent for direct oral FXa inhibitors is not approved in patients requiring emergency surgery [11].
Other strategies to enable surgery in anticoagulated patients include delaying the procedure, for which either laboratory tests (anticoagulation intensity or drug plasma concentrations) or pharmacokinetic (pk) considerations are taken into account [12,13]. However, delaying surgery may be associated with increased morbidity and mortality [14], e.g., after hip fracture.
Here, we provide data from the prospective German RADOA-registry (Reversal Agent use in patients treated with Direct Oral Anticoagulants or vitamin K antagonists Registry) on clinical practice, effectiveness, and safety of different treatment strategies in VKA- or DOAC-treated patients who needed emergency surgery.
2. Materials and Methods
The prospective RADOA-registry is a German observational, noninterventional, open-label, investigator-initiated, multicenter registry, which documents the management of severe bleeding and/or emergency surgery in patients treated with the vitamin K antagonist phenprocoumon or DOAC [15]. Here, we report data from the subgroup of patients who needed emergency surgery within 24 h after admission. Patients requiring surgery because of severe bleeding were excluded.
2.1. Patients
The inclusion criteria were:
- Age > 18 years.
- Patients anticoagulated with a DOAC or phenprocoumon needing an urgent surgical intervention (not caused by severe bleeding) within 24 h after admission.
2.2. Study Design and Oversight
Patients were followed prospectively up to 30 days after hospital admission. Participating centers were included only if they had 24 h interdisciplinary teams available to manage anticoagulant-related bleeding in specialized units (i.e., emergency departments and intensive care units). Patients were recruited at 10 German centers from April 2014 to March 2018. Clinical outcome measures were peri- and/or post-operative major bleeding, and requirement of hemostatic agents (PCC, FFP, tranexamic acid, etc.) or drug-specific antidotes.
The prespecified primary observation endpoint was 30-day in-hospital mortality (deaths that occurred within 30 days of hospitalization). Secondary observation endpoints were blood loss, number of blood transfusions, satisfaction of the surgeon with peri- and postoperative hemostasis, outcome after use versus no use of reversal agents, frequency of serious adverse events (SAE) depending on the type of anticoagulant, and delay of the procedures due to anticoagulation.
2.3. Definition of INR-Rebound
In patients treated with phenprocoumon, the INR was monitored according to the instructions of the treating physicians. An INR rebound was assumed if the INR value rose to >2.0 within 10 days after admission despite a drop in the INR-Value to <1.5 within 24 h after admission.
2.4. Definition of Major Bleeding
The occurrence and severity of peri- and postoperative bleeding < 24 h was judged by the surgeon, who performed the surgery according to the following definitions:
- good hemostasis (for the type of surgery performed)
- severe bleeding outside the surgical area with a drop in hemoglobin ≥ 2 g/dL or a need of >2 RBC transfusions within 24 h after admission
- bleeding in the surgical area requiring re-surgery
- bleeding in the surgical area that was unexpected and persistent and/or led to hemodynamic instability (drop in Hb value ≥ 2 g/dL or transfusion of >2 RBC) within 24 h after admission
The occurrence of severe bleeding > 24 h during the hospital stay (including clinically relevant non-major bleeding) was also documented.
2.5. Ethics
Approval of the study protocol was obtained from the institutional review boards of all participating centers. External independent monitoring ensured 100% site source data validity.
Due to the emergency nature of the conditions under investigation, patient information and informed consent should not interfere with or delay acute treatment. Therefore, with the approval of all ethics committees and institutional review boards, written informed consent was obtained from patients after the acute management phase. In the event of a patient’s inability to provide written informed consent, this was obtained from his/her legal representative. Data of patients who remained unconscious or died before a legal representative had been appointed were also included. This was explicitly approved by the ethical boards to prevent major bias caused by exclusion of the most severely affected patients [16]. The study complies with the Declaration of Helsinki. ClinicalTrials.gov (National Library of Medicine, 8600 Rockville Pike, Bethesda, MD 20894, USA) Identifier: NCT01722786.
