Abstract
Cell therapies are an emergent treatment for cerebral palsy (CP) with promising evidence demonstrating efficacy for improving gross motor function. However, families value improvements in a range of domains following intervention and the non-motor symptoms, comorbidities and complications of CP can potentially be targeted by cell therapies. We conducted a scoping review to describe all outcomes that have been reported in cell therapy studies for CP to date, and to examine what instruments were used to capture these. Through a systematic search we identified 54 studies comprising 2066 participants that were treated with a range of cell therapy interventions. We categorized the reported 53 unique outcome instruments and additional descriptive measures into 10 categories and 12 sub-categories. Movement and Posture was the most frequently reported outcome category, followed by Safety, however Quality of Life, and various prevalent comorbidities and complications of CP were infrequently reported. Notably, many outcome instruments used do not have evaluative properties and thus are not suitable for measuring change following intervention. We provide a number of recommendations to ensure that future trials generate high-quality outcome data that is aligned with the priorities of the CP community.
1. Introduction
Stem cell and cell therapies have been in clinical research for the treatment of cerebral palsy (CP) for more than 15 years [1]. There are a variety of cell types being investigated including umbilical cord blood, mesenchymal stem/stromal cells, and neural stem- or stem-like cells [2]. The principal target of cell therapies for the treatment of CP is remediation of the underlying brain injury thereby improving neuronal signaling, which could be achieved by either direct or indirect actions. Cell therapies are proposed to work via a variety of mechanisms for the treatment of CP. Depending on the cell type, therapeutic benefits may include reduction of inflammation, promotion of cell survival, stimulation of proliferation and migration of endogenous neural stem cells, replacement and/or regeneration of damaged brain cells, and promotion of angiogenesis [2].
Systematic reviews of randomized controlled trials have shown that improvement in gross motor skills/function, typically measured using the Gross Motor Function Measure (GMFM) [3], is the most common primary outcome assessed [4,5]. Promisingly, these studies have demonstrated that various cell therapies can produce a small but significant improvement in gross motor function [4,5], although these findings are limited by heterogeneity in various aspects (e.g., participants, interventions, outcomes). Furthermore, whilst the number of clinical studies and total number of participants with CP treated with cell therapies continues to climb (now >2427 participants across >77 published and unpublished studies) [1], there remains a high volume of lower-quality evidence employing poor study design and/or unvalidated outcome assessment tools, and thus more research is warranted.
Although CP is characterized by impairment of movement and/or posture, it is a highly heterogeneous condition, and individuals with CP often experience a range of comorbidities and/or co-occurring complications that can be just as disabling as the motor symptoms [6]. These include pain, intellectual impairment, epilepsy, behavior disorders, and vision and hearing impairments [6]. As such, there is an increasing focus within the CP field to understand these elements, and find ways to target them, with the overarching goal of improving the quality of life for people living with CP.
Individuals with CP and their families value a wide range of potential benefits following certain types of stem cell treatments [7] and other interventions [8,9]. These benefits often focus on activity and participation rather than necessarily remediating physical impairment. It is therefore important that clinical studies of cell therapies measure outcomes that are both scientifically valid and valued by individuals with CP and their families. In addition, outcomes should be measured using well-validated tools so that evidence generated from these studies can increase our confidence in study findings. To aid in this, a panel of international experts have compiled recommended CP-specific common data elements for use in clinical research studies [10]. However, these instruments may not always be consistently applied. As such, the purpose of this scoping review is to describe all outcomes that have been reported in cell therapy studies for CP to date, and to examine what instruments have been used to capture these outcomes.
2. Materials and Methods
A protocol for this review was registered on Open Science Framework (OSF) (identifier DOI 10.17605/OSF.IO/T9C8J [https://osf.io/t9c8j/?view_only=9b82c37725834a1da1a50bb199cf5091 (accessed on 14 November 2022)], registration date 8 July 2022). This scoping review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines [11] (Supplementary Table S1).
2.1. Inclusion and Exclusion Criteria
We included any type of study (both controlled and non-controlled studies, including case series/reports) in which participants with CP were treated with a cell therapy intervention specifically for the treatment of CP. If studies reported participants with various diagnoses, >50% must have had CP. There was no restriction on participant age. The full text of the study must also have been published in English (due to no translation services available), in a peer-reviewed journal. Studies were excluded from this review if they reported an organ graft or transplant, or were a secondary analysis of a study that was already included in this review.
2.2. Data Sources and Search Strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, April 2022), PubMed (MEDLINE) (1946 to 6 May 2022) and EMBASE (1947 to 6 May 2022) via OVID using the search strategy described in Supplementary Table S2. The search was conducted on 10th May 2022. De-duplicated results from OVID were exported into Covidence Systematic Review Software (http://www.covidence.org (accessed on 14 November 2022)). Database searching was also supplemented by hand searching, i.e., cross checking systematic review reference lists for potentially eligible articles, and new paper alerts were monitored for potentially eligible papers published after the formal search was conducted.
2.3. Study Selection and Data Extraction
Titles and/or abstracts of studies retrieved using the search strategy were screened independently by two reviewers (split between M.F.-E., M.C.B.P., C.F.). Full texts of studies were then retrieved and independently assessed for eligibility by two reviewers (split between M.F.-E., M.C.B.P., C.F.), with any disagreements resolved by the third screener.
A data extraction form was developed specifically for this review by the research team. Data extraction was performed independently by at least two members of the research team (M.F.-E., M.C.B.P., C.F.), with any discrepancies identified and resolved through discussion with the third extractor. Extracted data included details of study design, participants, intervention/s, comparator (if relevant), and outcome instrument/s.
