Abstract
Background: Peripheral arterial disease (PAD) and acute limb ischemia (ALI) pose an increasing strain on health care systems. The objective of this study was to describe the German health care landscape and to assess hospital utilization with respect to PAD and ALI. Methods: Secondary data analysis of diagnosis-related group statistics data (2009–2018) provided by the German Federal Statistical Office. Inclusion of cases encoded by the International Classification of Diseases (ICD-10) codes for PAD and arterial embolism or thrombosis. Construction of line diagrams and choropleth maps to assess temporal trends and regional distributions. Results: A total of 2,589,511 cases (median age 72 years, 63% male) were included, of which 2,110,925 underwent surgical or interventional therapy. Overall amputation rate was 17%, with the highest rates of minor (28%) and major amputations (15%) in patients with tissue loss. In-hospital mortality (overall 4.1%) increased in accordance to Fontaine stages and was the highest in patients suffering arterial embolism or thrombosis (10%). Between 2009 and 2018, the annual number of PAD cases with tissue loss (Fontaine stage IV) increased from 97,092 to 111,268, whereby associated hospital utilization decreased from 2.2 million to 2.0 million hospital days. Hospital incidence and hospital utilization showed a clustering with the highest numbers in eastern Germany, while major amputation rate and mortality were highest in northern parts of Germany. Conclusions: Increased use of endovascular techniques was observed, while hospital utilization to treat PAD with tissue loss has decreased. This is despite an increased hospital incidence. Addressing socioeconomic inequalities and a more homogeneous distribution of dedicated vascular units might be advantageous in reducing the burden of disease associated with PAD and ALI.
1. Introduction
Most likely due to an aging population and a growing burden of atherosclerotic risk factors, cardiovascular diseases are globally on the rise. Between 1990 and 2019, the number of affected people almost doubled to a total of 523 million with 18.6 million related deaths worldwide [1]. The upward movement is reflected in an increased number of people suffering from peripheral arterial disease (PAD), which has been rising globally from 164 to 202 million between 2000 and 2010, with the highest change rates observed in low- and middle-income countries [2]. While the largest share of people with PAD are asymptomatic or experience intermittent claudication, about 11% of all PAD patients suffer from chronic limb-threatening ischemia (CLTI), corresponding to a prevalence of 1.3% in a population aged ≥40 years [3]. The annual amputation rate rises with increasing PAD categories and was found to be 60% for Rutherford class 6 patients [4].
Aside from the individual patient’s suffering, PAD poses an enormous strain on health care systems, particularly due to the abundance of associated comorbidities. Over the past years, different approaches have been applied to further characterize the cohort of PAD patients in Germany. The multicentric prospective GermanVasc cohort study found that >1/3 of PAD patients suffered severe systemic disease, with 3% exhibiting a constant threat to life [5]. Health care-related expenditures for patients with PAD are significantly higher compared to those without [6]. A retrospective health insurance claims data analysis of patients insured by one of the largest insurance providers in Germany found that disease related reimbursement costs rose by 31% between 2008 and 2016 [7]. Mean costs were shown to rise with higher Rutherford classes [4]. Annual costs were particularly high in PAD patients after they underwent major amputation [8]. While PAD generally describes a chronic condition, acute-on-chronic arterial occlusion may occur and (besides other causes including embolism, peripheral arterial aneurysm, dissection, injury, vasculitis) is causative in a major part of cases presenting as acute limb ischemia (ALI) [9,10,11]. Therefore, ALI, evolving on the basis of PAD and often requiring urgent or emergent treatment, further challenges health care systems.
The present study was conducted to delineate the utilization of the German health care system to treat PAD and ALI patients with respect to different treatment modalities, temporal trends, and regional distribution. Analysis of time course and regional distribution of PAD burden will support health policy makers to improve planning and control the provision of regional health care services.
2. Materials and Methods
The principal methods of this study have been applied previously by our research group and are described elsewhere [12,13,14,15,16].
2.1. Data Extraction
Diagnosis-related group (DRG) statistics data of the German Federal Statistical Office (GFSO) from 2009–2018 were extracted for secondary data analysis.