2.6. Statistics
The statistical analysis focused on descriptive statistics (median, range, frequencies) and two-sided 95% confidence intervals. Primary analysis was the characterization of the primary endpoint (in-hospital mortality up to 30 days after admission) by a two-sided 95% confidence interval.
Data were given with median and 25–75% quartiles. Group comparisons between binary data were compared with the exact Fisher test and between ordinal or quantitative data with the Wilcoxon-Mann–Whitney test. Time to in-hospital mortality was compared with a log-rank test. All explorative tests used a significance level of alpha = 5%.
3. Results
3.1. Demographic Data of Included Patients
Overall, 78 patients were included, 44 were treated with a DOAC and 34 treated with VKA (phenprocoumon). In the DOAC-group, 52% (23/44) of patients were treated with apixaban, 32% (14/44) with rivaroxaban, 7% (3/44) with edoxaban und 9% (4/44) with dabigatran. Median age was 76 years. While nearly half of the DOAC-treated patients were women, only a quarter of the VKA-treated patients were female (p = 0.012). On admission, acute renal failure occurred to a similar extent in both groups (DOAC: 5% (2/44) vs. VKA: 9% (3/34); p > 0.20; see Table 1).

Table 1.
Characteristics of patients at baseline.
Indications for emergency surgery were mainly bone fractures (total: 35%, 27/78) or an acute abdomen (total: 30%, 23/78). Significantly more DOAC-treated patients suffered from bone fractures compared to VKA-treated patients (DOAC: 46%, 20/44 vs. VKA: 21%,7/34; p= 0.031). Numerically more VKA-treated patients needed open-heart surgery compared to the DOAC-treated patients (VKA: 21%, 7/34 vs. DOAC: 9%,4/44; p = 0.195).
Indication for oral anticoagulation was predominantly atrial fibrillation (total: 74%, 58/78) with a similar distribution in both groups (DOAC: 77%, 34/44; VKA: 71%, 24/34). The median CHADS-VASC Score was 5 in both groups (p > 0.20). The median bleeding risk score according to the modified HASBLED score (HASBLED score excluding the criterion “stability of INR”) was 2 in both groups. Concomitant treatment with antiplatelet or anti-inflammatory drugs was similar among both groups (antiplatelet drugs; DOAC: 14%, 6/44 vs. VKA: 15%, 5/34; p > 0.20; anti-inflammatory drugs; DOAC: 2%, 1/44 vs. VKA: 9%, 3/34; p > 0.20, see Table 1).
On admission, information on bleeding history was available for all VKA patients and for 43 of 44 DOAC patients. In total, 2 of 43 DOAC patients (5%) and 5 of 34 VKA patients (15%) had a history of bleeding (p = 0.23).
3.2. Management
Management was highly diverse (see Figure 1). While 80% of the VKA patients (27/34) received PCC, only 43% of the DOAC-patients (19/44) were treated with PCC (p = 0.002). FFP was also significantly more frequently applied in VKA-patients compared to DOAC-patients (35% (12/34) vs. 7% (3/44); p = 0.003). Vitamin K was less frequently applied in DOAC-treated patients compared to VKA-treated patients (14% (6/44) vs. 62% (21/34); p < 0.001). In total, 47% of the VKA patients (16/34) required RBC transfusions compared to 32% of the DOAC patients (14/44) (p = 0.241). Platelet concentrates were applied in both groups to a similar extend (DOAC: 14%, 6/44 vs. VKA: 24%, 8/34; p > 0.20). Moreover, 30% of the DOAC patients (13/44) received no hemostatic treatment compared to 3% (1/34) of the VKA patients (p = 0.002) (see Figure 1).
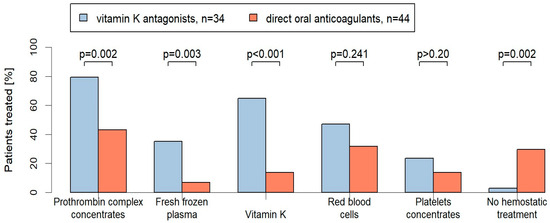
Figure 1.
