Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Assessment of PROMs
2.3. Statistical Analysis
3. Results
3.1. Baseline Demographics and Characteristics
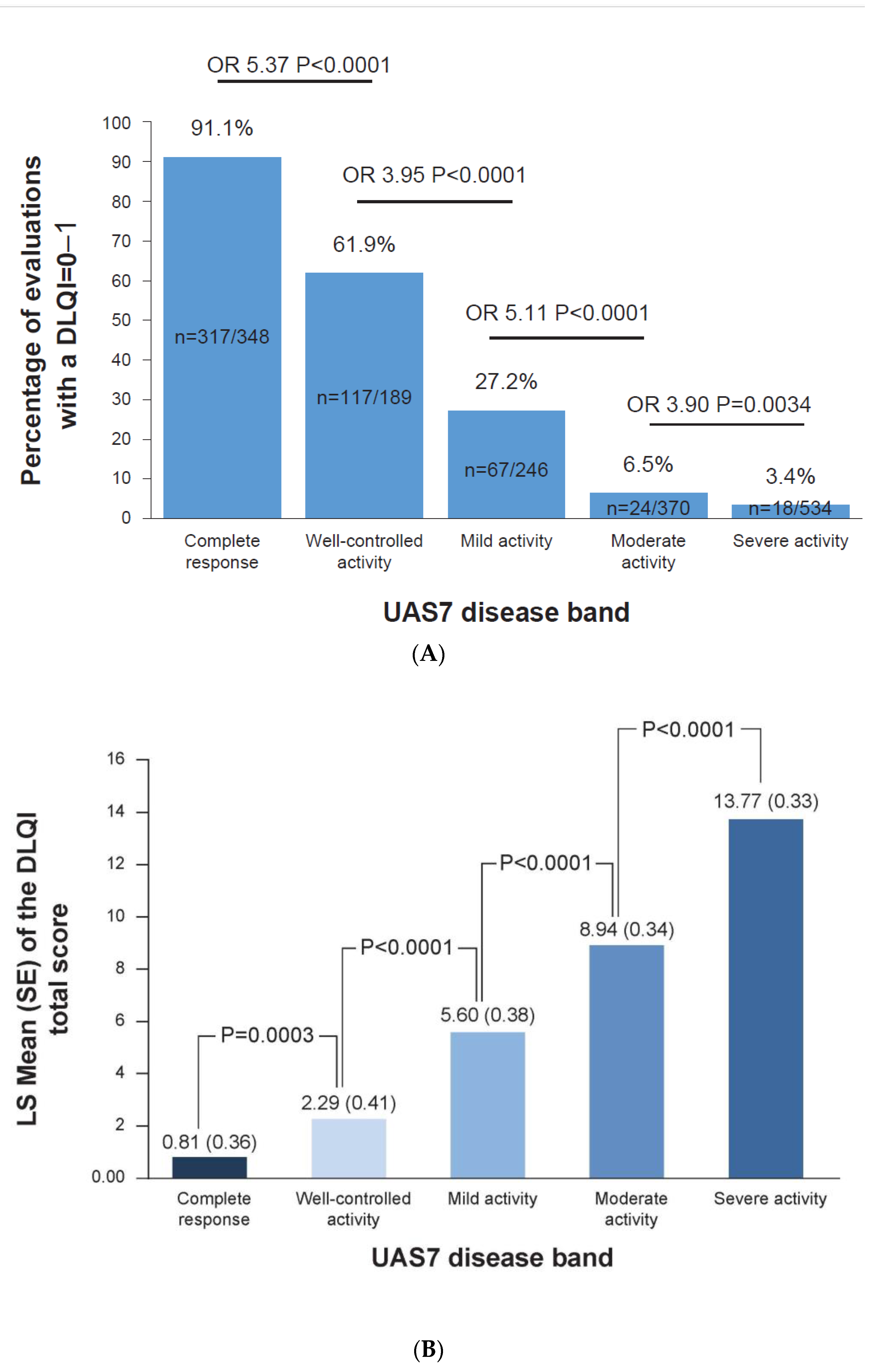
3.2. Complete Response to Treatment Is Linked to No Impact on DLQI
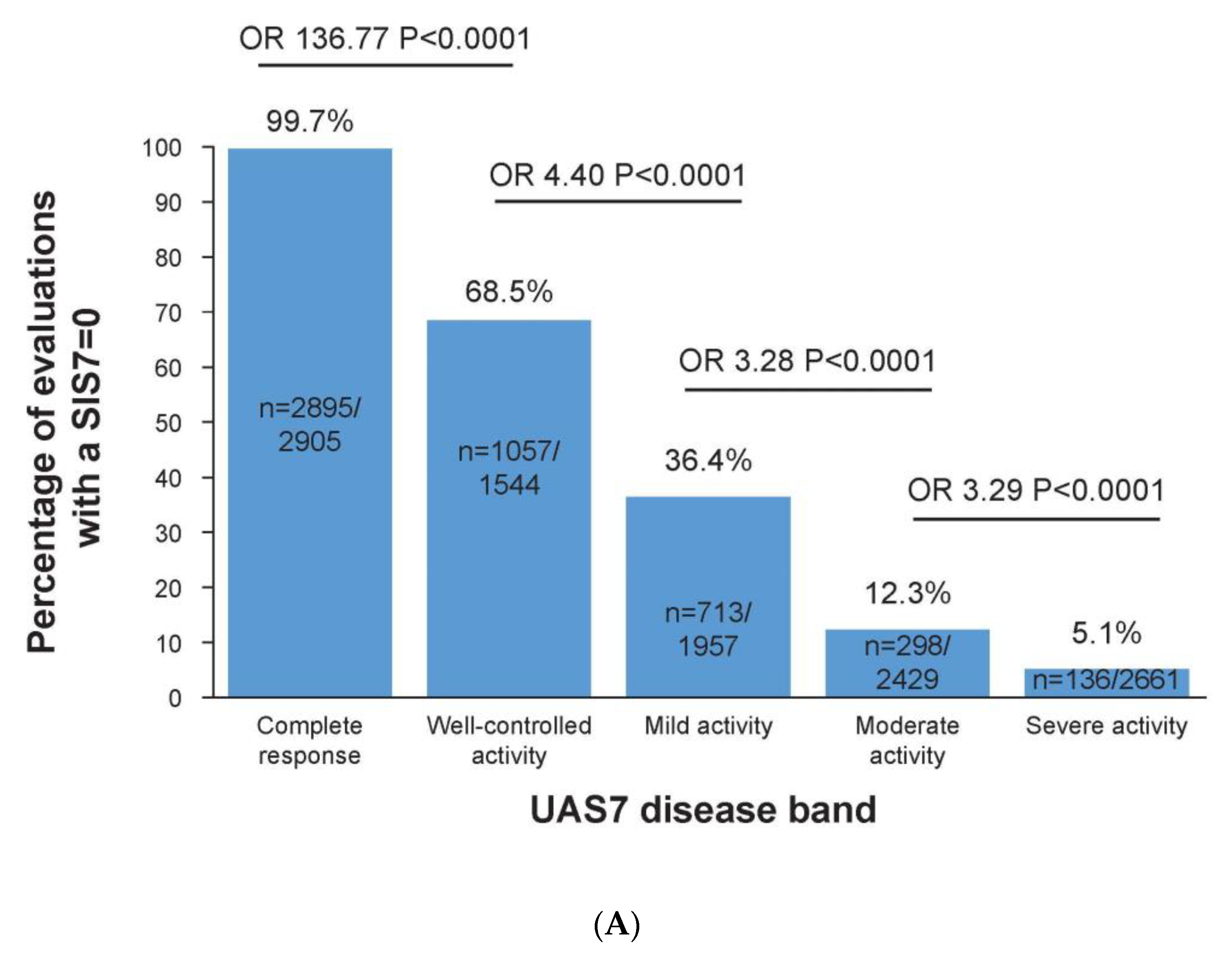
3.3. Complete Response to Treatment Is Linked to No Impact on Sleep
3.4. No Impairment of CSU on Life Is Linked to No Impact on Sleep and Daily Activities
3.5. Complete Response to Treatment Is Linked to No Impact on a Patients’ Work
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, K.; Maurer, M.; Grattan, C.; Nakonechna, A.; Abuzakouk, M.; Berard, F.; Sussman, G.; Gimenez-Arnau, A.M.; Ortiz de Frutos, J.; Knulst, A.; et al. ASSURE-CSU: A real-world study of burden of disease in patients with symptomatic chronic spontaneous urticaria. Clin. Transl. Allergy 2015, 5, 29. [Google Scholar] [CrossRef]
- Maurer, M.; Gimenez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.Y.; Chung, W.H.; et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Staubach, P.; Raap, U.; Richter-Huhn, G.; Baier-Ebert, M.; Chapman-Rothe, N. ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br. J. Dermatol. 2016, 174, 892–894. [Google Scholar] [CrossRef]
- Maurer, M.; Abuzakouk, M.; Berard, F.; Canonica, W.; Oude Elberink, H.; Gimenez-Arnau, A.; Grattan, C.; Hollis, K.; Knulst, A.; Lacour, J.P.; et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017, 72, 2005–2016. [Google Scholar] [CrossRef]
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.A.; Ensina, L.F.; Fomina, D.; Galvàn, C.A.; Godse, K.; Grattan, C.; et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Lawlor, F.; Simpson, J.; Morgan, M.; Greaves, M.W. The impact of chronic urticaria on the quality of life. Br. J. Dermatol. 1997, 136, 197–201. [Google Scholar] [CrossRef]
