Effects and Usefulness of Inspiratory Muscle Training Load in Patients with Advanced Lung Cancer with Dyspnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Characteristics of the Patients
2.3. Outcome Measures
2.4. Rehabilitation Program
2.5. Statistical Analysis
3. Results
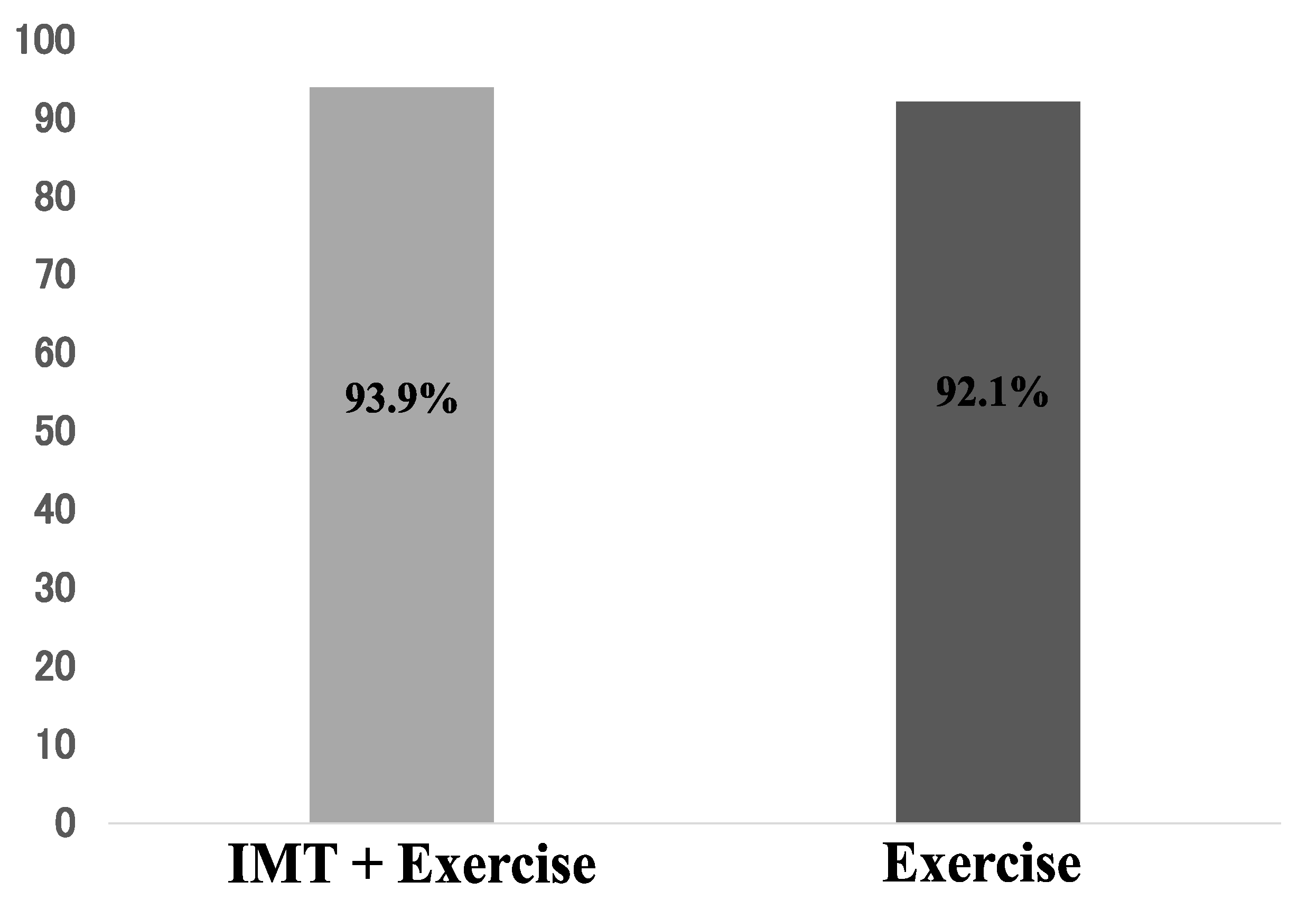
3.1. Adherence
3.2. Participant Characteristics
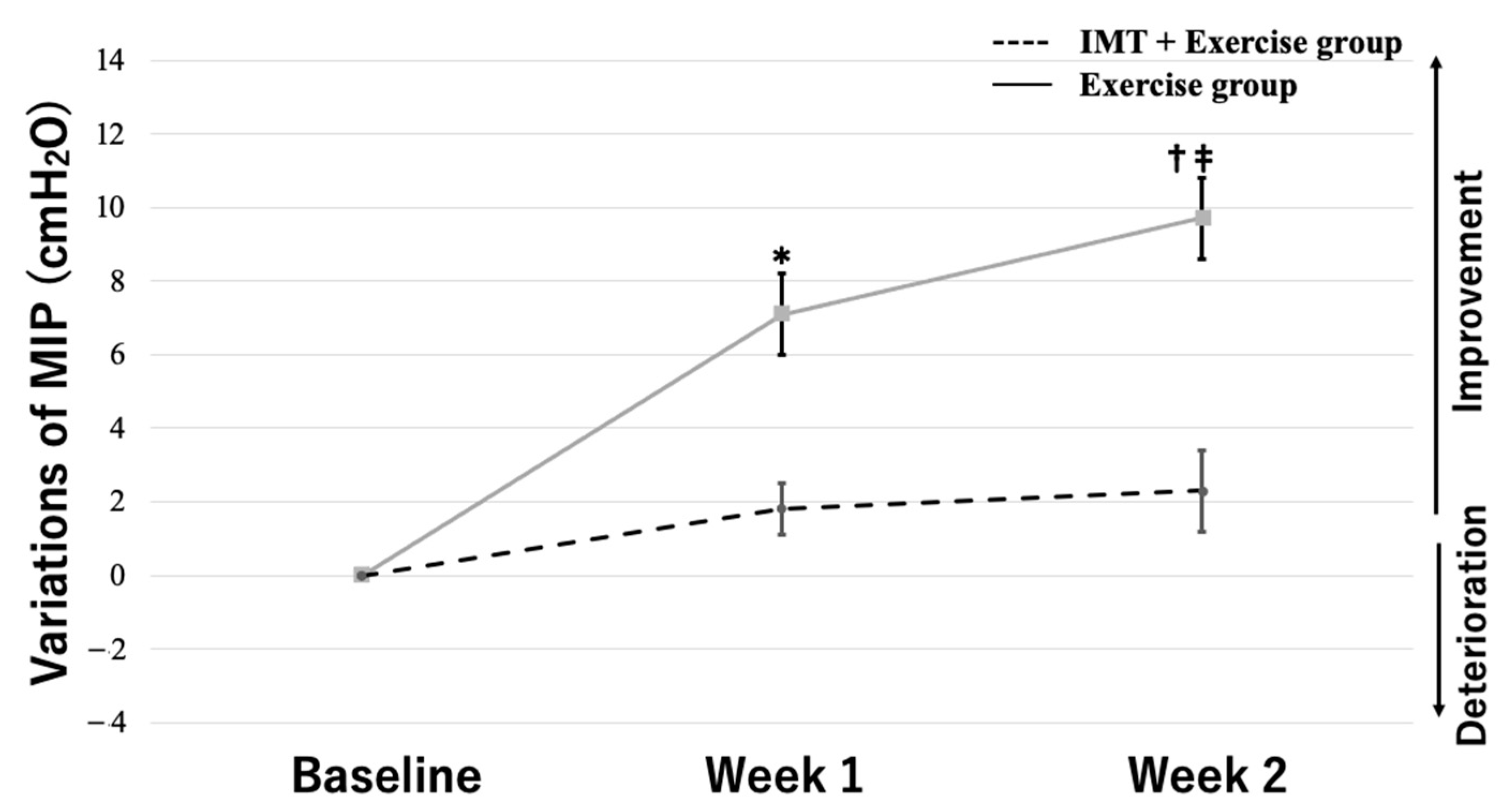
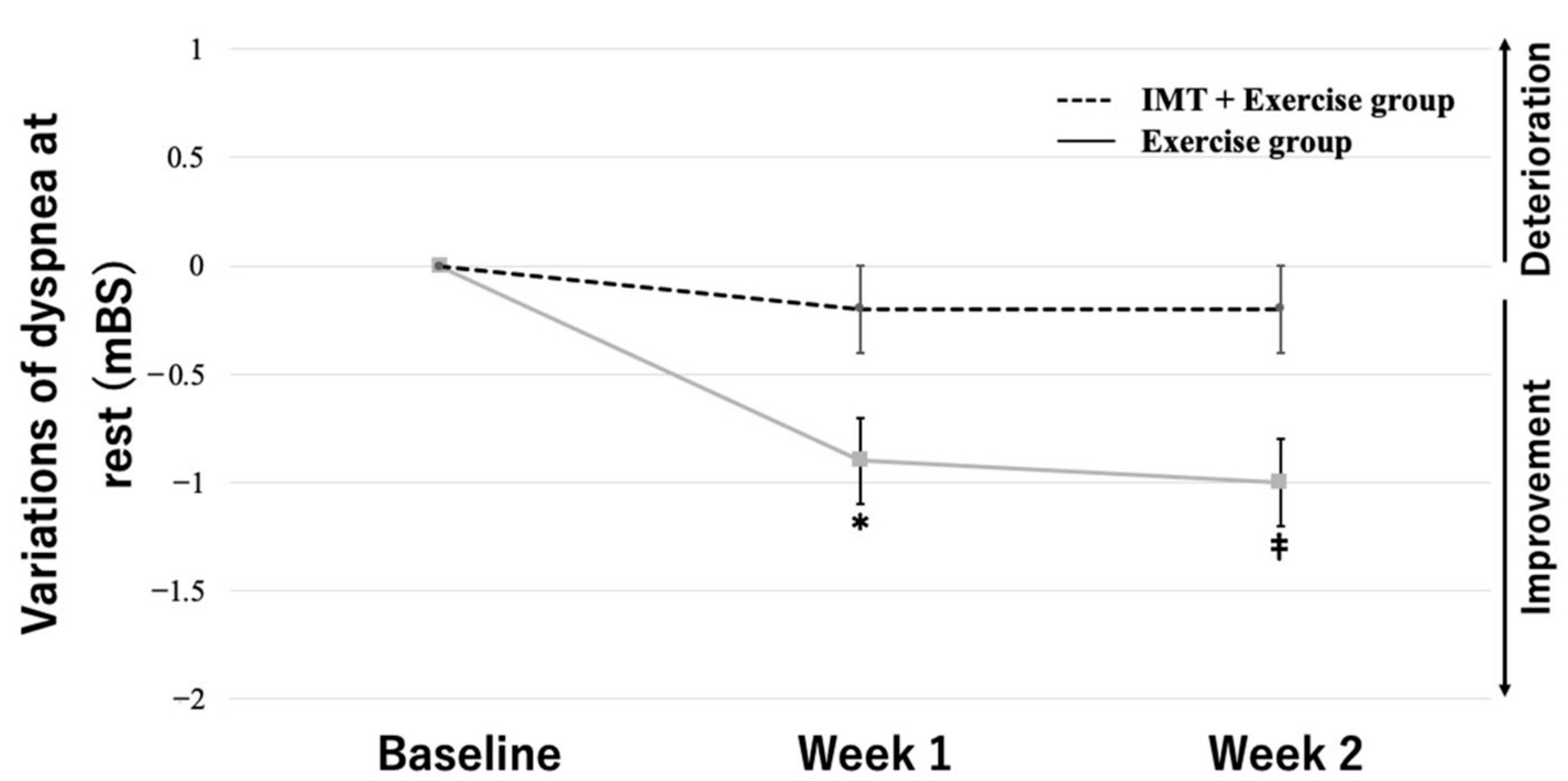
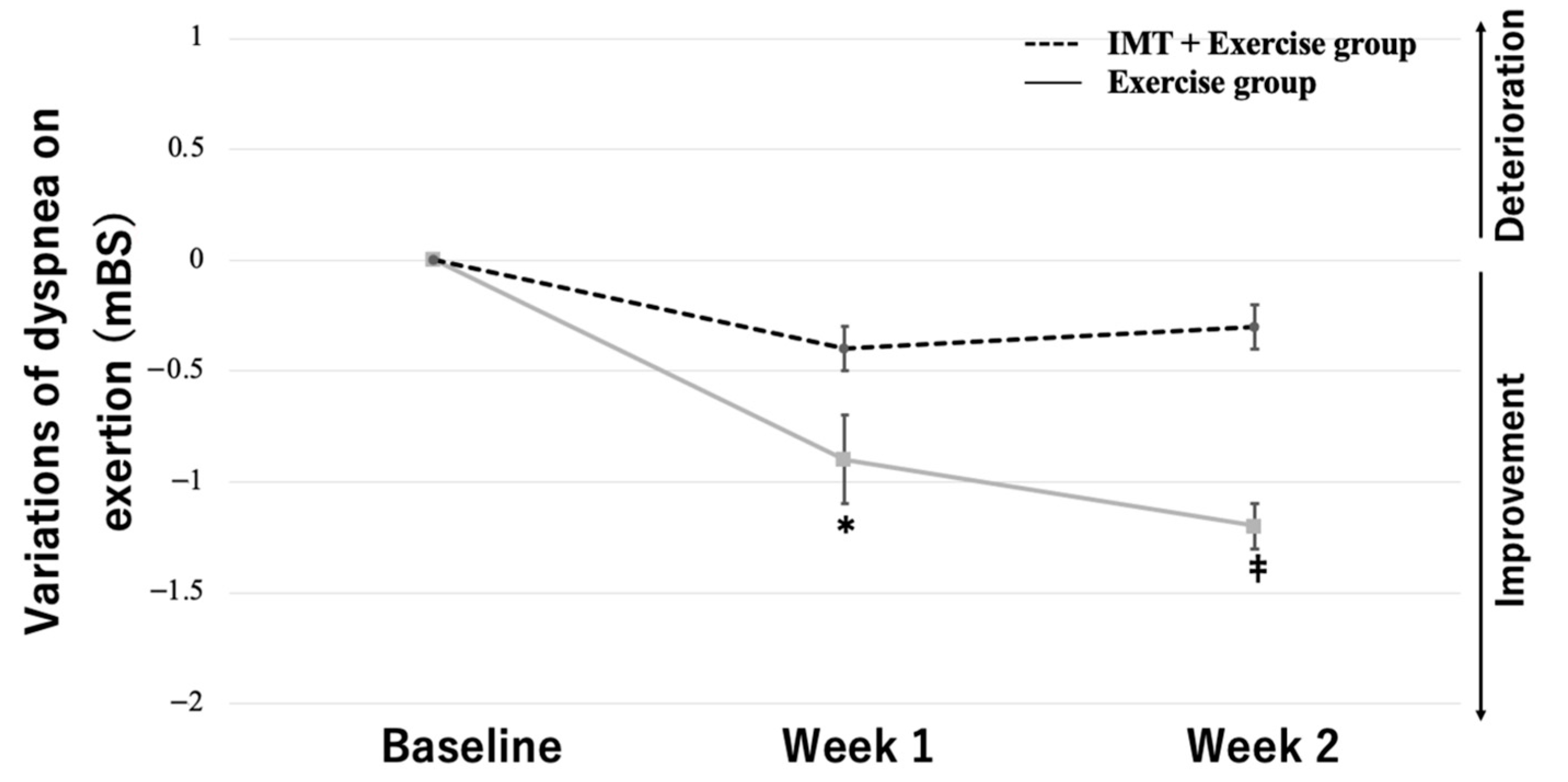
3.3. Two-Way Repeated-Measures ANOVA
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCCN. Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer Version 3. 2020. Available online: www.nccn.org (accessed on 11 February 2020).