As virtually all (excluding military and psychiatry services) case related data of patient hospital episodes are obliged to be submitted to the GFSO by the German Institute for the Hospital Remuneration System (InEK) by §21 Hospital Finance Act (Krankenhausentgeltgesetz), this analysis by approximation was a full survey of the German population and hospitals. Due to the completeness of the data, imputation of missing data was not necessary. Follow-up of each DRG case covered the period from hospital admission to discharge. Legal basis of the use of DRG statistics for secondary data analysis is the German Federal Statistics Act (§3a; §16). The study protocol was approved by the ethics committee of the Medical Faculty, Technical University of Munich (Reference 21/16 S). The study was conducted in compliance with good practice of secondary data analysis [17] and the manuscript was written in accordance to the STROSA guideline (2nd version) [18].
Controlled remote data processing was used to access data on the servers of the GFSO according to data protection rules [12,13,14,15].
Data of the GFSO and Federal Institute for Research on Building, Urban Affairs, and Spatial Development (BBSR; INKAR database) were linked. Sex and age-specific population, district type (settlement structure, defined by BBSR), and length of hospital stay were linked. To identify the treating hospital, the institutional identification (“Institutskennung”) was linked to individual hospital addresses (conducted by the GFSO). This allowed for the calculation of distances between the patients’ residencies (center of postal code area) and actual hospital location. Data protection regulations were realized by GFSO employees. Type of treating department was directly adopted from the GFSO DRG database.
2.2. Study Population
Administrative codes used in this analysis are listed in Tables S1 and S2. All DRG cases encoded by International Classification of Diseases (ICD-10) codes for PAD or arterial embolism or thrombosis (AET) were included (Figure 1). Disease stages were operationalized using the ICD-10 code (Table S1). In case of disparities between primary and secondary diagnoses, the main diagnosis was used for classification. As it was not possible to distinguish between arterial embolism and thrombosis, a relevant proportion of ALI cases will be attributable to causes other than PAD (e.g., cardiac or arterio-arterial embolism, peripheral arterial aneurysm, vasculitis, injury). To assess patients who underwent specific treatment, all cases encoded by German operation procedure codes (OPS) for open-surgical procedures (e.g., endarterectomy, various bypasses, surgical revisions), endovascular procedures (e.g., percutaneous transluminal angioplasty (PTA), stenting, implantation of covered stents), procedures to treat ALI (e.g., embolectomy, thrombectomy, thrombolysis), or amputations (e.g., forefoot amputation, below or above the knee amputation) were included. Cases transferred to another hospital without treatment were excluded.
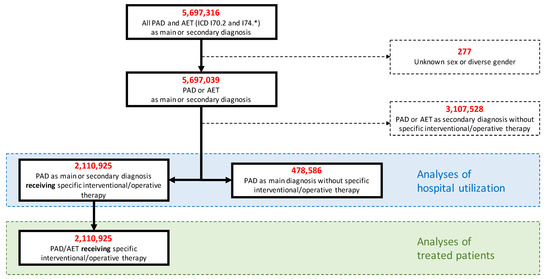
Figure 1.
Flowchart. PAD indicates peripheral arterial disease; AET, arterial embolism or thrombosis.
All analyses refer to cases rather than patients as a link to individual data was not feasible.
2.3. Patient Characteristics, Hospital Incidence and Utilization, Outcomes
To report time trends in hospital incidence, hospital utilization, and treatment modalities, data from 2009–2018 were used. As the study aimed at describing the absolute utilization of vascular services for medical demand planning or hospital planning, no age and sex standardization was performed regarding incidence, while it was performed for the analysis of mortality.
To demonstrate regional distribution of hospital incidence, hospital utilization, and outcomes, choropleth maps were constructed. Thereby, aggregated data from 2018 for each administrative district was illustrated in a defined color or color intensity. Moran’s I statistic was performed to assess for spatial autocorrelation. Hotspot statistics were performed using Getis-Ord Gi * statistics, which is a method for analyzing the local values of a dataset. The resulting Z-values and p-values allow for determination where regions with high or low values tend to form spatial clusters [19,20]. With this analysis, each spatial value is evaluated considering the neighboring values. Regions with a positive Z-value and p-values ≤ 0.1 were defined as hot spots (99% hot spot: p < 0.01; 95%: p = 0.01–0.05; 90% p = 0.051–0.1) while regions with a negative Z-value and with respective p-values were classified as cold spots.