Proportion of hemostatic treatments [%] in the VKA- and DOAC-treated patients with emergency operations. Two-sided p-values are given for comparison.
A delay of the procedures was observed to a similar extent in both groups (DOAC-group: 20%, 9/44; VKA-group: 21%, 7/34). In most cases, there was no delay (see Table 2).

Table 2.
Delay of procedures due to anticoagulation.
Use of Specific Antidotes
Overall, four patients had been treated with dabigatran. Two patients needed trauma surgery, one was operated on because of an acute abdomen and one needed a CNS emergency surgery. Two of these patients were treated with idaruzicumab (5 g intravenously), one preoperatively and one postoperatively. In all four patients, the surgeons categorized the postoperative bleeding situation as good. Of note, andexanet alfa was not available in Germany at the time of recruitment.
3.3. Bleeding Complications
In 90% of the patients (70/78), hemostasis was categorized by the surgeon as “good” during the first 24 h peri- and postoperatively (93% of the DOAC-treated patients: 41/44; 85% of the VKA-treated patients: 29/34; p = 0.29).
In 7% of the DOAC patients (3/44) and in 21% of the VKA patients (7/34), major or clinically relevant non-major bleeding at the surgical site occurred until day 30 after admission (p = 0.09; see Table 3).

Table 3.
Major and clinically relevant non-major bleedings in VKA- and DOAC-patients with emergency surgery.
Major bleeding complications did not differ depending on whether preoperative hemostatic treatment was applied or was not applied (7% (1/14) of the DOAC-patients vs. 19% (4/21) of the VKA-patients received preoperative hemostatic treatment compared to 7% (2/30) of the DOAC-patients vs. 24% (3/13) of the VKA-patients, who did not receive preoperative hemostatic treatment (p > 0.20)).
Most VKA-patients with bleeding complications (6/7) had an INR-level > 1.5 on admission but only three of these patients received PCC as a preoperative hemostatic treatment. An INR >1.5 was also observed in 24 of 26 non-bleeding VKA patients. The median INR on admission was comparable between VKA-patients without bleeding complications and VKA-patients with bleeding complications (INR 2.3 vs. INR 2.5, p = 0.923).
On admission, the median DOAC-concentration of the 3 DOAC-patients (3/44) with bleeding complication was numerically higher compared to the DOAC-treated patients without bleeding complications (41/44): 279 ng/mL vs. 104 ng/mL; p = 0.288. Preoperative PCC was only given to 1 of these patients with bleeding complications (see Table 3).
Postoperative hemostatic treatment until day 30 in patients with peri-surgery major bleeding was highly diverse. Most patients with bleeding complications received more than three different hemostatic treatments (see Table 3).
3.4. INR-Rebound
In 36% (12/33) of the VKA patients, an INR-Rebound was observed. Major bleeding occurred significantly more often in VKA patients with an INR-Rebound compared to those without an INR-Rebound (42% (5/12) vs. 5% (1/21); p = 0.016).
3.5. Thromboembolic Complications
None of the patients developed a venous or arterial thrombosis within 30 days after admission.
3.6. In-Hospital Mortality (until Day 30 after Admission)
In-hospital mortality was 13% (10/78) with no significant difference between the two treatment groups (DOAC-patients: 11%, 5/44; VKA-patients: 15%, 5/34; p > 0.20) (see Figure 2).
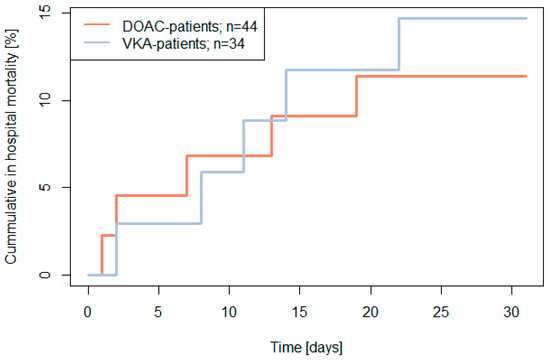
Figure 2.
