Outcomes of Laparoscopic Cesarean Scar Defect Repair: Retrospective and Observational Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Curtin, S.C.; Matthews, T.J. Births: Final data for 2013. Natl. Vital. Stat. Rep. 2015, 64, 1–65. [Google Scholar] [PubMed]
- Hoffmann, E.; Vahanian, S.; Martinelli, V.T.; Chavez, M.; Mesbah, M.; Nezhat, F.R. Combined Medical and Minimally Invasive Robotic Surgical Approach to the Treatment and Repair of Cesarean Scar Pregnancies. JSLS 2021, 25, e2021.00039. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J.; Orellana, R.; Dolmans, M.M. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil. Steril. 2017, 107, 289–296.e2. [Google Scholar] [CrossRef] [PubMed]
- Setubal, A.; Alves, J.; Osorio, F.; Guerra, A.; Fernandes, R.; Albornoz, J.; Sidiroupoulou, Z. Treatment for Uterine Isthmocele, A Pouchlike Defect at the Site of a Cesarean Section Scar. J. Minim. Invasive Gynecol. 2018, 25, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Falik, R.; Li, A. Surgical management of niche, isthmocele, uteroperitoneal fistula, or cesarean scar defect: A critical rebirth in the medical literature. Fertil. Steril. 2017, 107, 69–71. [Google Scholar] [CrossRef]
- Nezhat, C.G.L.; Soliemannjad, R.; Meshkat Razavi, G.; Nezhat, A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016, 28, 32–53. [Google Scholar]
- Antila-Langsjo, R.M.; Maenpaa, J.U.; Huhtala, H.S.; Tomas, E.I.; Staff, S.M. Cesarean scar defect: A prospective study on risk factors. Am. J. Obstet. Gynecol. 2018, 219, 458.e1. [Google Scholar] [CrossRef]
- Dodd, J.M.; Anderson, E.R.; Gates, S.; Grivell, R.M. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst. Rev. 2014, CD004732. [Google Scholar] [CrossRef]
- Raimondo, G.; Grifone, G.; Raimondo, D.; Seracchioli, R.; Scambia, G.; Masciullo, V. Hysteroscopic treatment of symptomatic cesarean-induced isthmocele: A prospective study. J. Minim. Invasive Gynecol. 2015, 22, 297–301. [Google Scholar] [CrossRef]
- van der Voet, L.F.; Bij de Vaate, A.M.; Veersema, S.; Brolmann, H.A.; Huirne, J.A. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 236–244. [Google Scholar] [CrossRef]