- NICE. CG24 Lung Cancer: Full Guideline. 2005. Available online: http://guidance.nice.org.uk/CG24/Guidance/pdf/English (accessed on 3 March 2022).
- Dudgeon, D.J.; Lertzman, M.; Askew, G.R. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J. Pain Symptom Manag. 2001, 21, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Bohlke, K.; Bao, T.; Campbell, T.C.; Coyne, P.J.; Currow, D.C.; Gupta, A.; Leiser, A.L.; Mori, M.; Nava, S.; et al. Management of Dyspnea in Advanced Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1389–1411. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Rice, S.J.; Belani, C.P. Pulmonary Rehabilitation in Lung Cancer. PMR 2016, 8, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-L.; Yu, C.-J.; Shih, J.-Y.; Yang, P.-C.; Wu, Y.-T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Mori, A.; Esaki, H.; Shiraki, T.; Uemura, H.; Okazawa, M.; Sakakibara, H. The effect of pulmonary rehabilitation in patients with post-tuberculosis lung disorder. Chest 2003, 123, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Perez, H.; Nana-Sinkam, P. Integrating pulmonary rehabilitation into the multidisciplinary management of lung cancer: A review. Respir. Med. 2015, 109, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.L.; Bauldoff, G.S.; Carlin, B.W.; Casaburi, R.; Emery, C.F.; Mahler, D.A.; Make, B.; Rochester, C.L.; Zuwallack, R.; Herrerias, C. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007, 131 (Suppl. S5), 4S–42S. [Google Scholar] [CrossRef]
- Casaburi, R.; Kukafka, D.; Cooper, C.B.; Witek, T.J.; Kesten, S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005, 127, 809–817. [Google Scholar] [CrossRef]
- Ozalevli, S.; Ilgin, D.; Kul Karaali, H.; Bulac, S.; Akkoclu, A. The effect of in-patient chest physiotherapy in lung cancer patients. Support. Care Cancer 2010, 18, 351–358. [Google Scholar] [CrossRef]
- Geddes, E.L.; Reid, W.D.; Brooks, D.; O’Brien, K.; Crowe, J. A Primer on Inspiratory Muscle Trainers. 2010. Available online: https://www.researchgate.net/profile/W-Darlene-Reid-2/publication/228373385_A_primer_on_inspiratory_muscle_trainers/links/0c960532858c72419b000000/A-primer-on-inspiratory-muscle-trainers.pdf (accessed on 27 March 2022).
