Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values
Abstract
1. Introduction
2. Materials and Methods
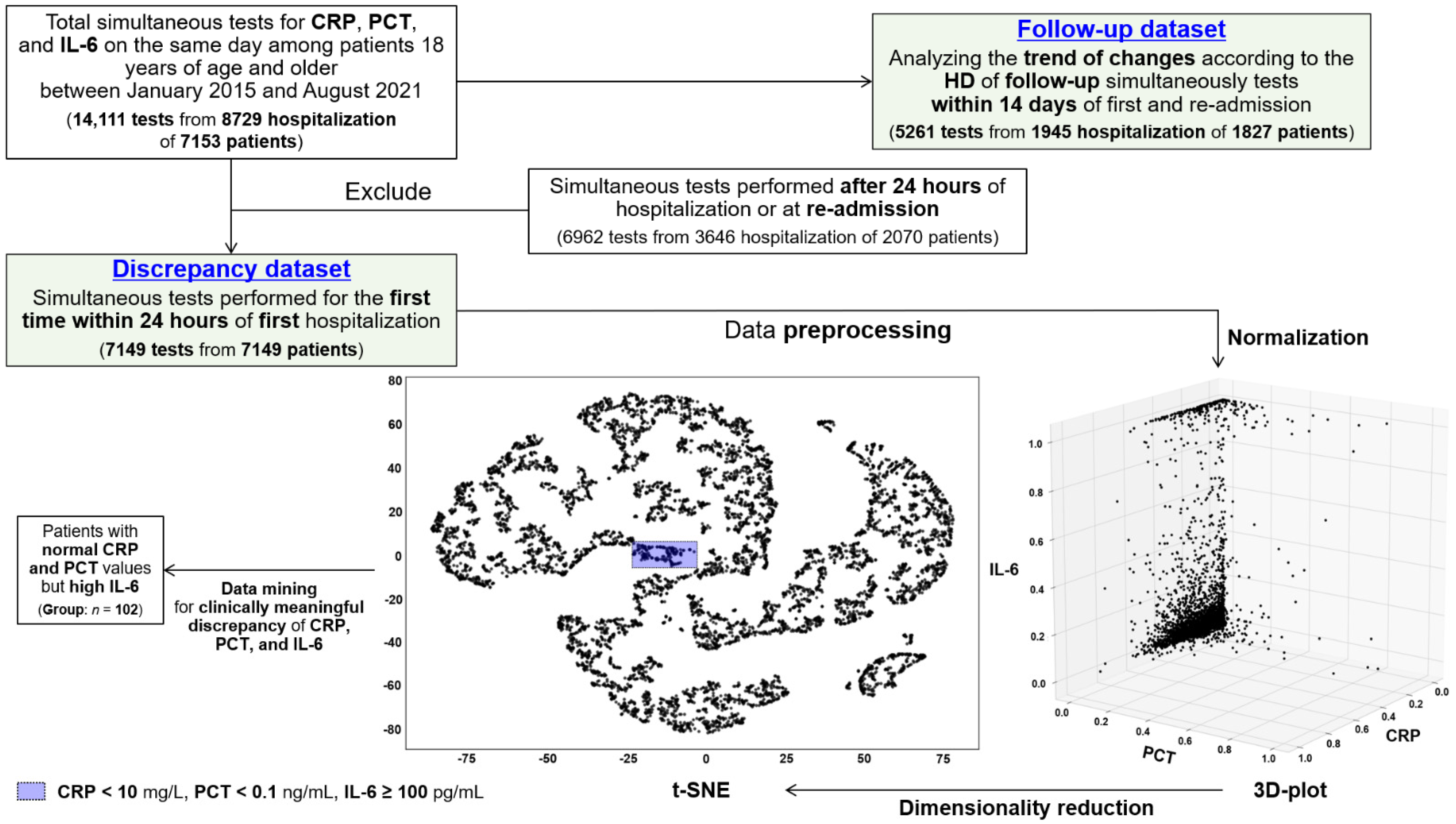
2.1. Study Design and Data Preprocessing
2.2. Group Selection and Data Collection
2.3. Definitions
2.4. Measurements of Inflammatory Markers
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Discrepancy and Follow-Up Dataset
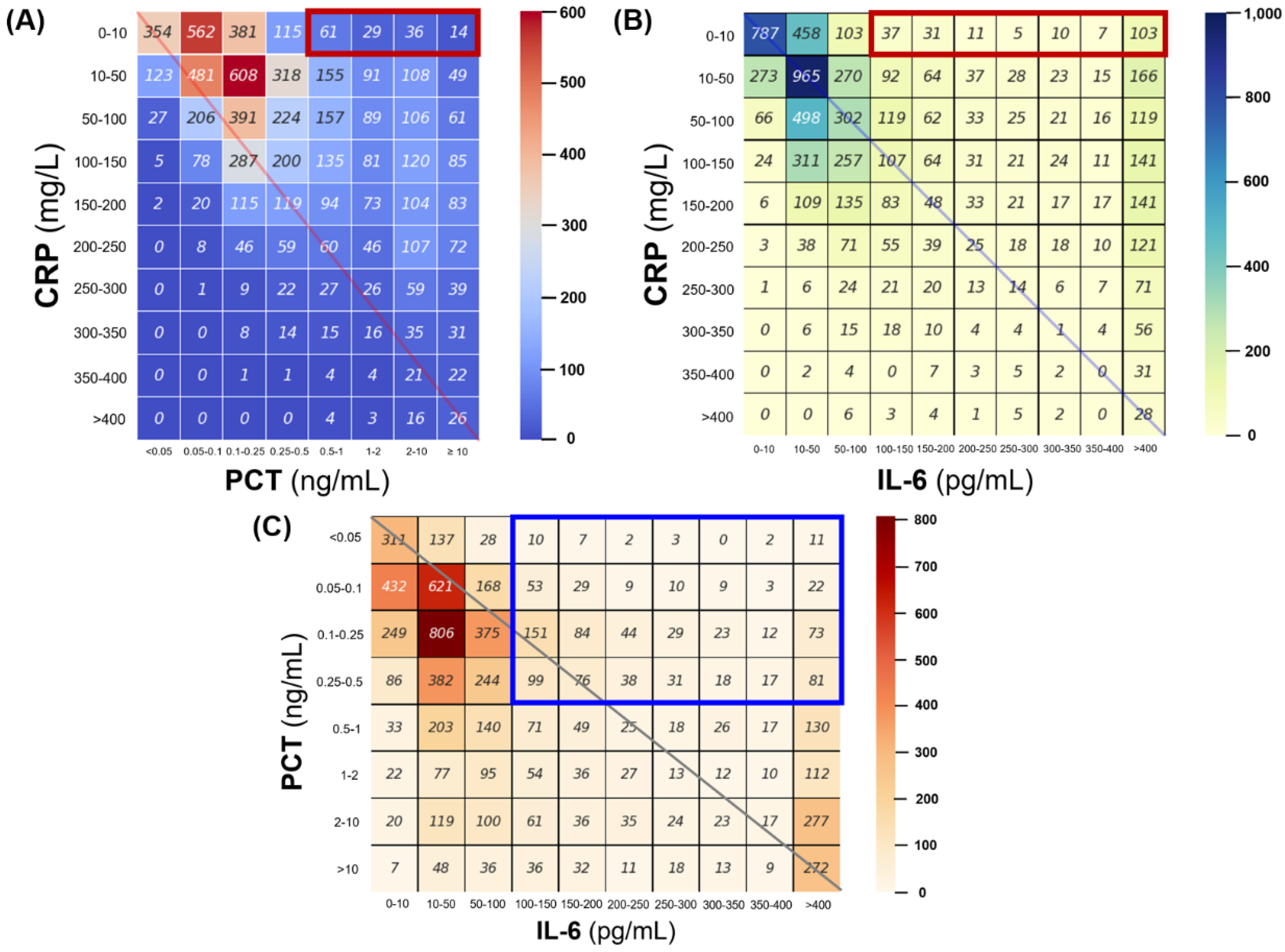
3.2. Concordance of CRP, Procalcitonin and IL-6 in Discrepancy Dataset
3.3. Characteristics of Patients with Normal CRP and PCT Levels but High IL-6 Levels According to the Occurrence of Infection
3.4. Changing Patterns of Concurrent CRP, Procalcitonin, and IL-6 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hausfater, P. Biomarkers and infection in the emergency unit. Med. Mal. Infect. 2014, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Bel Imam, M.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686. [Google Scholar] [CrossRef] [PubMed]
- Haag, E.; Molitor, A.; Gregoriano, C.; Müller, B.; Schuetz, P. The value of biomarker-guided antibiotic therapy. Expert Rev. Mol. Diagn 2020, 20, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Paudel, R.; Dogra, P.; Montgomery-Yates, A.A.; Coz Yataco, A. Procalcitonin: A promising tool or just another overhyped test? Int. J. Med. Sci. 2020, 17, 332–337. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Larsson, A.; Tydén, J.; Johansson, J.; Lipcsey, M.; Bergquist, M.; Kultima, K.; Mandic-Havelka, A. Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scand. J. Clin. Lab. Investig. 2020, 80, 156–161. [Google Scholar] [CrossRef]
- Kataoka, H.; Kono, H.; Patel, Z.; Kimura, Y.; Rock, K.L. Evaluation of the contribution of multiple damps and damp receptors in cell death-induced sterile inflammatory responses. PLoS ONE 2014, 9, e104741. [Google Scholar] [CrossRef]
- Bloomfield, S.M.; McKinney, J.; Smith, L.; Brisman, J. Reliability of s100b in predicting severity of central nervous system injury. Neurocrit. Care 2007, 6, 121–138. [Google Scholar] [CrossRef]
- Schuetz, P.; Hausfater, P.; Amin, D.; Amin, A.; Haubitz, S.; Faessler, L.; Kutz, A.; Conca, A.; Reutlinger, B.; Canavaggio, P.; et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: The multinational, prospective, observational triage study. Crit. Care 2015, 19, 377. [Google Scholar] [CrossRef]
- Memar, M.Y.; Baghi, H.B. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef]