The 30-day in hospital mortality (primary endpoint) by baseline treatment in patients with emergency surgery (two-sided log rank test p > 0.20).
There was only one patient in the VKA-group who potentially died due to bleeding (3%; 1/34; total group: 1%, 1/78). Other reasons for 30-day in-hospital mortality were sepsis (DOAC: 7%, 3/44 vs. VKA 12%, 4/34; p > 0.20), acute heart failure (DOAC: 2%, 1/44 vs. 6%, 2/34; p > 0.20) and respiratory insufficiency (DOAC: 2%, 1/44 vs. 3%, 1/34; p > 0.20) without any significant differences between both groups.
4. Discussion
To our knowledge, the RADOA-registry is the first multicenter registry which prospectively analysed the use of hemostatic agents in either DOAC- or VKA-anticoagulated patients requiring emergency surgery within 24 h after hospital admission. Our elderly patient population (median age: 76) was mainly anticoagulated because of atrial fibrillation. Most frequent indications for emergency surgery were bone fractures and acute abdomen.
Similar demographic data was presented in a recently published French registry, the GIHP-NACO-registry, which analyzed the management of urgent invasive procedures in patients treated with DOAC [17]. This registry included 478 DOAC-treated patients with a median age of 79, who needed an urgent invasive procedure within 48 h after admission to hospital.
Compared to our patient population, the French registry included a study population with a lower bleeding risk since 34% of their patients only needed a minor procedure such as pleural punctures, skin and organ biopsies, or tooth extraction. Moreover, 50% of the invasive procedures were classified as “expedited” which required treatment within days instead of hours. Although many of the procedures (43%) were delayed, excessive bleeding was observed in 13% of all procedures and hemostatic agents were only administered in 16% of the procedures.
This is in marked contrast to our registry, in which only 20% of the affected DOAC patients had their surgery postponed and 70% of DOAC-treated patients received hemostatic treatment. Although our DOAC-patients had more major surgeries compared to the French registry, only 7% of the RADOA-patients experienced major bleeding, potentially because of more aggressive prohemostatic treatment.
The difference in clinical severity between the two registries is also reflected by the 30-day in-hospital mortality rate. It was significantly lower in the French registry (5.9%) than in our registry (13%). Mortality in our registry was more comparable with the mortality rate in the REVERSE-AD study (13%) which investigated the effects of the specific antidote idarucizumab in dabigatran-treated patients needing emergency surgery (n = 202) [10]. Moreover, a similar 30-day mortality of 15% was presented in a small subgroup population (n = 21) of a Dutch cohort study that prospectively analyzed the management and outcome of DOAC-treated patients who needed urgent interventions within 8 h after admission to hospital [18].
In contrast to the French registry and the Dutch study, our registry also included the management of VKA-treated patients needing emergency surgery. Our results show that the hemostatic treatment was highly diverse with significant differences between the VKA- and the DOAC-groups. Not surprising, VKA-patients received PCC almost twice as often as the DOAC-patients. Also, FFP and Vitamin K was significantly more frequently applied in VKA-patients compared to DOAC-patients. In addition, 30% of the DOAC-treated patients did not receive any hemostatic treatment compared to only 3% of the VKA-treated patients (p = 0.002).
More patients in the VKA group had a major or clinically relevant non-major bleeding at the surgical site (VKA: 21% (7/34) vs. DOAC: 7% (3/44); p = 0.09) which was often triggered by an INR-rebound, a phenomenon which is frequently observed in phenprocoumon-treated patients due to its long half-life [19]. Only 3 of the 7 VKA patients with major bleeding peri- and/or postoperatively received PCC before the emergency surgery, despite 6 out of 7 having INR values of >1.5 before surgery.
Limitations
The RADOA registry is subject to all limitations that are typical for observational studies, including patient selection bias, subjective and non-standardized treatment decisions or reporting bias. In clinical routine, such registry data can also be influenced by variations in surgery indications or surgeons experience in performing the emergency procedure or evaluating the bleeding situation. The bleeding risks of different emergency surgeries are different, and an uneven distribution of different interventions may lead to different bleeding risks in the respective patient population.