2.4. Statistical Analyses
Absolute numbers and percentages are given for categorical variables (i.e., sex, comorbidities, treating department, minor and major amputation, in-hospital death, endovascular, and open surgical procedures). Medians with first (Q1) and third quartile (Q3) are given for continuous variables (i.e., age, modified Elixhauser Score, distance from residency to hospital, length of hospital stay, annual sum of days in hospital).
As the database is a full survey, calculation of confidence intervals or p values is not necessary.
Controlled remote data processing was carried out using SAS (version 9.4M4 for Microsoft Windows, Copyright 2016, SAS Institute Inc., Cary, NC, USA). Analyses and graphics were performed using R (version 4.0.5, the R Foundation, www.r-project.org (accessed on 11 March 2022)) and Microsoft Excel (Version 2013, Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Baseline Characteristics
Between 2009 and 2018, more than 2.5 million people were treated for peripheral arterial disease in Germany, whereby 2.1 million underwent interventional or surgical therapy (Table 1). With 39%, the highest proportion of cases was treated by general surgical departments, followed by vascular surgery (29%) and internal medicine departments (15%). The average length of stay was nine days if patients received interventional or surgical treatment and six days if treated conservatively. Overall mortality was 4.1%.

Table 1.
Characteristics of cases utilizing in-patient services for peripheral arterial disease and acute limb ischemia (2009–2018).
3.2. Characteristics of Interventionally or Surgically Treated Cases
Most patients undergoing interventional or surgical treatment ranged in Fontaine stage IIb, followed by stages IV and III (Table 2). The average patient age rose with Fontaine stages from 68 years (Fontaine stages I/IIa) to 76 years (Fontaine stage IV). The proportion of patients suffering chronic ischemic heart disease (30%), diabetes (45%), and renal disease (39%) was highest in Fontaine stage IV patients.

Table 2.
Characteristics of operatively or interventionally treated cases utilizing services for lower extremity artery disease from 2009 to 2018 by main diagnosis.
While vascular surgery and general surgery units predominantly treated cases ranging in Fontaine stages IIb upward, the highest proportion of cases treated in internal medicine units ranged from stages I to IIa.
Median length of stay increased from two days in Fontaine stages I or IIb to 14 days in stage IV. Similarly, in-hospital mortality increased with Fontaine stages from 0.1 to 5.8%. The highest mortality (10.1%) was found for patients suffering arterial embolism.
3.3. Procedural Characteristics of Interventionally or Surgically Treated Cases
Mild PAD (Fontaine stages I–IIb) was predominantly treated by above knee balloon angioplasty (64%) with below knee balloon angioplasty only being performed in 10% (Table 3).

Table 3.
Procedural characteristics of operatively treated patients utilizing services for lower extremity artery disease 2009–2018.
CLTI (Fontaine stages III and IV) involved a higher proportion of below knee balloon angioplasty (26%).
Regarding open surgical procedures, mild PAD was most commonly treated with femoral endarterectomy (20%). Bypass surgery was performed in 12% with the distal anastomosis below the knee in 2.9%.
For treatment of CLTI, bypass surgery was applied in 23%. In 14% of cases, the distal bypass anastomosis was situated below the knee.
In the presence of tissue loss (Fontaine stage IV), an amputation was necessary in 46%, of which two thirds (67%) were minor amputations.
Arterial embolism or thrombosis was most frequently treated by open surgical above knee procedures (28%), followed by balloon angioplasty above (21%) and below the knee (11%).
3.4. Temporal Trends of Cases, Hospital Utilization, and Procedures
Between 2009 and 2018, the overall number of PAD and ALI cases increased from 232,302 to 259,572 (Figure 2A). Increases were observed for cases in all but Fontaine stage III, with the largest gain (15%) in cases with tissue loss.
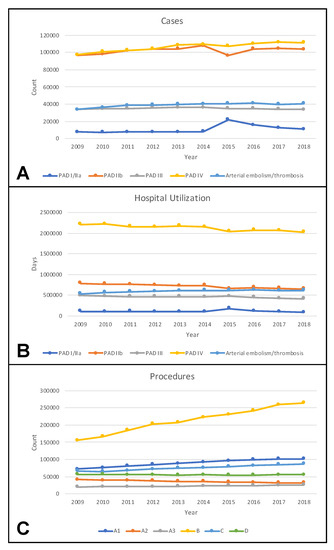
Figure 2.