2.4. Assigning Level of Evidence for Included Studies
The level of evidence for each included study was assigned according to Oxford Centre for Evidence-Based Medicine: Levels of Evidence [12].
2.5. Categorization of Cell Interventions
Cell interventions were sorted into six categories: (1) Umbilical cord blood; (2) Mesenchymal stem/stromal cells; (3) Bone marrow cells, hematopoietic stem cells and peripheral blood cells (including mononuclear cell fragment, enriched/expanded cells from bone marrow or umbilical cord blood, and peripheral blood mononuclear cells); (4) Neural stem cells/neural-like cells (including neural stem cells (NSCs), neural progenitor cells, olfactory ensheathing cells and mesenchymal stem/stromal cell-derived NSC-like cells); (5) Immune cells (M2-like macrophages); and (6) Fetal cells/embryonic stem cells.
2.6. Categorization of Instruments (into Outcome Domains)
For this review, members of the research team (M.F.-E., M.C.B.P., I.H., P.K., C.S., D.C.) determined outcome domain categories and sub-categories for sorting the reported outcome instruments. This process involved consideration of all the extracted outcome instruments followed by group discussion to reach consensus on which outcome categories/sub-categories to include. All outcome instruments were then assigned to these categories/sub-categories according to the outcome domain/s they were designed to assess, again via group discussion between multiple members of the research team (M.F.-E., M.C.B.P., P.K., I.H., C.S., D.C.) to reach agreement.
Outcome instruments that spanned more than one outcome domain, i.e., encompassing multiple reported sub-domains, were assigned to various categories/sub-categories according to these sub-domains. Any instrument for which the outcome being assessed could not be determined (or agreed), the tool was a multi-domain measure but was only reported as a total score, or the instrument did not fit with any other outcome sub/category, were designated as Other. Any reported descriptive/observational outcomes were subsequently categorized into the same outcome sub/categories through discussion and agreement.
2.7. Outcome Instrument Properties
Outcome instrument properties including format (i.e., who completed the assessment and the nature of it), primary purpose (i.e., predictive, discriminative, evaluative or classification) and population designed for, were determined from various information sources including test manuals/handbooks, systematic reviews, and websites, as necessary.
The categories used for instrument format followed that of the U.S. Food and Drug Administration (FDA) types of clinical outcome assessments, namely: Patient (or self)-reported, Clinician (or therapist)-reported, Observer (e.g., parent/carer/teacher)-reported, and Performance-based measures [13]. In this review we have used the term ‘Parent/other’ to denote the Observer group. Additionally, the report type was specified as Questionnaire, Interview (including semi-structured interview) or Observation.
2.8. Calculating Total Number of Participants per Outcome Sub/Category
For calculating the number of participants assessed for each outcome sub/category, n’s were collapsed or compounded as such: In studies that utilized multiple assessment tools within a single outcome sub-category (e.g., Gross Motor measured using GMFM, Gross Motor Function Classification System Expanded and Revised (GMFCS) [14], etc.), the number of participants assessed for Gross Motor was collapsed, meaning that n’s were not counted more than once for that sub-category. E.g., if 10 participants were evaluated using the GMFCS and GMFM, the n for Gross Motor would remain at 10. However, if the same 10 study participants were also assessed for Fine Motor and Upper Limb using the Fine Motor Function Measure (FMFM) [15], this would result in a total compounded n of 20 for the Movement and Posture category.
3. Results
3.1. Search Results
Following the literature search and de-duplication, 1145 records were identified. After title and abstract screening, 93 full-text reports were reviewed and 50 met eligibility [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. A further four eligible reports were identified through hand searching [66,67,68,69]. In addition, during data extraction and preparation of the manuscript, two new studies were identified [70,71] that also met eligibility and were included. Moreover, a study that was initially included was retracted [30] and was therefore subsequently excluded from this review. Thus, finally, 55 reports were included. These 55 reports represented 54 studies since Amanat 2021 [17] and Zarrabi 2022 [64] are two reports of the same clinical trial (clinical trial registration identifier NCT03795974) and share the same control group. The PRISMA [72] flow chart of the search process is presented in Figure 1.
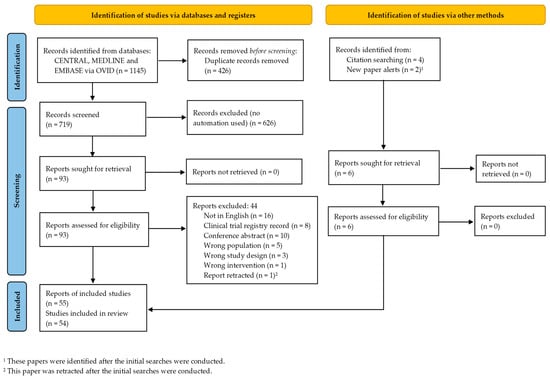
Figure 1.
PRISMA flow diagram of study selection.
3.2. Study Characteristics
A summary of the included studies is presented in Table 1, including details of study design, participants, intervention/s and comparator/s, and outcome instrument/s.

Table 1.
Details of included studies.
3.3. Types of Studies
Of the 54 studies included, 17 (32%) were controlled studies: 14 were randomized controlled trials, with three of these being a cross-over design, and three were non-randomized controlled studies. A further 18 studies (33%) were single-arm and 18 (33%) were case series or case reports, including four studies [19,26,50,56] that were retrospective analyses including ‘therapeutic experiments’ and a ‘post-registration clinical investigation’. In addition, one study (2%) [27] was a non-randomized dose comparison trial. Accordingly, the majority of included studies (n = 37, 69%) were deemed to be Level 4 evidence with n = 3 being Level 3, and n = 14 were Level 2.