- Jakubovic, B.D.; Vecillas, L.L.; Jimenez-Rodriguez, T.W.; Sanchez-Sanchez, S.; Castells, M. Drug hypersensitivity in the fast lane: What clinicians should know about phenotypes, endotypes, and biomarkers. Ann. Allergy Asthma Immunol. 2020, 124, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.F.; Wu, C.C.; Liu, S.H.; Chen, K.F. Comparison of the accuracy of neutrophil cd64, procalcitonin, and c-reactive protein for sepsis identification: A systematic review and meta-analysis. Ann. Intensive Care 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P. C-reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.H.; Olsen, S.H.; Ahmad, E.; Bigelow, N.C. Neutrophil cd64 is an improved indicator of infection or sepsis in emergency department patients. Arch. Pathol. Lab. Med. 2006, 130, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The c-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet. Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and c-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and c-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell. Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Ratzinger, F.; Haslacher, H.; Perkmann, T.; Schmetterer, K.G.; Poeppl, W.; Mitteregger, D.; Dorffner, G.; Burgmann, H. Sepsis biomarkers in neutropaenic systemic inflammatory response syndrome patients on standard care wards. Eur. J. Clin. Investig. 2015, 45, 815–823. [Google Scholar] [CrossRef]
- Kamat, I.S.; Ramachandran, V.; Eswaran, H.; Guffey, D.; Musher, D.M. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin. Infect. Dis. 2020, 70, 538–542. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of america and the american thoracic society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Aloisio, E.; Dolci, A.; Panteghini, M. Procalcitonin: Between evidence and critical issues. Clin. Chim. Acta 2019, 496, 7–12. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Pike, F.; Yealy, D.M.; Huang, D.T.; Shapiro, N.I.; Angus, D.C. Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock: Analysis of the protocol-based care for early septic shock study. Crit. Care Med. 2017, 45, 438–445. [Google Scholar] [CrossRef]
- Iwase, S.; Nakada, T.A.; Hattori, N.; Takahashi, W.; Takahashi, N.; Aizimu, T.; Yoshida, M.; Morizane, T.; Oda, S. Interleukin-6 as a diagnostic marker for infection in critically ill patients: A systematic review and meta-analysis. Am. J. Emerg. Med. 2019, 37, 260–265. [Google Scholar] [CrossRef]