Temporal trends of cases (A), hospital utilization (B), and procedures (C) in Germany between 2009 and 2018. PAD indicates peripheral arterial disease with respective Fontaine stage; A1, local reconstructions including endarterectomy and patch angioplasty; A2, bypass procedures; A3, surgical revisions; B, balloon angioplasty and stenting; C, surgical and endovascular procedures to treat acute ischemia including (rotational) thrombectomy and thrombolysis; D, amputations.
Regarding arterial embolism or thrombosis, there was a rise from 33,619 to 40,624 cases.
Hospital utilization was measured in hospital days and decreased for PAD across all Fontaine stages (Figure 2B).
Regarding ALI, a rise of arterial embolism or thrombosis was found from 536,350 to 617,025 days.
With respect to applied procedures, the largest increase was found for endovascular procedures, while a decline in bypass surgery was observed (Figure 2C).
3.5. Regional Distribution of Cases, Hospital Utilization, and Outcomes
Choropleth maps were constructed to assess regional distributions for the year 2018. Clustering in northeastern German districts was observed for cases, hospital days, and procedures per 100,000 inhabitants (Figure 3).
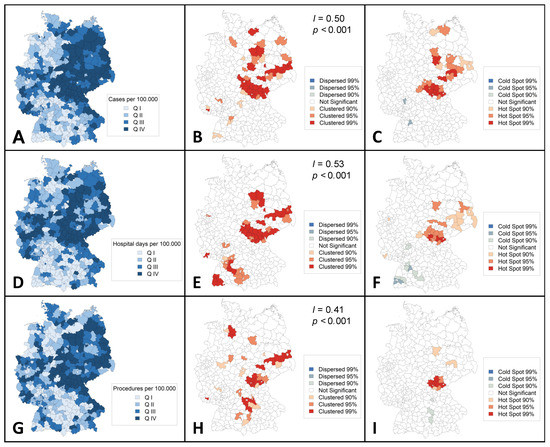
Figure 3.
Regional distribution of cases and hospital utilization (aggregated 2018) in Germany. Choropleth maps showing regional distribution of cases (A–C), hospital days (D–F), and procedures (G–I), all per 100,000 inhabitants. Moran’s I statistics (B,E,F) were performed to assess for spatial autocorrelation. Getis-Ord Gi * statistics (C,F,I) were performed to identify hotspots. Q indicates quartile; I, Global Moran’s I; p, p-value.
The Getis-Ord Gi * analysis revealed 30 administrative regions as hot spots of PAD and ALI incidence, of which 11 were classified as 99% hot spots (p-level < 0.01: Coburg, Hof, Kronach, Nordsachsen, Anhalt-Bitterfeld, Saalekreis, Schmalkalden-Meiningen, Hildburghausen, Ilm-Kreis, Sonneberg, and Saalfeld-Rudolstadt), 13 as 95% hot spots (p-level = 0.01–0.05: Fulda, Kulmbach, Elbe-Elster, Potsdam-Mittelmark, Mecklenburgische Seenplatte, Leipzig country, Magdeburg, Harz, Wittenberg, Suhl, Wartburgkreis, Gotha, and Saale-Orla-Kreis), and six as 90% hot spots (p-level = 0.051–0.1: Dahme-Spreewald, Spree-Neiße, Vorpommern-Greifswald, Leipzig city, Dessau-Roßlau, and Stendal). Groß-Gerau (p = 0.042) and Böblingen (p = 0.045) were identified as 95% cold spots of incidence.
Three administrative regions appeared to be 99% hot spots in terms of hospital days per 100,000 inhabitants (p-level < 0.01: Hof, Kronach, and Hildburghausen), 12 as 95% hot spots (p-level = 0.01–0.05: Coburg, Kulmbach, Dahme-Spreewald, Anhalt-Bitterfeld, Saalekreis, Kyffhäuserkreis, Schmalkalden-Meiningen, Gotha, Ilm-Kreis, Sonneberg, Saalfeld-Rudolstadt, and Saale-Orla-Kreis), and 10 as 90% hot spots (p-level = 0.051–0.1: Essen, Fulda, Saarlouis, Elbe-Nelster, Spree-Neiße, Erzgebirgskreis, Bautzem, Meißen, Nordsachsen, and Suhl). Ten regions were identified as cold spots (95%; p-level = 0.01–0.05: Böblingen, and Breisgau-Hochschwarzwald; 90%; p-level = 0.051–0.1: Darmstadt-Dieburg, Offenbach, Calw, Enzkreis, Emmendingen, Schwarzwald-Baar-Kreis, Reutlingen, and Biberach).