3.4. Types of Participants
Collectively, data from 2066 participants was reported, and all studies exclusively included participants with CP. Most studies enrolled/treated participants of various type and topography, and all GMFCS severity levels were represented. Whilst the majority of studies recruited/treated children and youth (up to 18 years) with CP, participant ages ranged from 6 months to 35 years (Table 1).
3.5. Types of Interventions
The majority of studies administered one cellular intervention, however four studies in five reports [17,36,64,66,71] investigated two different cell therapies head-to-head to give a total of 58 cell regimens administered.
For these 58 cell regimens, the classification into the various cell therapy types was: 31% bone marrow cells, hematopoietic stem cells and peripheral blood cells (n = 18); 29% mesenchymal stem/stromal cells (n = 17); 26% umbilical cord blood (n = 15); 7% neural stem cells/neural-like cells (n = 4); 3% immune cells (n = 2); and 3% fetal cells/embryonic stem cells (n = 2). The source of cells was autologous in 32 studies (55%) and allogeneic in 25 studies (43%). The donor origin of the cells could not be determined in one study (2%) [25].
Cell interventions were delivered by various routes. Intrathecal (n = 25, 43%) or intravenous (n = 20, 34%) delivery was the most common. A further three studies (5%) used a combination of the two. Exclusive direct transplantation into the brain (intracerebral, intra-cerebroventricular) was used infrequently (n = 3, 5%), and all were for studies that administered neural stem cells/neural-like cells. In addition, one study (2%) [61] used intrathecal +/− intra-parenchymal brain administration for mesenchymal stem/stromal cells. The remainder of the studies (n = 6, 10%) utilized various routes (or a combination of routes) including but not limited to intra-arterial or intramuscular delivery (Table 1).
3.6. Types of Outcome Measures
Instruments measuring treatment outcomes were grouped into ten categories: (1) Movement and Posture; (2) Cognition and General Development; (3) Communication and Language; (4) Behavior; (5) Activities of Daily Living; 6) Quality of Life; (7) Brain Structure and Function; (8) Biomarkers; (9) Safety and (10) Other. Four of these categories were further split into a total of 12 sub-categories (Table 1, Figure 2). The 12 sub-categories were Gross Motor, Fine Motor and Upper Limb, Spasticity, Muscle Strength and General Motor within Movement and Posture; Communication and Language within Communication and Language; Adaptive Behavior, Executive Function and Social-Emotional within Behavior; and Neuroimaging and Seizures/Electrical Brain Activity within Brain Structure and Function.
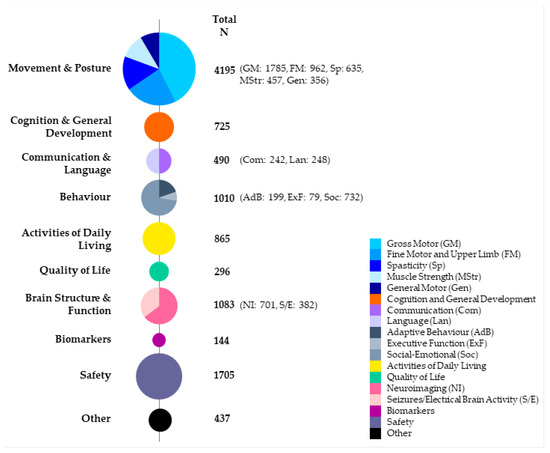
Figure 2.
Number of participants assessed for each outcome category and sub-category across all included studies. N, number of participants.
Unsurprisingly, Movement and Posture was the most frequently reported outcome category (n = 4195) (Figure 2). Indeed, all included studies reported on Movement and Posture except Feng 2015 [26], which exclusively reported safety. Within Movement and Posture, measures of Gross Motor were the most common, followed by Fine Motor and Upper Limb then Spasticity. Safety was the next most common category (n = 1705) and was specifically reported in all but 11 studies. Reported safety data included adverse event reporting, routine laboratory and clinical assessments (e.g., bloods/biochemistry, X-ray), and neuroimaging conducted exclusively for safety. Brain Structure and Function (n = 1083), Behavior (n = 1010) and Activities of Daily Living (n = 865) were all also commonly reported outcome categories (Figure 2). In contrast, a relatively small proportion of participants were assessed for Biomarkers and Quality of Life. Of the four studies that conducted biomarker analysis these comprised assessment of various cytokine and growth factor levels including interferon (IFN)-γ, interleukin (IL)-17, IL-4, brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF) [22], BDNF [23], pentraxin 3 (PTX-3), IL-8, and IL-10 [33] and PTX-3, IL-8, tumor necrosis factor (TNF)-α, and IL-1β [41].
Examining the data by either study design or cell intervention type revealed a similar pattern, with Movement and Posture consistently the most frequently reported outcome category, followed by Safety, and a relatively similar distribution of participants across outcome sub/categories (Supplementary Figures S1 and S2).
Across the included studies there were 53 unique instruments reported, although not all were true outcome measure, i.e., responsive to change (Table 1 and Table 2). This number does not include measures of Safety or Biomarkers since these are commonly reported in various ways and could not be synthesized, nor descriptive/observational outcomes. The categorization of all instruments into outcome sub-/categories is shown in Table 2. Notably, 12/53 of the captured instruments had multiple sub-domains that were reported and hence were included across several outcome categories/sub-categories in Table 2.

Table 2.
Outcome instruments used in cerebral palsy cell therapy studies with details.