- Cong, S.; Ma, T.; Di, X.; Tian, C.; Zhao, M.; Wang, K. Diagnostic value of neutrophil cd64, procalcitonin, and interleukin-6 in sepsis: A meta-analysis. BMC Infect. Dis. 2021, 21, 384. [Google Scholar] [CrossRef]
- Zhou, P.; Waresi, M.; Zhao, Y.; Lin, H.C.; Wu, B.; Xiong, N.; Li, H.; Huang, Q.; Luo, X.; Li, J. Increased serum interleukin-6 level as a predictive biomarker for atrial fibrillation: A systematic review and meta-analysis. Rev. Port. Cardiol. 2020, 39, 723–728. [Google Scholar] [CrossRef]
- Ilmarinen, P.; Tuomisto, L.E.; Niemelä, O.; Danielsson, J.; Haanpää, J.; Kankaanranta, T.; Kankaanranta, H. Comorbidities and elevated il-6 associate with negative outcome in adult-onset asthma. Eur. Respir. J. 2016, 48, 1052–1062. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Liu, W.; Zhang, X.; Xue, L. The diagnostic value of interleukin 6 as a biomarker for gastric cancer: A meta-analysis and systematic review. Medicine 2021, 100, e27945. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and specificity: Insights from the interleukin 6 family of cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Yin, Y.L.; Guo, W.Z.; Ma, Y.Q.; Wang, Y.B.; Shu, C.; Dong, L.Q. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Fatemi, Y.; Bergl, P.A. Diagnostic stewardship: Appropriate testing and judicious treatments. Crit. Care Clin. 2022, 38, 69–87. [Google Scholar] [CrossRef]
- Takahashi, W.; Nakada, T.A.; Yazaki, M.; Oda, S. Interleukin-6 levels act as a diagnostic marker for infection and a prognostic marker in patients with organ dysfunction in intensive care units. Shock 2016, 46, 254–260. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- Molano Franco, D.; Arevalo-Rodriguez, I.; Roqué, I.F.M.; Montero Oleas, N.G.; Nuvials, X.; Zamora, J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst. Rev. 2019, 4, Cd011811. [Google Scholar] [CrossRef] [PubMed]
- Shimazui, T.; Matsumura, Y.; Nakada, T.A.; Oda, S. Serum levels of interleukin-6 may predict organ dysfunction earlier than sofa score. Acute Med. Surg. 2017, 4, 255–261. [Google Scholar] [CrossRef]
- Coster, D.; Wasserman, A.; Fisher, E.; Rogowski, O.; Zeltser, D.; Shapira, I.; Bernstein, D.; Meilik, A.; Raykhshtat, E.; Halpern, P.; et al. Using the kinetics of c-reactive protein response to improve the differential diagnosis between acute bacterial and viral infections. Infection 2020, 48, 241–248. [Google Scholar] [CrossRef]