Eleven regions were classified as hot spots in terms of the number of procedures per 100,000 inhabitants (99%; p-level < 0.01: Coburg, Hof, Kronach, Hildburghausen, Sonneberg, and Saalfeld-Rudolstadt; 95%; p-level = 0.01–0.05: Kulmbach, and Saale-Orla-Kreis; 90%; p-level = 0.051–0.1: Lichtenfels, Nordsachsen, and Saalekreis), while three were classified as 90% cold spots (p-level = 0.051–0.1: Fürth, Augsburg, and Donau-Ries).
Regarding major amputations and mortalities, a rise was observed toward northern regions of Germany (Figure 4).
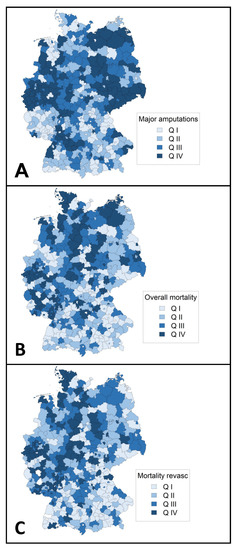
Figure 4.
Regional distribution of outcomes (aggregated 2018) in Germany. Choropleth maps showing major amputation rate per 1000 peripheral arterial disease or arterial embolism or thrombosis cases (A), regional overall mortality (B), and regional mortality in patients who underwent revascularization (C). Mortalities were adjusted for age, sex, Elixhauser score, and Fontaine stage. Q indicates quartile.
With respect to the specialty of the treating center, it was found that PAD patients were predominantly treated by surgical divisions across Germany (Figure S1). Particularly for PAD in Fontaine stages I and II, regions exist where PAD patients are treated predominantly by other specialties (Figure S1B). For the treatment of CLTI (Figure S1C) or ALI (Figure S1D), only in a few areas were patients treated predominantly by non-surgical specialties.
Although it has to be taken into account that even large (but not independent) vascular units might be administratively coded as general surgical units, it was found that regions where PAD patients are treated predominantly by dedicated vascular (i.e., vascular surgery or angiology) units are situated, especially in western Germany (Figure S2). In most of Germany, these patients are treated predominantly by less specialized (e.g., cardiology, internal medicine, general surgery) departments.
4. Discussion
The present study represents a full survey to assess the German health care landscape with respect to patient characteristics, time trends, and regional distributions to treat PAD and ALI.
It was demonstrated that patients with PAD often suffer multiple comorbidities and are at high risk for amputation (17%) and in-hospital death (4.1%). The complexity of patients increases with the severity of PAD, as higher Fontaine stages are associated with more comorbidities, higher amputation rates, and higher mortality. Between 2009 and 2018, an increase in PAD cases was observed, applying especially to the most demanding group of patients with PAD involving tissue loss (Figure 2A). Different studies have found a rise in PAD prevalence over the past years [2,7,21]. Apart from an elevated life expectancy, this might be due to an increasing prevalence of diabetes mellitus [22].
Notably, between 2014 and 2015, a notch was found in the curve of cases for PAD in Fontaine stage III with a contrary movement for Fontaine I/IIa cases (Figure 2A). Coding errors might be the reason as the coding system was changed in 2015 (Table S1) and some cases in Fontaine stage III might have been coded as Fontaine stage I/IIa.
Despite a remarkable increase in PAD cases with tissue loss, the associated hospital utilization declined (Figure 2B). On one hand, this might be explained by a progressive use of endovascular techniques (Figure 2C), typically requiring shorter hospital stays. On the other hand, hospitals are increasingly confronted with economic pressure and are therefore inclined to limit hospital stays to a minimum.