More than one instrument was used across the studies for the majority of outcome categories/sub-categories. For example, Gross Motor was assessed using 12 different tools, Cognition and General Development by 11, and Activities of Daily Living by eight different instruments (Table 2). In contrast, Executive Function was assessed using just a single instrument, the Behavior Rating Inventory of Executive Function (BRIEF) [74], in a single study.
The most commonly reported instrument was the GMFM (n = 1163), with this measure reported for 56% of all included participants in this review. The Pediatric Evaluation of Disability Inventory (PEDI)/PEDI-Computer Adaptive Test (PEDI-CAT) [75] (n = 573), GMFCS (n = 533) and magnetic resonance imaging (MRI) with or without diffusion tensor imaging (DTI) (n = 525) were also frequently used (Table 2).
Of note, study participants were often assessed using more than one instrument within an outcome category/sub-category (Table 1). This was particularly true for Gross Motor. For example, Rah 2017 [49] assessed participants using the GMFM, GMFCS, PEDI and Denver Development Screening Test (DDST) [76], all measures of gross motor capacity and/or performance. Although many studies also just used single instruments to assess various outcome domains (Table 1). Furthermore, the total number of instruments used per study varied substantially. Whereas Feng 2015 [26] only assessed safety, Min 2020 [41] administered 18 instruments (including safety and biomarker assessments) (Table 1).
Descriptive Outcomes
In addition to the above reported outcome instruments, there were numerous descriptive/observational outcomes reported. For instance, 24/54 (44%) studies included purely descriptive outcome/s for at least one outcome category/sub-category (Table 1). Some studies were heavily weighted to reporting descriptive outcomes almost exclusively, particularly case series/reports or single-arm studies. Moreover, some outcome sub-categories were more often reported via descriptive means than an outcome instrument. For example, as mentioned above, although Executive Function was assessed using the BRIEF in only one study, it was captured descriptively in another five studies.
Of particular note are the descriptive/observational outcomes classified under the Other category. These covered a range of outcomes including sleep, sensory (sensory processing/smell), vision, hearing, appetite and immunity, as well as overarching/comprehensive assessments of participant condition/well-being (Table 1).
3.7. Outcome Instrument Properties
The properties of all reported instruments including format, primary purpose and population designed for are shown in Table 2. The largest proportion of instruments (55%) were either exclusively, or partially, Performance-based measures. Clinician-reported measures were the next most commonly utilized, representing 26% of instruments, and these were typically Observations. Other measures were either Parent/other-reported or could be completed interchangeably by a clinician, parent/other or the participant themselves. Only one instrument was exclusively Self-reported (Modified Rankin Scale). In general, across the various outcome sub-/categories, there was a mix between Performance-based and Clinician-reported measures, although Brain Structure and Function was exclusively Clinician Observation. The three Quality of Life instruments were all Parent/other or Self-reported, and most Behavior and Activities of Daily Living assessment tools included input from Parent/other (Table 2).
Of the 53 instruments, 33 (62%) were determined to be evaluative measures, 14 discriminative and/or predictive, and three were classification systems. Of particular note, all instruments within Brain Structure and Function were designated as discriminative/predictive, and the Language and Communication outcome tools were also primarily non-evaluative. Finally, 14 (26%) of the instruments were specifically designed for a CP-population, mostly within the Movement and Posture category. An additional six were designed for adult and/or pediatric rehabilitation and the remainder are for non-specific (general) populations.
When comparing the 53 reported outcome instruments against the highly recommended tools within the common data elements for CP [10], only six instruments overlapped: the GMFM, Tardieu Scale [77], Bayley Scales of Infant and Toddler Development (BSID) [78], Wechsler Intelligence Scale for Children (WISC) [79], BRIEF, and the Cerebral Palsy Quality of Life Questionnaire (CP QOL) [80]. Of note, the GMFCS, MACS and CFCS are also recommended in the common data elements, but as classification systems.
4. Discussion
Stem cells and cell therapies offer great potential as a treatment for CP, with efficacy demonstrated in systematic reviews [4,5]. Improvements in gross motor function have been the most commonly studied outcome in randomized controlled trials, however individuals with CP and their families cite improvements in various domains to be of value [7,9]. We conducted this scoping review to describe all outcomes reported in cell therapy studies for CP to date. From this, we wanted to understand whether clinical study outcomes align with common comorbidities and complications of CP, and hence whether they are meeting the expectations of trial participants and their families. Furthermore, we aimed to examine the instruments that are being used to assess these outcomes, to determine whether they are being captured appropriately.
We found that, across 54 included studies comprising >2000 participants, a large range of outcome domains/categories were reported. Notably, Movement and Posture was the most commonly assessed outcome category, captured in 98% of included studies. This is understandable given that CP is clinically characterized by motor and postural impairments. Movement and posture are routinely measured within CP clinical studies investigating a whole host of interventions, with several validated instruments with good psychometric properties available for the CP population [81]. Safety was the next most common outcome domain. Again, not surprising since clinical studies must necessarily focus on assessing and reporting the safety of experimental intervention/s. Specifically, Phase 1, 2 clinical trials are important for understanding how a drug interacts with the human body, and to identify adverse events. Subsequent Phase 3 clinical trials, including larger numbers of participants, are important to show long-term or rare side effects. Importantly, previous systematic reviews have reported an encouraging safety profile for cell therapy treatments in individuals with CP [4,5], giving confidence to the field in pursuing these novel interventions.