- Bernstein, D.; Coster, D.; Berliner, S.; Shapira, I.; Zeltser, D.; Rogowski, O.; Adler, A.; Halutz, O.; Levinson, T.; Ritter, O.; et al. C-reactive protein velocity discriminates between acute viral and bacterial infections in patients who present with relatively low crp concentrations. BMC Infect. Dis. 2021, 21, 1210. [Google Scholar] [CrossRef] [PubMed]
- Holzknecht, M.; Tiller, C.; Reindl, M.; Lechner, I.; Troger, F.; Hosp, M.; Mayr, A.; Brenner, C.; Klug, G.; Bauer, A.; et al. C-reactive protein velocity predicts microvascular pathology after acute st-elevation myocardial infarction. Int. J. Cardiol. 2021, 338, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Banai, A.; Levit, D.; Morgan, S.; Loewenstein, I.; Merdler, I.; Hochstadt, A.; Szekely, Y.; Topilsky, Y.; Banai, S.; Shacham, Y. Association between c-reactive protein velocity and left ventricular function in patients with st-elevated myocardial infarction. J. Clin. Med. 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Levinson, T.; Wasserman, A. C-reactive protein velocity (crpv) as a new biomarker for the early detection of acute infection/inflammation. Int. J. Mol. Sci. 2022, 23, 8100. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total (n = 102) | Infection Diagnosed within 3 Days of Admission | p-Values | |
|---|---|---|---|---|
| Yes (n = 36) | No (n = 66) | |||
| Age, years | 67.1 ± 15.6 | 69.4 ± 16.5 | 65.9 ± 15.1 | 0.281 |
| Sex, male | 55 (53.9) | 20 (55.6) | 35 (53.0) | 0.838 |
| BMI, kg/m2 | 23.0 ± 3.9 | 23.2 ± 3.6 | 22.8 ± 4.0 | 0.697 |
| Underlying co-morbiditiesa | ||||
| NIDDM | 18 (17.6) | 10 (27.8) | 8 (12.1) | 0.047 |
| Chronic heart diseases | 6 (5.9) | 2 (5.6) | 4 (6.1) | 0.917 |
| Chronic lung diseases | 5 (4.9) | 2 (5.6) | 3 (4.5) | 0.581 |
| Chronic liver diseases | 18 (17.6) | 5 (13.9) | 13 (19.7) | 0.590 |
| Chronic renal diseases | 6 (5.9) | 1 (2.8) | 5 (7.6) | 0.420 |
| Solid cancers | 33 (32.4) | 8 (22.2) | 25 (37.9) | 0.125 |
| Charlson comorbidity index | 4 (3–6) | 5 (1–7) | 4 (3–5) | 0.427 |
| Vaccination history b | 31 (30.4) | 12 (33.3) | 19 (28.8) | 0.637 |
| Clinical presentations at admission | ||||
| Fever c | 62 (60.8) | 28 (77.8) | 34 (51.5) | 0.011 |
| qSOFA | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.842 |
| Altered mentation, GCS < 15 | 24 (23.5) | 5 (13.9) | 19 (28.8) | 0.142 |
| RR ≥ 22 breaths/min | 23 (22.5) | 10 (27.8) | 13 (19.7) | 0.459 |
| Systolic BP ≤ 100 mmHg | 58 (56.9) | 21 (58.3) | 37 (56.1) | 0.664 |
| Fever and shock c | 20 (19.6) | 8 (22.2) | 12 (18.2) | 0.795 |
| Laboratory tests d | ||||
| CRP (mg/L) | 3.5 ± 2.7 | 3.3 ± 2.7 | 3.9 ± 2.7 | 0.320 |
| Procalcitonin (ng/mL) | 0.15 ± 0.10 | 0.15 ± 0.11 | 0.14 ± 0.08 | 0.441 |