- Lotters, F.; Van Tol, B.; Kwakkel, G.; Gosselink, R. Effects of controlled inspiratory muscle training in patients with COPD: A meta-analysis. Eur. Respir. J. 2002, 20, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Hossein Pour, A.H.; Gholami, M.; Saki, M.; Birjandi, M. The effect of inspiratory muscle training on fatigue and dyspnea in patients with heart failure: A randomized, controlled trial. Jpn. J. Nurs. Sci. 2020, 17, e12290. [Google Scholar] [CrossRef] [PubMed]
- Budweiser, S.; Moertl, M.; Jörres, R.A.; Windisch, W.; Heinemann, F.; Pfeifer, M. Respiratory muscle training in restrictive thoracic disease: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2006, 87, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.K.; Romer, L.M. Dyspnoea in health and obstructive pulmonary disease: The role of respiratory muscle function and training. Sport. Med. 2004, 34, 117–132. [Google Scholar] [CrossRef]
- Bailey, C.D.; Wagland, R.; Dabbour, R.; Caress, A.; Smith, J.; Molassiotis, A. An integrative review of systematic reviews related to the management of breathlessness in respiratory illnesses. BMC Pulm. Med. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Charalambous, A.; Taylor, P.; Stamataki, Z.; Summers, Y. The effect of resistance inspiratory muscle training in the management of breathlessness in patients with thoracic malignancies: A feasibility randomised trial. Support. Care Cancer 2015, 23, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.G.; Kanner, R.E. Spirometry. Clin. Rev. Allergy Immunol. 2009, 37, 137–152. [Google Scholar] [CrossRef]
- Charususin, N.; Gosselink, R.; Decramer, M.; Demeyer, H.; McConnell, A.; Saey, D.; Maltais, F.; Derom, E.; Vermeersch, S.; Heijdra, Y.F.; et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018, 73, 942–950. [Google Scholar] [CrossRef]
- Langer, D.; Charususin, N.; Jácome, C.; Hoffman, M.; McConnell, A.; Decramer, M.; Gosselink, R. Efficacy of a Novel Method for Inspiratory Muscle Training in People With Chronic Obstructive Pulmonary Disease. Phys. Ther. 2015, 95, 1264–1273. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Takahashi, H.; Sugawara, K.; Satake, M.; Shioya, T.; Kagaya, H.; Kawatani, M. Effects of low-intensity exercise training (Chronic Obstructive Pulmonary Disease Sitting Calisthenics) in patients with stable chronic obstructive pulmonary disease. Jpn. J. Compr. Rehabil. Sci. 2011, 2, 5–12. [Google Scholar] [CrossRef]
- Brocki, B.C.; Andreasen, J.J.; Langer, D.; Souza, D.S.R.; Westerdahl, E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: A randomized controlled trial. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: A pilot randomized clinical trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Lee, S.H.; Kim, S.A.; Kim, A.H.; Gelvosa, M.N.; Cheon, H.; Jeon, J.Y. The effects of inspiratory muscle training with pulmonary rehabilitation on NSCLC patients during radiation therapy: A pilot clinical study. Thorac. Cancer 2023. [Google Scholar] [CrossRef]