4.1. Alignment of Reported Outcomes with Symptoms, Comorbidities and Complications of CP
Some interesting observations were noted when evaluating the reported outcome categories against frequently occurring symptoms, comorbidities and complications of CP [6]. Firstly, whilst many common impairments and functional limitations were captured in the included studies (e.g., walking, talking, epilepsy (seizures), intellect and behavior), the frequency with which these were reported often differed markedly from their prevalence in the CP population. For example, as previously mentioned, gross motor was captured for 86% of participants as expected for a condition defined by limitations to movement and posture. However, other comorbidities/functional limitations with high prevalence in CP were underrepresented. These include intellectual disability (1 in 2 children with CP, but only assessed for 35% of participants), speech impairment (1 in 3 children with CP, but only assessed for 24% of participants), behavior disorders (1 in 4 children with CP, but only assessed for 49% of participants) and epilepsy (seizures) (1 in 4 children with CP, but only assessed for 18% of participants) [6]. In addition, some comorbidities and complications were reported for only a minority of participants using primarily descriptive measures, or not reported at all, despite being commonly occurring, in particular vision impairment (1 in 4 children with CP), pain (3 in 4 children with CP) and sleep disorders (1 in 5 children with CP) [6]. While questions relating to pain and sleep are included in measures of quality of life, these contribute towards the construct of quality of life rather than being assessments of pain or sleep in their own right. Quality of Life was captured for only 14% of participants, and of these, more than a third were assessed using health-related-specific quality of life measures. We know that quality of life is influenced by a broad array of factors (i.e., more than health), including socioeconomic status and community life, impacted by social policy such as inclusion, participation, community, and accessibility [82]. Given that quality of life was identified as the most important domain for improvement following intervention via a Delphi survey of youth with CP, parents of children with CP, and medical professionals [9], it is interesting that this was not captured more broadly. We advocate that outcome measures that assess overarching quality of life, with responsiveness to change, such as the CPCHILD [83] for children with severe physical disability [10,84], should be included in future studies.
Important to consider is why many of these prevalent comorbidities, complications and functional limitations are not typically reported in clinical studies of cell therapies to date. Whilst it may be due to a lack of availability or knowledge of suitable/appropriate measurement tools for these outcomes, it is also possible that it is not scientifically plausible for cell therapies to target all of these domains. Indeed, there is some debate in the field as to what potential benefits various cell therapies are actually capable of bestowing [85]. Whilst cell therapies have been under investigation for decades (both clinically and pre-clinically), a comprehensive understanding of the mechanism/s of action for each cell type is still being uncovered. For example, it is accepted that neural stem cells can differentiate into neurons, oligodendrocytes and astrocytes to potentially replace lost or damaged brain cells. On the other hand, cell types including mesenchymal stem/stromal cells and hematopoietic stem cells, which were frequently administered in the studies included in this review, are more ambiguous in their mechanism/s of action for CP [4]. Moreover, how various potential mechanisms of action may relate to the likelihood of improvement across different outcome domains (e.g., gross motor vs. cognition vs. pain) remains unknown. Despite these uncertainties, accumulating high-quality evidence exists to support the efficacy of various cell therapies for improving gross motor function in CP, and there is lower-quality evidence suggesting that cell therapies can have wide-ranging effects across many other domains. This includes various anecdotes and descriptive measures, and while this information can be useful in providing hints at potential areas of efficacy, these subjective reports should be verified using valid tools, in well-designed and powered clinical trials, to determine if they are indeed true effects of a cell treatment. Furthermore, a thorough review of the clinical literature across various conditions that share some of the common comorbidities and complications of CP may help identify additional beneficial effects of cell therapies on these treatment targets.
Another reason why common comorbidities, complications and functional limitations of CP are absent in clinical trials may be a ‘carry-over’ from preclinical (primarily rodent/small animal) research. A known limitation of many animal models is the inadequacy to faithfully replicate the complexity of human disease [86], in addition to difficulties assessing traditionally self-reported outcomes, such as pain [87]. Thus, some outcomes may get overlooked when translating promising cell therapies from the ‘bench’ to the clinic. This highlights the importance of consumer engagement and co-design in medical research, to ensure that research, in particular clinical trials, are informed by community priorities, whilst remaining balanced with what scientists believe, and evidence tells us, cell therapies can feasibly achieve. We therefore recommend that future trials are designed in collaboration with consumer and community representatives to ensure included outcomes are aligned with consumer priorities.
4.2. Appropriate Outcome Instrument Selection in Cell Therapy Clinical Studies for CP
Regardless of the outcome domain/s being assessed, it is vitally important that psychometrically sound and appropriate instruments are utilized. This will ensure that data generated from costly and time-consuming clinical trials is high quality and will not lead to incorrect conclusions about the efficacy (or lack thereof) of a particular intervention. This review revealed a large number (>50) of instruments used across the included studies. Encouragingly, many were ‘gold standard’ CP outcome measures, with responsiveness to change, such as the GMFM and the PEDI/PEDI-CAT, which were the two most frequently utilized measures. In contrast, it was concerning that the GMFCS was used to capture change following intervention for a substantial number of participants (the third most frequent outcome tool used). Whilst the GMFCS is a widely used tool for the classification of gross motor function in children with CP, it is not an evaluative measure (i.e., it was not designed, nor shown to be, responsive to change), and is thus not appropriate to be used as an instrument to detect change following an intervention. Interestingly, two other classification tools were also used: the Manual Ability Classification Scale (MACS) [88] and the Communication Function Classification System (CFCS) [89]. We recommend that these classification systems are not used as outcome assessment instruments in future studies.