Moreover, the small number of included patients limits the transferability of the results to the corresponding patient population. Randomized, controlled and prospective studies with higher numbers of study population are needed to verify our results.
5. Conclusions
Despite heterogeneous management approaches, 93% of the DOAC-treated patients and 85% of the VKA-treated patients showed good bleeding control during and after emergency surgery within 24 h after admission. A delay of the procedure was needed in only 20% of the patients. The 30-day in-hospital mortality was comparable in both patient groups. DOAC-treated patients required less prohemostatic treatment than VKA patients.
Author Contributions
Conceptualization, E.L.-L.; methodology, J.L. and E.L.-L.; validation, E.H.; formal analysis, E.H.; investigation, J.L., I.B., S.L., S.K., O.G., U.N.-G., B.Z., C.v.H., A.S., J.B.-W., S.S., P.M. and A.G.; writing—original draft preparation, J.L. and E.L.-L.; writing—review and editing, J.L., E.L.-L. and E.H.; visualization, E.H.; supervision, E.L.-L.; project administration, E.L.-L.; funding acquisition E.L.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Bayer, Bristol-Myers Squibb/Pfizer, DAIICHI Sankyo, and CSL Behring. The funders had no role in the design of the registry, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The leading ethical committee was the ethical committee of the university hospital Frankfurt (Ethikkommission des Fachbereichs Medizin Goethe Universität Frankfurt.): ethical review board statement: protocol code 215/13, date of approval: 1 August 2013. The institutional review boards of the participating hospitals followed these recommendations.
Informed Consent Statement
Written informed consent was obtained from patients after the acute management phase. This was approved by all ethics committees and institutional review boards. In case of a patient’s inability to provide written informed consent, this was obtained from his/her legal representative. Data of patients who remained unconscious or died before a legal representative was contacted were allowed to be included which was explicitly approved by the ethical boards to prevent major bias.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to thank the following: Sina Hehn, Department of Medicine II/University Cancer Center Frankfurt, University Hospital Frankfurt, Germany, who provided support in the development of the online Case Report Form of the Registry; Sebastian Harder, Department of Clinical Pharmacology, University Hospital Frankfurt, Germany, who gave advice on ethical issues; Hartmut Clausnizer, Institute of Clinical Chemistry, Thrombosis & Hemostasis Treatment Center, University Hospital, Kiel-Lübeck, Germany; and Norbert Weiler, Department of Anesthesiology & Intensive Care, University Hospital Schleswig Holstein, Campus Kiel, Germany, who recruited patients.
Conflicts of Interest
I.B. received speaker’s honoraria from CSL Behring, LFB biomedicaments, Octapharma AG, AstraZeneca GmbH and Siemens Healthcare and performed contract research for Siemens Healthcare and Behnk Elektronik GmbH & Co. and is supported by means of medical writing from CSL Behring and Portola Pharmaceuticals and is a member of the advisory board/expert testimony of LFB biomedicaments, Portola Pharmaceuticals, Siemens Healthcare and CSL Behring. S.K. has received lecture honoraria and advisory fees from Bayer AG, Boehringer Ingelheim, MSD, Actelion, and Daiichi-Sankyo; and institutional research support from Bayer AG, Boehringer Ingelheim, MSD, Actelion, and Daiichi-Sankyo. O.G. has received research funding from Astra Zeneca (Alexion), Bayer Healthcare, Boehringer Ingelheim, Biotest, CSL Behring, Octapharma, Novo Nordisk, Nycomed, and Portola. He has also received honoraria for lectures and consultancy support from Bayer Healthcare, Boehringer Ingelheim, CSL Behring, Ferring, Octapharma, Sanofi, Shire, Takeda, Pfizer, and Portola. U.N.-G. has received lecture honoraria and advisory fees from Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Octapharma, and LFB. C.v.H. has received honoraria for lectures and consultancy work potentially related to this topic, as well as travel reimbursements from Bayer GmbH, Biotest GmbH, Pfizer GmbH, Daiichi Sankyo, CSL Behring, NovoNordisk GmbH, and HICC GbR. J. B.