| IL-6 (pg/mL) | 947.9 ± 699.6 | 1449.5 ± 791.2 | 674.3 ± 149.2 | 0.007 |
| Creatinine (mg/dL) | 1.3 ± 1.0 | 0.9 ± 0.5 | 1.5 ± 1.1 | 0.031 |
| PT, INR | 1.4 ± 0.7 | 1.2 ± 0.5 | 1.5 ± 0.7 | 0.054 |
| Bacteremia or fungemia e | 9 (8.8) | 9 (25.0) | ― | ― |
| Non-infectious conditions | ― | |||
| Anaphylaxis or drug-related AR | 2 (2.0) | ― | 2 (3.0) | |
| Cancer or anti-neoplastic therapy | 21 (20.6) | ― | 21 (31.8) | |
| Cardiogenic shock | 17 (16.7) | ― | 17 (25.8) | |
| Chronic organ failure | 2 (2.0) | ― | 2 (3.0) | |
| CNS diseases f | 3 (2.9) | ― | 3 (4.5) | |
| Connective tissue disease | 1 (1.0) | ― | 1 (1.5) | |
| Hypovolemic shock g | 7 (6.9) | ― | 7 (10.6) | |
| Post-invasive procedures | 10 (9.8) | ― | 10 (15.2) | |
| Trauma including fracture | 3 (2.9) | ― | 3 (4.5) | |
| Antimicrobial therapy | ||||
| Within 24 h of admission | 67 (65.7) | 25 (69.4) | 42 (63.6) | 0.664 |
| Within 48 h of admission | 71 (69.6) | 28 (77.8) | 43 (65.2) | 0.260 |
| Outcomes | ||||
| Hospital stays—days | 5.0 (1.5–12.5) | 7.1 (2.5–14.6) | 2.7 (1.5–8.7) | 0.023 |
| Invasive MV treatment | 31 (30.4) | 6 (16.7) | 25 (37.9) | 0.041 |
| ICU admission—yes | 27 (26.5) | 7 (19.4) | 20 (30.3) | 0.253 |
| In-hospital all-cause mortality | 21 (20.6) | 7 (19.4) | 14 (21.2) | 0.833 |
| Variables | OR | 95% CI | p-Values |
|---|---|---|---|
| Underlying diseases—yes | |||
| NIDDM | 2.86 | 0.91–8.97 | 0.072 |
| Fever at admission—yes | 2.70 | 1.03–7.05 | 0.043 |
| Laboratory tests within 24 h of admission | |||
| IL-6 > 500 pg/mL a | 1.70 | 0.70–4.12 | 0.242 |
| Creatinine < 0.9 mg/dL a | 0.84 | 0.34–2.08 | 0.710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-H.; Lee, K.-H.; Song, Y.-G.; Han, S.-H. Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values. J. Clin. Med. 2022, 11, 7324. https://doi.org/10.3390/jcm11247324
Lee E-H, Lee K-H, Song Y-G, Han S-H. Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values. Journal of Clinical Medicine. 2022; 11(24):7324. https://doi.org/10.3390/jcm11247324
Chicago/Turabian StyleLee, Eun-Hwa, Kyoung-Hwa Lee, Young-Goo Song, and Sang-Hoon Han. 2022. "Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values" Journal of Clinical Medicine 11, no. 24: 7324. https://doi.org/10.3390/jcm11247324
APA StyleLee, E.-H., Lee, K.-H., Song, Y.-G., & Han, S.-H. (2022). Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values. Journal of Clinical Medicine, 11(24), 7324. https://doi.org/10.3390/jcm11247324