Excluding the classification tools, two-thirds of all instruments reported had evaluative properties, making them suitable as outcome assessment instruments. Some outcome categories however, were primarily assessed using inappropriate instruments in terms of their evaluative properties, e.g., Language and Communication. There are various reasons why inappropriate instruments may be used in clinical trials, including a lack of knowledge, training, or access (e.g., funding). Alternatively, there may as yet be no widely accepted, and validated, evaluative tools for assessing that particular outcome in CP. There are excellent reviews that have identified valid and reliable measures for use in studies of children and youth with CP [81]. However, if suitable tools do not exist, we propose that these areas are not ready for measurement within clinical trials or that individualized goal setting tools might be considered.
Another consideration for selection of outcome domain/s and assessment tools relates to the heterogeneity of CP. Some may argue that the inherent variability between individuals with CP precludes the inclusion and measurement of particular outcomes because they may not be relevant for a large proportion of trial participants, e.g., hearing or vision impairment, or epilepsy. Yet, there is precedent for the use of individualized outcome measures, for example the Goal Attainment Scale (GAS) [90] or Canadian Occupational Performance Measure (COPM) [91] within clinical trials to importantly capture change that matters to the child and family. The use of such measures may enhance the relevancy of captured outcomes for a given participant, help to limit the total number of assessments, thereby reducing respondent burden, and improve sensitivity to detect meaningful change. Thus, it would be interesting to see whether such measures could be used in future trials.
4.3. Mechanisms of Cell Therapies and Ensuing Effects
CP is caused by an interference, lesion, or abnormality of the developing brain which manifests as a disorder of movement and/or posture. Repairing the underlying brain injury, via direct or indirect mechanisms, to promote increased neuronal signaling and function is the aim of cell therapies for CP. As such, it is recognized that improvements in brain structure or connectivity following cell intervention could directly improve motor function. It is important to acknowledge however that links exist between motor skills and some comorbidities of CP. Figure 3 shows a schema of the proposed effects of stem cells for CP including therapeutic targets leading to remediation of the underlying brain injury, and resultant effects on various comorbidities, leading to the ultimate goal of improving quality of life. We wish to specifically highlight that changes in brain structure and connectivity producing improvements in motor function may have secondary effects on a number of motor-associated CP comorbidities (e.g., pain, sleep, drooling and speech). This may therefore mean that, in fact, improvements in various outcomes of importance to individuals with CP and their families may be more achievable than widely believed. In addition, the non-motor-associated comorbidities of CP (e.g., cognition, behavior) may be indirectly targeted by cell treatments.
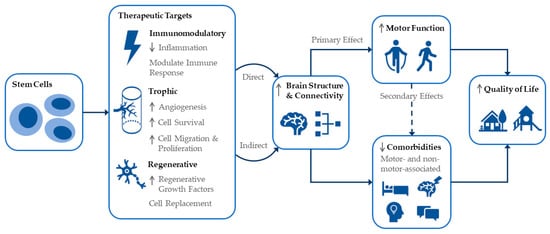
Figure 3.
Schematic representation of cell intervention effects and interlinked outcomes for CP including quality of life.
4.4. Limitations
We acknowledge some limitations of this scoping review including that due to our decision to include all study designs, there is a significant amount of lower-quality evidence included. In addition, extracted outcome instruments may have been categorized in varying ways, and, for simplicity of reporting, some sub-categories of outcomes were consolidated during the sorting process, despite arguably representing distinct outcome sub-domains. Finally, we did not extract nor report on the efficacy of cell therapies for any of the outcome categories, as this was outside the scope of this review.
5. Conclusions
Stem cells are an emerging intervention for CP with potential to target a wide variety of outcome domains. We found that movement and posture and safety were the predominant outcomes assessed in cell therapy clinical studies, despite many other outcomes, including quality of life, being of high importance to individuals with CP and their families. Moreover, amongst the considerable number of outcome instruments employed in clinical studies, many are not appropriate for use as measures of change following intervention. We provide several recommendations to ensure that future trials collect scientifically valid, high-quality outcome data that also meets the expectations of the CP community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247319/s1, Figure S1: Number of participants assessed for each outcome category and sub-category split by study design, excluding the dose comparison study, Figure S2: Number of participants assessed for each outcome category and sub-category split by intervention type, Table S1: PRISMA-ScR Checklist, Table S2: Search strategy.
Author Contributions
Conceptualization, M.F.-E., M.C.B.P. and I.N.; methodology, M.F.-E., M.C.B.P., I.H., P.K., C.S., D.C. and S.R.; formal analysis, M.F.-E.; investigation, M.F.-E. and M.C.B.P.; data curation, M.F.-E., M.C.B.P., I.H., P.K., C.S., D.C., S.R., A.R.G., C.M. and I.N.; writing—original draft preparation, M.F.-E.; writing—review and editing, M.C.B.P., I.H., P.K., C.S., D.C., S.R., A.R.G., C.M. and I.N.; visualization, M.F.-E.; supervision, M.F.-E., P.K., S.R. and I.N.; project administration, M.F.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Open Science Framework https://osf.io/t9c8j/?view_only=9b82c37725834a1da1a50bb199cf5091 (accessed on 14 November 2022).