- Ertaş, R.; Erol, K.; Hawro, T.; Yılmaz, H.; Maurer, M. Sexual Functioning Is Frequently and Markedly Impaired in Female Patients with Chronic Spontaneous Urticaria. J. Allergy. Clin. Immunol. Pract. 2020, 8, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Nam, Y.H.; Park, C.S.; Kim, M.Y.; Jo, E.J.; Park, H.K.; Kim, H.K. Anxiety, depression, and stress in Korean patients with chronic urticaria. Korean J. Intern. Med. 2020, 35, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Arnau, A.; Maurer, M.; Bernstein, J.; Staubach, P.; Barbier, N.; Hua, E.; Severin, T.; Joubert, Y.; Janocha, R.; Balp, M.M. Ligelizumab improves sleep interference and disease burden in patients with chronic spontaneous urticaria. Clin. Transl. Allergy 2022, 12, e12121. [Google Scholar] [CrossRef]
- Maurer, M.; Weller, K.; Bindslev-Jensen, C.; Giménez-Arnau, A.; Bousquet, P.J.; Bousquet, J.; Canonica, G.W.; Church, M.K.; Godse, K.V.; Grattan, C.E.; et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy 2011, 66, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, A.M.; Maurer, M.; Bernstein, J.A.; Barbier, N.; Hua, E.; Severin, T.; Janocha, R.; Balp, M.-M. Complete symptom control in chronic spontaneous urticaria patients leads to improved sleep and quality of life: Results from the Phase 2b ligelizumab study. In Proceedings of the 29th European Academy of Dermatology and Venereology Congress, Virtual, 29–31 October 2020. [Google Scholar]
- Yosipovitch, G.; Ansari, N.; Goon, A.; Chan, Y.H.; Goh, C.L. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br. J. Dermatol. 2002, 147, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sussman, G.; Abuzakouk, M.; Berard, F.; Canonica, W.; Oude Elberink, H.; Gimenez-Arnau, A.; Grattan, C.; Hollis, K.; Hunter, S.; Knulst, A.; et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: Analyses from ASSURE-CSU. Allergy 2018, 73, 1724–1734. [Google Scholar] [CrossRef]
- Balp, M.M.; Vietri, J.; Tian, H.; Isherwood, G. The Impact of Chronic Urticaria from the Patient’s Perspective: A Survey in Five European Countries. Patient 2015, 8, 551–558. [Google Scholar] [CrossRef]
- Gimenez-Arnau, A.M.; Spector, S.; Antonova, E.; Trzaskoma, B.; Rosen, K.; Omachi, T.A.; Stull, D.; Balp, M.M.; Murphy, T. Improvement of sleep in patients with chronic idiopathic/spontaneous urticaria treated with omalizumab: Results of three randomized, double-blind, placebo-controlled studies. Clin. Transl. Allergy 2016, 6, 32. [Google Scholar] [CrossRef]