- Bij de Vaate, A.J.; Brolmann, H.A.; van der Voet, L.F.; van der Slikke, J.W.; Veersema, S.; Huirne, J.A. Ultrasound evaluation of the Cesarean scar: Relation between a niche and postmenstrual spotting. Ultrasound Obstet. Gynecol. 2011, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Osser, O.V.; Jokubkiene, L.; Valentin, L. Cesarean section scar defects: Agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet. Gynecol. 2010, 35, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Osser, O.V.; Jokubkiene, L.; Valentin, L. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet. Gynecol. 2009, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Regnard, C.; Nosbusch, M.; Fellemans, C.; Benali, N.; van Rysselberghe, M.; Barlow, P.; Rozenberg, S. Cesarean section scar evaluation by saline contrast sonohysterography. Ultrasound Obstet. Gynecol. 2004, 23, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.; Hansen, W.F.; Van Voorhis, B.J.; Syrop, C.H. Detection of cesarean scars by transvaginal ultrasound. Obstet. Gynecol. 2003, 101, 61–65. [Google Scholar] [PubMed]
- Nezhat, C.; Grace, L.A.; Razavi, G.M.; Mihailide, C.; Bamford, H. Reverse Vesicouterine Fold Dissection for Laparoscopic Hysterectomy After Prior Cesarean Deliveries. Obstet. Gynecol. 2016, 128, 629–633. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists (the College) and the Society for Maternal–Fetal Medicine; Caughey, A.B.; Cahill, A.G.; Guise, J.M.; Rouse, D.J. Safe prevention of the primary cesarean delivery. Am. J. Obstet. Gynecol. 2014, 210, 179–193. [Google Scholar] [CrossRef]
- Tower, A.M.; Frishman, G.N. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J. Minim. Invasive Gynecol. 2013, 20, 562–572. [Google Scholar] [CrossRef]
- Poidevin, L.O. The value of hysterography in the prediction of cesarean section wound defects. Am. J. Obstet. Gynecol. 1961, 81, 67–71. [Google Scholar] [CrossRef]
- Tanimura, S.; Funamoto, H.; Hosono, T.; Shitano, Y.; Nakashima, M.; Ametani, Y.; Nakano, T. New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. J. Obstet. Gynaecol. Res. 2015, 41, 1363–1369. [Google Scholar] [CrossRef]
- Wang, C.B.; Chiu, W.W.; Lee, C.Y.; Sun, Y.L.; Lin, Y.H.; Tseng, C.J. Cesarean scar defect: Correlation between Cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet. Gynecol. 2009, 34, 85–89. [Google Scholar] [CrossRef] [PubMed]