Acknowledgments
The authors would like to thank Charlotte Frazer (C.F.) for assistance with study screening and data extraction.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paton, M.C.B.; Finch-Edmondson, M.; Fahey, M.C.; London, J.; Badawi, N.; Novak, I. Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J. Paediatr. Child Health 2021, 57, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Kurtzberg, J. Stem cell therapies in cerebral palsy and autism spectrum disorder. Dev. Med. Child Neurol. 2021, 63, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved Scaling of the Gross Motor Function Measure for Children with Cerebral Palsy: Evidence of Reliability and Validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, S.; Boucard, C.; Schoeberlein, A.; Guzman, R.; Limacher, A.; Surbek, D.; Mueller, M. Stem cell treatment and cerebral palsy: Systemic review and meta-analysis. World J. Stem Cells 2019, 11, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Walker, K.; Hunt, R.W.; Wallace, E.M.; Fahey, M.; Badawi, N. Concise Review: Stem Cell Interventions for People with Cerebral Palsy: Systematic Review with Meta-Analysis. Stem Cells Transl. Med. 2016, 5, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Hines, M.; Goldsmith, S.; Barclay, R. Clinical Prognostic Messages from a Systematic Review on Cerebral Palsy. Pediatrics 2012, 130, e1285–e1312. [Google Scholar] [CrossRef]
- Smith, M.J.; Finch-Edmondson, M.; Miller, S.L.; Webb, A.; Fahey, M.C.; Jenkin, G.; Paton, M.C.B.; McDonald, C.A. Acceptability of neural stem cell therapy for cerebral palsy: Survey of the Australian cerebral palsy community. Stem Cell Res. Ther. 2022; provisionally accepted. [Google Scholar] [CrossRef]
- Almoajil, H.; Toye, F.; Dawes, H.; Pierce, J.; Meaney, A.; Baklouti, A.; Poverini, L.; Hopewell, S.; Theologis, T. Outcomes of importance to children and young adults with cerebral palsy, their parents and health professionals following lower limb orthopaedic surgery: A qualitative study to inform a Core Outcome Set. Health Expect. 2022, 25, 925–935. [Google Scholar] [CrossRef]
- Vargus-Adams, J.N.; Martin, L.K. Measuring what matters in cerebral palsy: A breadth of important domains and outcome measures. Arch. Phys. Med. Rehabil. 2009, 90, 2089–2095. [Google Scholar] [CrossRef]
- Schiariti, V.; Fowler, E.; Brandenburg, J.E.; Levey, E.; McIntyre, S.; Sukal-Moulton, T.; Ramey, S.L.; Rose, J.; Sienko, S.; Stashinko, E.; et al. A common data language for clinical research studies: The National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 1.0 recommendations. Dev. Med. Child Neurol. 2018, 60, 976–986. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 24 October 2022).
- U.S. Food and Drug Administration. Clinical Outcome Assessment. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338448/def-item/glossary.clinical-outcome-assessment/ (accessed on 27 October 2022).
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, H.; Su, Y.; Yang, H.; Zhang, J. Study on reliability and unidimension of the Fine Motor Function Measure Scale for children with cerebral palsy. Chin. J. Evid.-Based Pediatr. 2008, 3, 110–118. [Google Scholar]
- Abi Chahine, N.H.; Wehbe, T.W.; Hilal, R.A.; Zoghbi, V.V.; Melki, A.E.; Habib, E.B.B. Treatment of Cerebral Palsy with Stem Cells: A Report of 17 Cases. Int. J. Stem Cells 2016, 9, 90–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amanat, M.; Majmaa, A.; Zarrabi, M.; Nouri, M.; Akbari, M.G.; Moaiedi, A.R.; Ghaemi, O.; Zamani, F.; Najafi, S.; Badv, R.S.; et al. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: A randomized double-blind sham-controlled clinical trial. Stem Cell Res. Ther. 2021, 12, 439. [Google Scholar] [CrossRef]
- Bansal, H.; Singh, L.; Verma, P.; Agrawal, A.; Leon, J.; Sundell, I.B.; Koka, P.S. Administration of autologous bone marrow-derived stem cells for treatment of cerebral palsy patients: A proof of concept. J. Stem Cells 2016, 11, 37–49. [Google Scholar]
- Boruczkowski, D.; Zdolinska-Malinowska, I. Wharton’s jelly mesenchymal stem cell administration improves quality of life and self-sufficiency in children with cerebral palsy: Results from a retrospective study. Stem Cells Int. 2019, 2019, 7402151. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Xi, H.; Xie, Z.; Liu, R.; Jiang, Z.; Zhang, F.; Liu, Y.; Chen, D.; Wang, Q.; et al. Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: A randomized controlled clinical trial. Cell Transplant. 2010, 19, 185–191. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.; Fang, F.; Xu, R.; Wang, Y.; Hu, X.; Fan, L.; Liu, H. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J. Transl. Med. 2013, 11, 21. [Google Scholar] [CrossRef]
- Chernykh, E.R.; Kafanova, M.Y.; Shevela, E.Y.; Sirota, S.I.; Adonina, E.I.; Sakhno, L.V.; Ostanin, A.A.; Kozlov, V.V. Clinical experience with autologous M2 macrophages in children with severe cerebral palsy. Cell Transplant. 2014, 23, S97–S104. [Google Scholar] [CrossRef]
- Chernykh, E.; Shevela, E.; Kafanova, M.; Sakhno, L.; Polovnikov, E.; Ostanin, A. Monocyte-derived macrophages for treatment of cerebral palsy: A study of 57 cases. J. Neurorestoratol. 2018, 6, 41–47. [Google Scholar] [CrossRef]