Notably, the upward trend of PAD cases with tissue loss was not paralleled by an increase in amputations (Figure 2C). A retrospective study of German health insurance claims data more specifically demonstrated that major amputations decreased by 15%, while minor amputations rose by 28% between 2008 and 2016 [7]. This observation was confirmed by an observational study on administrative data provided by the Statistical Office of the European Union (EUROSTAT) [23]. The stable number of overall amputations might be explained by improvements in surgical and especially in endovascular techniques, allowing for revascularization of very distal below knee pathologies.
In this study, regional clustering was found for hospital incidence, hospital utilization, and procedures toward northeastern Germany (Figure 3). Previous studies have reported on the clustering of other conditions related to atherosclerotic disease such as carotid artery disease or abdominal aortic aneurysm disease [24,25,26]. A pooled data analysis from the German Health Update showed variations in lifetime prevalence of major cardiovascular disease disfavoring federal states in eastern Germany [27]. Clustering is probably influenced by the distribution of risk factors, environmental factors, socioeconomic factors, and other determinants of variation (e.g., demand, supply, data inaccuracy) [24]. In the case of PAD, a higher proportion of ≥65 year old individuals [26], higher prevalence of tobacco smoking [28], and lower individual income [29] in northeastern German federal states might be important drivers. Therefore, addressing socioeconomic inequalities is likely to reduce the prevalence of PAD and the associated burden of disease. Hospitals and authorities of regions identified as hot spots in the Getis-Ord Gi * statistics should use the results as inducement for further investigation regarding hospital planning and the implementation of vascular units.
Remarkably, there seem to be large parts of the country—especially in the eastern parts of Germany, where the incidence for PAD is highest—in which PAD and ALI patients are predominantly treated by other than dedicated vascular units (Figure S2). This observation has to be interpreted with caution, as even large vascular units might be integrated into general surgical units and coded accordingly. Nevertheless, to encounter the increasing burden of disease associated with PAD and ALI, it might be advantageous to augment the number of vascular units, distribute them more homogenously across the country, and direct patient flows toward them.
Limitations
This study had a number of limitations, which in part have been previously reported [12,16].
The underlying dataset contained administrative data with hospital remuneration as the primary purpose. Despite regular controls by the Medical Review Board of the Statutory Health Insurance Funds, underreporting of comorbidities not affecting hospital remuneration with falsely low comorbidity rates cannot be ruled out. Nevertheless, hospital incidence and death were considered reliable outcomes. As there was no individual patient identifier, double counts could not be excluded, however, the main purpose of this study was to evaluate hospital utilization. For this question, double counts of individual patients do not seem relevant. The same accounts for follow-up after hospital discharge.
Coding errors or intentional up-coding cannot be excluded. However, due to the high number of cases, their impact on the whole cohort was considered negligible. Furthermore, adequacy of coding is regularly checked by the Medical Review Board of the Statutory Health Insurance Funds. Although a relevant proportion of ALI cases may not be attributed to an underlying PAD, this study aimed to describe the overall utilization of peripheral vascular treatment services in Germany. As the study design did not allow for disentanglement of arterial thrombosis from embolism, a relevant proportion of included ALI cases will not be associated with PAD. Type of treating department was directly adopted from the DRG data. Due to differences in local organization structures, even large (but not independent) vascular units might be administratively coded as general surgical units (e.g., department for general, visceral, and vascular surgery). Furthermore, it was not possible to extract information on symptom status for ALI cases (e.g., Rutherford classification). As only one ICD-10 code existed for PAD staged Fontaine I and IIa between 2005 and 2015 (Table S1), it was not possible to categorize symptoms by intermittent claudication and CLTI, respectively.
Due to data protection regulations, Moran’s I statistics and Getis-Ord Gi * statistics could not be performed for all clinical outcomes.
Despite the listed limitations, the present study represents a full survey comprising virtually all patients suffering PAD and AET and utilizing in-patient services in Germany between 2009 and 2018, therefore minimizing the risk of selection bias.
Differences regarding incidence and mortality of PAD and AET are most likely due to the different age structure of the regional population. As the main purpose of this paper was the absolute (not the relative) burden of disease and hospital utilization, age and sex standardization would not have been appropriate.