-W. has received personal honoraria (lectures, advisory boards) and travel support from AstraZeneca, Bayer, Daiichi Sankyo, Janssen, LEO Pharma, Norgine, Sanofi and Portola/Alexion and institutional research support from AstraZeneca, Bayer, Daiichi Sankyo, Janssen, LEO, Pfizer, and Portola/Alexion. S.S. has received honoraria for lectures from Bayer, Boehringer, Daiichi Sankyo, and Pfizer, grants, and honoraria from BMS. P.M.’s department has received grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt’s Patient Blood Management program. P.M. received honoraria for scientific lectures from Vifor Pharma, CSL Behring, and Pharmacosmos. A. G. reports grants and non-financial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, Werfen, nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V., E.L.-L. has received lecture honoraria and advisory fees from Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Portola, CSL Behring, Norgine, Roche, Leo, Astra Zeneca and Aspen and institutional research support from Bayer AG, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Werfen and CSL Behring. The other authors report no conflicts of interest.
References
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Spinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Investigators, E.; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Siegal, D.M.; Freedman, D.; Ansell, J. Urgent procedures or surgeries in patients receiving oral anticoagulants: A systematic literature review. J. Thromb. Thrombolysis 2023, 55, 197–202. [Google Scholar] [CrossRef]
- Pollack, C.V., Jr.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Birschmann, I.; Kuhn, J.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Gottl, U.; Lucks, J.; Zydek, B.; von Heymann, C.; et al. Pharmacokinetics of Direct Oral Anticoagulants in Emergency Situations: Results of the Prospective Observational RADOA-Registry. Thromb. Haemost. 2022, 122, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Gressenberger, P. Reversal strategies in patients treated with direct oral anticoagulants. Vasa 2019, 48, 389–392. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Abdulla, K.; Yang, H.; Sundaresan, S.; Doering, P.; Vaswani, S.G.; Thavorn, K.; Forster, A.J. Association of delay of urgent or emergency surgery with mortality and use of health care resources: A propensity score-matched observational cohort study. CMAJ 2017, 189, E905–E912. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E. Direct oral anticoagulants (DOAC)—Management of emergency situations. Hamostaseologie 2017, 37, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Herrmann, E.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Goettl, U.; Lucks, J.; Zydek, B.; Heymann, C.V.; Birschmann, I.; et al. Severe Hemorrhage Associated With Oral Anticoagulants. Dtsch. Arztebl. Int. 2020, 117, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Godon, A.; Gabin, M.; Levy, J.H.; Huet, O.; Chapalain, X.; David, J.S.; Tacquard, C.; Sattler, L.; Minville, V.; Memier, V.; et al. Management of urgent invasive procedures in patients treated with direct oral anticoagulants: An observational registry analysis. Thromb. Res. 2022, 216, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Bavalia, R.; Abdoellakhan, R.; Brinkman, H.J.M.; Brekelmans, M.P.A.; Hamulyak, E.N.; Zuurveld, M.; Hutten, B.A.; Westerweel, P.E.; Olie, R.H.; Ten Cate, H.; et al. Emergencies on direct oral anticoagulants: Management, outcomes, and laboratory effects of prothrombin complex concentrate. Res. Pract. Thromb. Haemost. 2020, 4, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Lindhoff-Last, E.; Birschmann, I.; Bidenharn, A.J.; Kuhn, J.; Lindau, S.; Konstantinides, S.; Grottke, O.; Nowak-Gottl, U.; Lucks, J.; Zydek, B.; et al. Pharmacokinetics of Phenprocoumon in Emergency Situations-Results of the Prospective Observational RADOA-Registry (Reversal Agent Use in Patients Treated with Direct Oral Anticoagulants or Vitamin K Antagonists Registry). Pharmaceuticals 2022, 15, 1437. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).