- Mlynek, A.; Magerl, M.; Hanna, M.; Lhachimi, S.; Baiardini, I.; Canonica, G.W.; Brzoza, Z.; Kasperska-Zajac, A.; Rogala, B.; Zalewska-Janowska, A.; et al. The German version of the Chronic Urticaria Quality-of-Life Questionnaire: Factor analysis, validation, and initial clinical findings. Allergy 2009, 64, 927–936. [Google Scholar] [CrossRef]
- Stull, D.; McBride, D.; Tian, H.; Gimenez Arnau, A.; Maurer, M.; Marsland, A.; Balp, M.M.; Khalil, S.; Grattan, C. Analysis of disease activity categories in chronic spontaneous/idiopathic urticaria. Br. J. Dermatol. 2017, 177, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Eyerich, K.; Eyerich, S.; Ferrer, M.; Gutermuth, J.; Hartmann, K.; Jakob, T.; Kapp, A.; Kolkhir, P.; Larenas-Linnemann, D.; et al. Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 321–333. [Google Scholar] [CrossRef]
- Brzoza, Z.; Badura-Brzoza, K.; Maurer, M.; Hawro, T.; Weller, K. Chronic spontaneous urticaria activity, impact and control as well as their changes are strongly linked, and these links are not affected by angioedema or comorbid inducible urticaria-Results from the validation of the Polish Urticaria Control Test. World Allergy Organ. J. 2022, 15, 100635. [Google Scholar] [CrossRef]
- Weller, K.; Siebenhaar, F.; Hawro, T.; Altrichter, S.; Schoepke, N.; Maurer, M. Clinical Measures of Chronic Urticaria. Immunol. Allergy Clin. North. Am. 2017, 37, 35–49. [Google Scholar] [CrossRef]
- Mathias, S.D.; Crosby, R.D.; Rosen, K.E.; Zazzali, J.L. The minimal important difference for measures of urticaria disease activity: Updated findings. Allergy Asthma Proc. 2015, 36, 394–398. [Google Scholar] [CrossRef]
- Mathias, S.D.; Dreskin, S.C.; Kaplan, A.; Saini, S.S.; Spector, S.; Rosen, K.E. Development of a daily diary for patients with chronic idiopathic urticaria. Ann. Allergy Asthma. Immunol. 2010, 105, 142–148. [Google Scholar] [CrossRef]
- Młynek, A.; Zalewska-Janowska, A.; Martus, P.; Staubach, P.; Zuberbier, T.; Maurer, M. How to assess disease activity in patients with chronic urticaria? Allergy 2008, 63, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Lewis, V.; Finlay, A.Y. 10 years experience of the Dermatology Life Quality Index (DLQI). J. Investig. Dermatol. Symp. Proc. 2004, 9, 169–180. [Google Scholar] [CrossRef]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Salek, M.S.; Finlay, A.Y. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J. Invest. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef]