5. Conclusions
The present study underlines the complexity of PAD and ALI patients as they are afflicted with various comorbidities and high mortality and morbidity. Therefore, PAD poses a major challenge to the German health care system. It was shown that the hospital incidence of PAD with tissue loss has been increasing. Nevertheless, and possibly due to an increased use of endovascular techniques, hospital utilization to treat PAD with tissue loss has decreased. Clustering of PAD and ALI was found disfavoring northeastern Germany. Addressing socioeconomic inequalities and a more homogeneous distribution of dedicated vascular units might be advantageous in reducing the burden of disease associated with PAD and ALI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11082116/s1, Table S1: Definition of cases according to ICD-10-GM; Table S2: Case definitions of surgical procedures (A1–3), endovascular procedures (B), procedures to treat arterial embolism or thrombosis (C), and amputations (D), according to the Operation and Procedure Classification System (OPS) between 2005 and 2008 (source: www.dimdi.de (accessed on 11 March 2022)); Figure S1: Regional distribution of treating units in Germany: surgical (vascular or general) units vs. other (angiology, cardiology, internal medicine) units; Figure S2: Regional distribution of treating units in Germany: dedicated vascular (vascular surgery or angiology) units vs. all other (cardiology, internal medicine, general surgery) units.
Author Contributions
Conceptualization, M.T., C.K., H.-H.E. and A.K.; Methodology, M.T., C.K., S.H. and A.K.; Software, B.B. and S.H.; Validation, M.T., C.K. and A.K.; Formal Analysis, B.B. and S.H.; Investigation, B.B. and S.H.; Resources, B.B., S.H. and A.K.; Data Curation, B.B., S.H. and A.K.; Writing—Original Draft Preparation, M.T. and C.K.; Writing—Review & Editing, M.T., C.K., B.B., S.H., H.-H.E. and A.K.; Visualization, B.B., S.H. and C.K.; Supervision, M.T., H.-H.E. and A.K.; Project administration, M.T., C.K. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the ethics committee of the Medical Faculty, Technical University of Munich (Reference 21/16 S).
Informed Consent Statement
Patient consent was waived, as individuals cannot be identified.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from the German Federal Statistical Office and are available from the authors with the permission of the German Federal Statistical Office.
Acknowledgments
We thank Melanie Heiliger, Jutta Spindler, and Sabine Nemitz from the German Federal Statistical Office for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Nehler, M.R.; Duval, S.; Diao, L.; Annex, B.H.; Hiatt, W.R.; Rogers, K.; Zakharyan, A.; Hirsch, A.T. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J. Vasc. Surg. 2014, 60, 686–695 e682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Luders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Kotov, A.; Peters, F.; Debus, E.S.; Zeller, T.; Heider, P.; Stavroulakis, K.; Remig, J.; Gussmann, A.; Hoffmann, J.; Friedrich, O.; et al. The prospective GermanVasc cohort study. Vasa 2021, 50, 446–452. [Google Scholar] [CrossRef]
- Scully, R.E.; Arnaoutakis, D.J.; DeBord Smith, A.; Semel, M.; Nguyen, L.L. Estimated annual health care expenditures in individuals with peripheral arterial disease. J. Vasc. Surg. 2018, 67, 558–567. [Google Scholar] [CrossRef]
- Kreutzburg, T.; Peters, F.; Riess, H.C.; Hischke, S.; Marschall, U.; Kriston, L.; L’Hoest, H.; Sedrakyan, A.; Debus, E.S.; Behrendt, C.A. Editor’s Choice—Comorbidity Patterns Among Patients with Peripheral Arterial Occlusive Disease in Germany: A Trend Analysis of Health Insurance Claims Data. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, J.A.; Katzen, B.T.; Neville, R.F.; Lookstein, R.A.; Zeller, T.; Miller, L.E.; Jaff, M.R. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J. Am. Heart Assoc. 2018, 7, e009724. [Google Scholar] [CrossRef]
- Callum, K.; Bradbury, A. ABC of arterial and venous disease: Acute limb ischaemia. BMJ 2000, 320, 764–767. [Google Scholar] [CrossRef] [Green Version]