- van der Voet, L.F.; Vervoort, A.J.; Veersema, S.; BijdeVaate, A.J.; Brolmann, H.A.; Huirne, J.A. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, A.J.; Van der Voet, L.F.; Witmer, M.; Thurkow, A.L.; Radder, C.M.; van Kesteren, P.J.; Quartero, H.W.; Kuchenbecker, W.K.; Bongers, M.Y.; Geomini, P.M.; et al. The HysNiche trial: Hysteroscopic resection of uterine caesarean scar defect (niche) in patients with abnormal bleeding, a randomised controlled trial. BMC Womens Health 2015, 15, 103. [Google Scholar] [CrossRef]
- Bujold, E.; Goyet, M.; Marcoux, S.; Brassard, N.; Cormier, B.; Hamilton, E.; Abdous, B.; Sidi, E.A.L.; Kinch, R.; Miner, L.; et al. The role of uterine closure in the risk of uterine rupture. Obstet. Gynecol. 2010, 116, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Chaillet, N.; Boutin, A.; Moore, L.; Jastrow, N.; Brassard, N.; Gauthier, R.J.; Hudic, I.; Shipp, T.D.; Weimar, C.H.; et al. Single-versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int. J. Gynaecol. Obstet. 2011, 115, 5–10. [Google Scholar] [CrossRef]
- Bij de Vaate, A.J.; van der Voet, L.F.; Naji, O.; Witmer, M.; Veersema, S.; Brolmann, H.A.; Bourne, T.; Huirne, J.A. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: Systematic review. Ultrasound Obstet. Gynecol. 2014, 43, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Gubbini, G.; Centini, G.; Nascetti, D.; Marra, E.; Moncini, I.; Bruni, L.; Petraglia, F.; Florio, P. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J. Minim. Invasive Gynecol. 2011, 18, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Vikhareva Osser, O.; Valentin, L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1119–1126. [Google Scholar] [CrossRef]
- Marotta, M.L.; Donnez, J.; Squifflet, J.; Jadoul, P.; Darii, N.; Donnez, O. Laparoscopic repair of post-cesarean section uterine scar defects diagnosed in nonpregnant women. J. Minim. Invasive Gynecol. 2013, 20, 386–391. [Google Scholar] [CrossRef]
- Tulandi, T.; Cohen, A. Emerging Manifestations of Cesarean Scar Defect in Reproductive-aged Women. J. Minim. Invasive Gynecol. 2016, 23, 893–902. [Google Scholar] [CrossRef]