- Finlay, A.Y. Current severe psoriasis and the rule of tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef]
- Reilly, M.C.; Zbrozek, A.S.; Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993, 4, 353–365. [Google Scholar] [CrossRef]
- Reinhold, J.; Giménez-Arnau, A.; Maurer, M.; Bernstein, J.A.; Barbier, N.; Hua, E.; Severin, T.; Balp, M.-M. Sleep and quality of life improves with better control of urticaria symptoms: Ligelizumab Phase-2b studies. In Proceedings of the ACAAI 2020 Annual Scientific Meeting, Virtual, 23–28 October 2020. [Google Scholar]
- Pedro, A.; Laires, N.J.A.K.; Barbier, N.; Ortmann, C.-E.; Serge, S.M.-M.B.; Weller, K. Complete Control of Wheals and Itch in CSU Significantly Correlates with Better Sleep Quality: Analysis from a Worldwide Real-World Database (AWARE Study). In Proceedings of the European Academy of Dermatology and Venereology Congress, Vienna, Austria, 29 September–2 October 2021. [Google Scholar]
- Erol, K.; Ertaş, Ş.K.; Ertaş, R. Fatigue Is Common and Predicted by Female Gender and Sleep Disturbance in Patients with Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. Pract. 2021, 9, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Laires, P.A.; Salameh, P.; Asero, R.; Bizjak, M.; Košnik, M.; Dissemond, J.; van Doorn, M.; Hawro, T.; Kasperska-Zajac, A.; et al. The Benefit of Complete Response to Treatment in Patients with Chronic Spontaneous Urticaria-CURE Results. J. Allergy Clin. Immunol. Pract. 2023, 11, 610–620. [Google Scholar] [CrossRef]
- Maurer, M.; Metz, M.; Bindslev-Jensen, C.; Bousquet, J.; Canonica, G.W.; Church, M.K.; Godse, K.V.; Grattan, C.E.; Hide, M.; Kocaturk, E.; et al. Definition, aims, and implementation of GA(2) LEN Urticaria Centers of Reference and Excellence. Allergy 2016, 71, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Cherrez-Ojeda, I.; Vanegas, E.; Cherrez, A.; Felix, M.; Weller, K.; Magerl, M.; Maurer, R.R.; Mata, V.L.; Kasperska-Zajac, A.; Sikora, A.; et al. Chronic urticaria patients are interested in apps to monitor their disease activity and control: A UCARE CURICT analysis. Clin. Transl. Allergy 2021, 11, e12089. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (N = 382) † |