- Bjorck, M.; Earnshaw, J.J.; Acosta, S.; Bastos Goncalves, F.; Cochennec, F.; Debus, E.S.; Hinchliffe, R.; Jongkind, V.; Koelemay, M.J.W.; Menyhei, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 173–218. [Google Scholar] [CrossRef] [Green Version]
- Kulezic, A.; Acosta, S. Epidemiology and Prognostic Factors in Acute Lower Limb Ischaemia: A Population Based Study. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kuhnl, A.; Erk, A.; Trenner, M.; Salvermoser, M.; Schmid, V.; Eckstein, H.H. Incidence, Treatment and Mortality in Patients with Abdominal Aortic Aneurysms. Dtsch. Arztebl. Int. 2017, 114, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krautz, C.; Nimptsch, U.; Weber, G.F.; Mansky, T.; Grutzmann, R. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann. Surg. 2018, 267, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grutzmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef]
- Nimptsch, U.; Mansky, T. Trends in acute inpatient stroke care in Germany--an observational study using administrative hospital data from 2005-2010. Dtsch. Arztebl. Int. 2012, 109, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Trenner, M.; Kuehnl, A.; Reutersberg, B.; Salvermoser, M.; Eckstein, H.H. Nationwide analysis of risk factors for in-hospital mortality in patients undergoing abdominal aortic aneurysm repair. Br. J. Surg. 2018, 105, 379–387. [Google Scholar] [CrossRef]
- Swart, E.; Gothe, H.; Geyer, S.; Jaunzeme, J.; Maier, B.; Grobe, T.G.; Ihle, P. Good Practice of Secondary Data Analysis (GPS): Guidelines and recommendations. Gesundheitswesen 2015, 77, 120–126. [Google Scholar]
- Swart, E.; Bitzer, E.M.; Gothe, H.; Harling, M.; Hoffmann, F.; Horenkamp-Sonntag, D.; Maier, B.; March, S.; Petzold, T.; Rohrig, R.; et al. A Consensus German Reporting Standard for Secondary Data Analyses, Version 2 (STROSA-STandardisierte BerichtsROutine fur SekundardatenAnalysen). Gesundheitswesen 2016, 78, e161. [Google Scholar] [CrossRef] [Green Version]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, C.A.; Sigvant, B.; Szeberin, Z.; Beiles, B.; Eldrup, N.; Thomson, I.A.; Venermo, M.; Altreuther, M.; Menyhei, G.; Nordanstig, J.; et al. International Variations in Amputation Practice: A VASCUNET Report. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehnl, A.; Salvermoser, M.; Erk, A.; Trenner, M.; Schmid, V.; Eckstein, H.H. Spatial Analysis of Hospital Incidence and in Hospital Mortality of Abdominal Aortic Aneurysms in Germany: Secondary Data Analysis of Nationwide Hospital Episode (DRG) Data. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Kuhnl, A.S.M.; Knipfer, E.; Zimmermann, A.; Schmid, V.; Eckstein, H.H. Regional frequency variation of revascularization procedures for carotid stenosis in Germany. Secondary data analysis of DRG data from 2012 to 2014. Gefasschirurgie: Zeitschrift fur vaskulare und endovaskulare Chirurgie: Organ der Deutschen und der Osterreichischen Gesellschaft fur Gefasschirurgie unter Mitarbeit der Schweizerischen Gesellschaft fur Gefasschirurgie. Gefässchirurgie 2018, 23, 519–528. [Google Scholar] [CrossRef]
- Deutsche Herzstiftung. Deutscher Herzbericht 2020. Available online: https://www.herzstiftung.de/system/files/2021-06/Deutscher-Herzbericht-2020.pdf (accessed on 10 March 2022).
- Dornquast, C.; Kroll, L.E.; Neuhauser, H.K.; Willich, S.N.; Reinhold, T.; Busch, M.A. Regional Differences in the Prevalence of Cardiovascular Disease. Dtsch. Arztebl. Int. 2016, 113, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Kotz, D.; Bockmann, M.; Kastaun, S. The Use of Tobacco, E-Cigarettes, and Methods to Quit Smoking in Germany. Dtsch. Arztebl. Int. 2018, 115, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Hans Böckler Stiftung. WSI Verteilungsmonitor—Verfügbare Pro-Kopf-Einkommen, Regional. Available online: https://www.boeckler.de/pdf/wsi_vm_verfuegbare_einkommen.pdf (accessed on 10 March 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).