- Api, M.; Boza, A.; Gorgen, H.; Api, O. Should Cesarean Scar Defect Be Treated Laparoscopically? A Case Report and Review of the Literature. J. Minim. Invasive Gynecol. 2015, 22, 1145–1152. [Google Scholar] [CrossRef]
- Grace, L.; Nezhat, A. Should Cesarean Scar Defect Be Treated Laparoscopically? A Case Report and Review of the Literature. J. Minim. Invasive Gynecol. 2016, 23, 843. [Google Scholar] [CrossRef] [PubMed]
- Gubbini, G.; Casadio, P.; Marra, E. Resectoscopic correction of the "isthmocele" in women with postmenstrual abnormal uterine bleeding and secondary infertility. J. Minim. Invasive Gynecol. 2008, 15, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Li, A.; Abed, S.; Balassiano, E.; Soliemannjad, R.; Nezhat, A.; Nezhat, C.H.; Nezhat, F. Strong Association Between Endometriosis and Symptomatic Leiomyomas. JSLS J. Soc. Laparoendosc. Surg. 2016, 20, e2016.00053. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.T.; Osias, J.; Velasco, A.; Charles, R.; Nezhat, C. Laparoscopic repair of a uteroperitoneal fistula. JSLS J. Soc. Laparoendosc. Surg. 2003, 7, 367–369. [Google Scholar] [CrossRef]
- Kok, N.; Wiersma, I.C.; Opmeer, B.C.; de Graaf, I.M.; Mol, B.W.; Pajkrt, E. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 42, 132–139. [Google Scholar] [CrossRef]
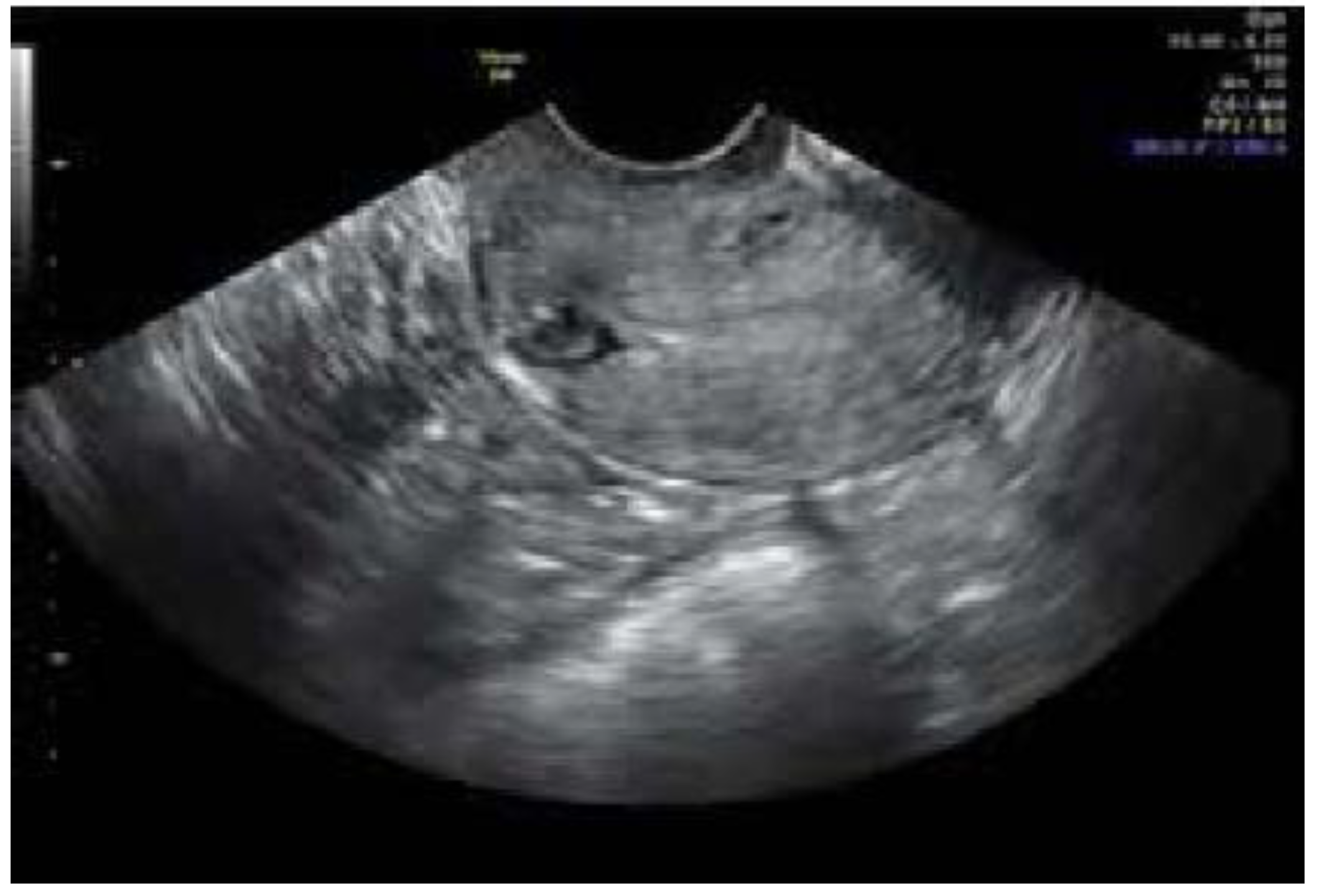
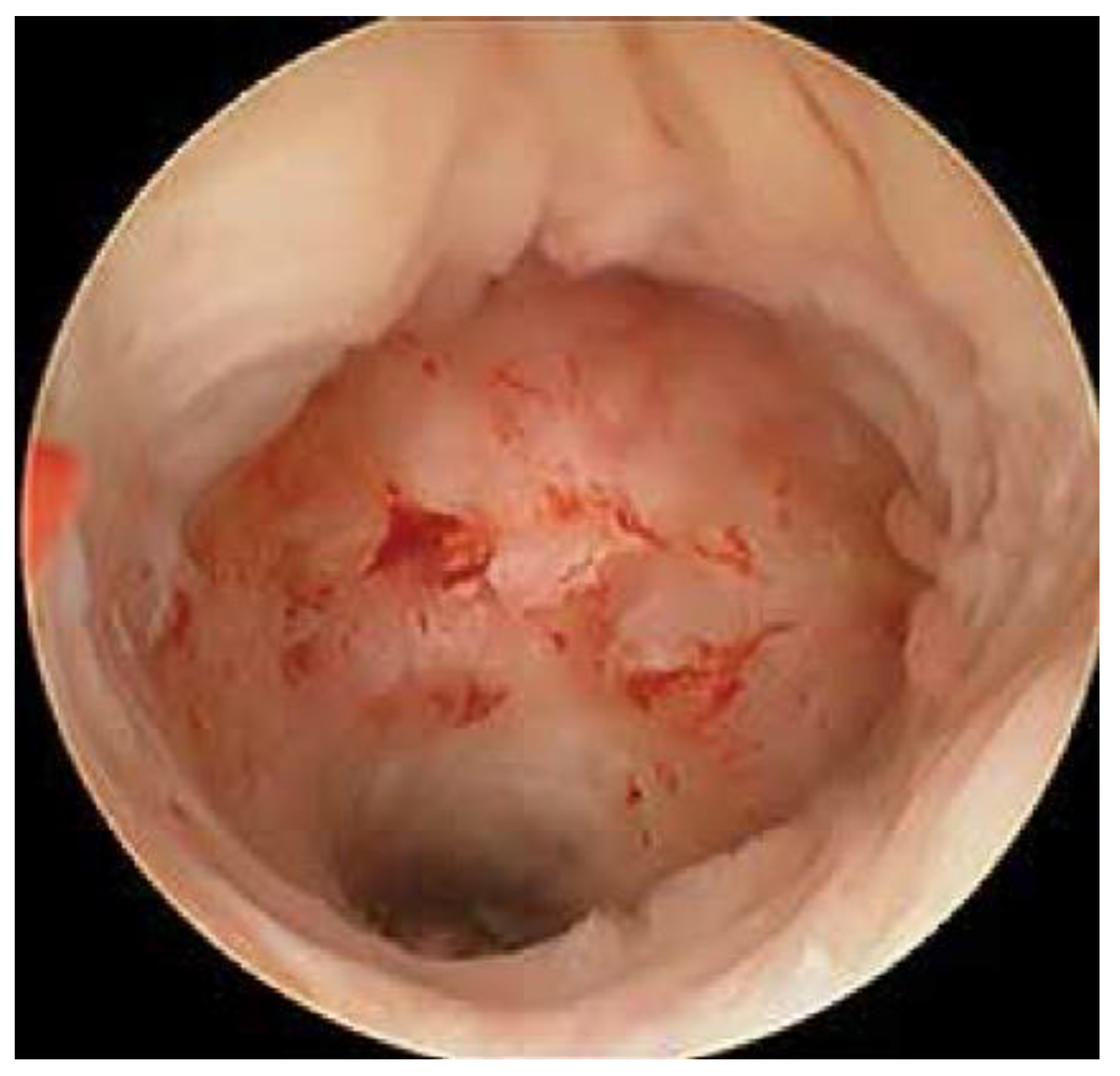

| Characteristics | Total (n = 27) | Percent | |
|---|---|---|---|
| Age | ≤30 | 1 | 3.7% |
| 31–35 | 11 | 40.7% | |
| >35 | 15 | 55.6% | |
| Average BMI | 23.1 | ||
| Previous LSC with TOE | 11 | 40% | |
| Primary Chief Complaint | Pain | 10 | 37% |
| Irregular bleeding or spotting | 2 | 7% | |
| Infertility | 4 | 14% | |
| Pain and bleeding | 7 | 26% | |
| Pain and infertility | 3 | 11% | |
| Irregular bleeding and infertility | 1 | 3% | |
| Pre-operative IVF attempts | 9/17 | 52% | |
| Pre-operative IUI attempts | 8/17 | 29.6% | |
| Parity | 1 | 20 | 74% |
| ≥2 | 7 | 26% | |
| History of Smoking | 1 | 3.7% | |
| History of Diabetes Mellitus | 2 | 7.4% | |
| Symptoms | Irregular vaginal bleeding | 18 | 67% |
| Pelvic pain | 22 | 81% | |
| Urinary symptoms | 12 | 44% | |