|---|---|
| Age, years ± SD | 43.3 ± 12.5 |
| Female, n (%) | 286 (75) |
| BMI, kg/m2 ± SD ‡ | 27.91 ± 6.5 |
| Race, n (%) § | |
| Native American | 1 (0.3) |
| Asian | 76 (20) |
| Black | 8 (2) |
| White | 283 (74) |
| Other | 12 (3) |
| IgE level, IU/mL | |
| Median | 87.2 |
| Range | 0–14, 100 |
| Weekly itch severity score ± SD ¶ | 13.1 ± 4.1 |
| Weekly hives severity score ± SD ¶ | 17.3 ± 4.4 |
| UAS7 ± SD †† | 30.4 ± 7.4 |
| Positive CU Index n (%) ‡‡ | 145 (38) |
| Mean duration of CSU, years ± SD | 4.3 ± 6.0 |
| Background medication, n (%) | |
| Locally approved dose of H1-antihistamine | 164 (43) |
| Escalated dose of locally approved H1-antihistamine | 218 (57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernstein, J.A.; Giménez-Arnau, A.; Maurer, M.; Staubach, P.; Barbier, N.; Hua, E.; Severin, T.; Laires, P.A.; Balp, M.-M. Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria. J. Clin. Med. 2023, 12, 3561. https://doi.org/10.3390/jcm12103561
Bernstein JA, Giménez-Arnau A, Maurer M, Staubach P, Barbier N, Hua E, Severin T, Laires PA, Balp M-M. Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria. Journal of Clinical Medicine. 2023; 12(10):3561. https://doi.org/10.3390/jcm12103561
Chicago/Turabian StyleBernstein, Jonathan A., Ana Giménez-Arnau, Marcus Maurer, Petra Staubach, Nathalie Barbier, Eva Hua, Thomas Severin, Pedro A. Laires, and Maria-Magdalena Balp. 2023. "Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria" Journal of Clinical Medicine 12, no. 10: 3561. https://doi.org/10.3390/jcm12103561
APA StyleBernstein, J. A., Giménez-Arnau, A., Maurer, M., Staubach, P., Barbier, N., Hua, E., Severin, T., Laires, P. A., & Balp, M.-M. (2023). Why a Complete Response Is the Treatment Aim in Chronic Spontaneous Urticaria. Journal of Clinical Medicine, 12(10), 3561. https://doi.org/10.3390/jcm12103561