- Oxberry, S.G.; Bland, J.M.; Clark, A.L.; Cleland, J.G.; Johnson, M.J. Minimally clinically important difference in chronic breathlessness: Every little helps. Am. Heart J. 2012, 164, 229–235. [Google Scholar] [CrossRef]
- Ray, A.D.; Williams, B.T.; Mahoney, M.C. Respiratory Muscle Training Improves Exercise Performance and Quality of Life in Cancer Survivors: A Pilot Study. Rehabil. Oncol. 2017, 35, 81–89. [Google Scholar] [CrossRef]

| Variable | IMT + Exercise Group (n = 31) | Exercise Group (n = 35) | p-Value |
|---|---|---|---|
| Age (years) | 69.1 ± 7.1 | 70.7 ± 7.4 | 0.478 |
| Men/women, n (%) | 16 (52)/15 (48) | 21 (60)/14 (40) | 0.437 |
| BMI (kg/m2) | 18.2 ± 1.7 | 18.3 ± 2.2 | 0.638 |
| Type of cancer | |||
| Non-small cell lung cancer, n (%) | 20 (65) | 24 (69) | 0.732 |
| Small cell lung cancer, n (%) | 11 (35) | 11 (31) | |
| Cancer stage | |||
| Stage III, n (%) | 16 (52) | 23 (66) | 0.177 |
| Stage IV, n (%) | 15 (48) | 12 (34) | |
| PS | 1.2 ± 1.2 | 1.3 ± 1.0 | 0.898 |
| BI | 92.6 ± 7.7 | 91.2 ± 6.9 | 0.515 |
| Medication | |||
| Line | 2.7 ± 1.3 | 2.7 ± 1.1 | 0.99 |
| Chemotherapy, n (%) | 9 (29) | 15 (43) | 0.372 |
| Molecular target drugs, n (%) | 10 (32) | 8 (23) | |
| ICI, n (%) | 8 (26) | 8 (23) | |
| Noting, n (%) | 4 (13) | 4 (11) | |
| Supplemental O2, n (%) | 5 (24) | 8 (23) | 0.517 |
| Physiologic | |||
| VC (%) | 52.6 ± 13.4 | 56.7 ± 18.7 | p = 0.04 |
| FVC (L) | 1.4 ± 0.3 | 1.8 ± 0.5 | p < 0.001 |
| FEV1.0 (%) | 68.9 ± 9.1 | 70.1 ± 14.9 | 0.746 |
| Outcome | |||
| MIP (cmH2O) | 33.2 ± 7.2 | 39.9 ± 11.2 | p = 0.023 |
| Dyspnea at rest (mBS) | 2.3 ± 0.9 | 2.0 ± 1.0 | 0.344 |
| Dyspnea on exertion (mBS) | 3.8 ± 0.8 | 3.9 ± 1.0 | 0.556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, Y.; Yamaga, T.; Yamamoto, S.; Matsumori, K.; Ichiyama, T.; Hanaoka, M.; Ikegami, S.; Horiuchi, H. Effects and Usefulness of Inspiratory Muscle Training Load in Patients with Advanced Lung Cancer with Dyspnea. J. Clin. Med. 2023, 12, 3396. https://doi.org/10.3390/jcm12103396
Sakai Y, Yamaga T, Yamamoto S, Matsumori K, Ichiyama T, Hanaoka M, Ikegami S, Horiuchi H. Effects and Usefulness of Inspiratory Muscle Training Load in Patients with Advanced Lung Cancer with Dyspnea. Journal of Clinical Medicine. 2023; 12(10):3396. https://doi.org/10.3390/jcm12103396
Chicago/Turabian StyleSakai, Yasunari, Takayoshi Yamaga, Shuhei Yamamoto, Keiji Matsumori, Takashi Ichiyama, Masayuki Hanaoka, Shota Ikegami, and Hiroshi Horiuchi. 2023. "Effects and Usefulness of Inspiratory Muscle Training Load in Patients with Advanced Lung Cancer with Dyspnea" Journal of Clinical Medicine 12, no. 10: 3396. https://doi.org/10.3390/jcm12103396
APA StyleSakai, Y., Yamaga, T., Yamamoto, S., Matsumori, K., Ichiyama, T., Hanaoka, M., Ikegami, S., & Horiuchi, H. (2023). Effects and Usefulness of Inspiratory Muscle Training Load in Patients with Advanced Lung Cancer with Dyspnea. Journal of Clinical Medicine, 12(10), 3396. https://doi.org/10.3390/jcm12103396