| Dysmenorrhea | 19 | 70% | |
| Existing Conditions | Infertility < 1 year | 2 | 7% |
| Infertility ≥ 1 year | 11 | 41% | |
| Previous surgical diagnosis of endometriosis | 12 | 44% | |
| Modality of Diagnosing Niche | Sonogram | 19 | 70% |
| Sonohysterogram | 3 | 11% | |
| MRI | 1 | 3.7% | |
| Not diagnosed | 4 | 15.3% | |
| Surgical Approach of Repair | Hysteroscopy only | 1 | 3.7% |
| Laparoscopy and hysteroscopy | 23 | 85.2% | |
| Hysterectomy | 3 | 11.1% | |
| Number of Cesarean Deliveries | 1 | 20 | 74% |
| ≥2 | 7 | 26% |
| Outcomes | Total (n = 27) | ||
|---|---|---|---|
| Symptoms | Persist | 1 | 3.7% |
| Improve | 11 | 40.7% | |
| Resolved | 15 | 55.6% | |
| Pregnancy Rate * | 11/15 | 73.3% | |
| Live Birth Rate * | 9/15 | 60% | |
| Delivery Route | Vaginal Birth After Cesarean | 1 | 6.6% |
| Repeat Cesarean Delivery | 8 | 53.3% | |
| Number of Spontaneous Miscarriages | 2 | 13.3% | |
| Post-Operative Complications | Umbilical Hernia | 1 | 3.7% |
| Umbilical Infection | 1 | 3.7% | |
| Uterine rupture | 0 | ||
| Cesarean scar pregnancy | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nezhat, C.; Zaghi, B.; Baek, K.; Nezhat, A.; Nezhat, F.; Lindheim, S.; Nezhat, C. Outcomes of Laparoscopic Cesarean Scar Defect Repair: Retrospective and Observational Study. J. Clin. Med. 2023, 12, 3720. https://doi.org/10.3390/jcm12113720
Nezhat C, Zaghi B, Baek K, Nezhat A, Nezhat F, Lindheim S, Nezhat C. Outcomes of Laparoscopic Cesarean Scar Defect Repair: Retrospective and Observational Study. Journal of Clinical Medicine. 2023; 12(11):3720. https://doi.org/10.3390/jcm12113720
Chicago/Turabian StyleNezhat, Camran, Benjamin Zaghi, Kelly Baek, Azadeh Nezhat, Farr Nezhat, Steven Lindheim, and Ceana Nezhat. 2023. "Outcomes of Laparoscopic Cesarean Scar Defect Repair: Retrospective and Observational Study" Journal of Clinical Medicine 12, no. 11: 3720. https://doi.org/10.3390/jcm12113720
APA StyleNezhat, C., Zaghi, B., Baek, K., Nezhat, A., Nezhat, F., Lindheim, S., & Nezhat, C. (2023). Outcomes of Laparoscopic Cesarean Scar Defect Repair: Retrospective and Observational Study. Journal of Clinical Medicine, 12(11), 3720. https://doi.org/10.3390/jcm12113720