- Crompton, K.; Novak, I.; Fahey, M.; Badawi, N.; Lee, K.J.; Mechinaud-Heloury, F.; Edwards, P.; Colditz, P.; Soosay Raj, T.; Hough, J.; et al. Safety of sibling cord blood cell infusion for children with cerebral palsy. Cytotherapy 2022, 24, 931–939. [Google Scholar] [CrossRef]
- Dong, H.; Li, G.; Shang, C.; Yin, H.; Luo, Y.; Meng, H.; Li, X.; Wang, Y.; Lin, L.; Zhao, M. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am. J. Transl. Res. 2018, 10, 901–906. [Google Scholar]
- Feng, M.; Lu, A.; Gao, H.; Qian, C.; Zhang, J.; Lin, T.; Zhao, Y. Safety of Allogeneic Umbilical Cord Blood Stem Cells Therapy in Patients with Severe Cerebral Palsy: A Retrospective Study. Stem Cells Int. 2015, 2015, 325652. [Google Scholar] [CrossRef]
- Fu, X.; Hua, R.; Wang, X.; Wang, P.; Yi, L.; Yu, A.; Yang, J.; Li, Y.; An, Y. Synergistic Improvement in Children with Cerebral Palsy Who Underwent Double-Course Human Wharton’s Jelly Stem Cell Transplantation. Stem Cells Int. 2019, 2019, 7481069. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.; Zhang, C.; Wang, Y.; Zhang, R.; Tu, Z.; Wang, H.; Zhou, X.; Xiao, Z.; Liu, Z.; et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Res. Ther. 2020, 11, 43. [Google Scholar] [CrossRef]
- Hassan, M.A.; Gabr, H.; Fathi, S.; Ramzy, G.M.; El-Hassany, A.H.; Abd El-Ghaffar, N.A. Stem cell transplantation in Egyptian patients with cerebral palsy. Egypt. J. Neurol. Psychiatry Neurosurg. 2012, 49, 117–122. [Google Scholar]
- He, S.; Luan, Z.; Qu, S.; Qiu, X.; Xin, D.; Jia, W.; Shen, Y.; Yu, Z.; Xu, T. Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children. Neural Regen. Res. 2012, 7, 2529–2535. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C.; Gu, J.; Wu, W.; Shen, Z.; Zhou, X.; Lu, H. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children with Cerebral Palsy. Cell Transplant. 2018, 27, 325–334. [Google Scholar] [CrossRef]
- Jensen, A.; Hamelmann, E. First Autologous Cord Blood Therapy for Pediatric Ischemic Stroke and Cerebral Palsy Caused by Cephalic Molding during Birth: Individual Treatment with Mononuclear Cells. Case Rep. Transplant. 2016, 2016, 1717426. [Google Scholar] [CrossRef]
- Kang, M.; Min, K.; Jang, J.; Kim, S.C.; Kang, M.S.; Jang, S.J.; Lee, J.Y.; Kim, S.H.; Kim, M.K.; An, S.A.; et al. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem Cells Dev. 2015, 24, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, K.V.; Moon, J.H.; Jun, H.J.; Kang, H.R.; Oh, S.I.; Kim, H.S.; Um, J.S.; Kim, M.J.; Choi, Y.Y.; et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J. Transl. Med. 2012, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, A.; Zhang, F.; Dai, G.; Cheng, H.; Wang, X.; An, Y. Treatment of one case of cerebral palsy combined with posterior visual pathway injury using autologous bone marrow mesenchymal stem cells. J. Transl. Med. 2012, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, X.; Dai, G.; Wang, X.; Zhang, Z.; Cheng, H.; Zheng, P.; An, Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Liu, W.; Qu, S.; Du, K.; He, S.; Wang, Z.; Yang, Y.; Wang, C.; Gong, X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012, 21, S91–S98. [Google Scholar] [CrossRef]
- Mancias-Guerra, C.; Marroquin-Escamilla, A.R.; Gonzalez-Llano, O.; Villarreal-Martinez, L.; Jaime-Perez, J.C.; Garcia-Rodriguez, F.; Valdes-Burnes, S.L.; Rodriguez-Romo, L.N.; Barrera-Morales, D.C.; Sanchez-Hernandez, J.J.; et al. Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: An open-label phase I trial. Cytotherapy 2014, 16, 810–820. [Google Scholar] [CrossRef]
- Maric, D.M.; Radomir, M.; Milankov, Z.; Stanojevic, I.; Vojvodic, D.; Velikic, G.; Susnjevic, S.; Maric, D.L.; Abazovic, D. Encouraging effect of autologous bone marrow aspirate concentrate in rehabilitation of children with cerebral palsy. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2330–2342. [Google Scholar] [CrossRef]
- Min, K.; Song, J.; Kang, J.Y.; Ko, J.; Ryu, J.S.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Oh, D.; Kim, M.K.; et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double-blind, randomized, placebo-controlled trial. Stem Cells 2013, 31, 581–591. [Google Scholar] [CrossRef]
- Min, K.; Suh, M.R.; Cho, K.H.; Park, W.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Rhie, S.; Choi, J.I.; Kim, H.J.; et al. Potentiation of cord blood cell therapy with erythropoietin for children with CP: A 2 × 2 factorial randomized placebo-controlled trial. Stem Cell Res. Ther. 2020, 11, 509. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, A.T.; Vu, C.D.; Ngo, D.V.; Bui, A.V. Outcomes of autologous bone marrow mononuclear cells for cerebral palsy: An open label uncontrolled clinical trial. BMC Pediatr. 2017, 17, 104. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, H.P.; Nguyen, T.K. The effects of bone marrow mononuclear cell transplantation on the quality of life of children with cerebral palsy. Health Qual. Life Outcomes 2018, 16, 164. [Google Scholar] [CrossRef]
- Okur, S.C.; Erdogan, S.; Demir, C.S.; Gunel, G.; Karaoz, E. The effect of Umbilical Cord-derived Mesenchymal stem cell transplantation in a patient with cerebral palsy: A case report. Int. J. Stem Cells 2018, 11, 141–147. [Google Scholar] [CrossRef]
- Padma, M.V.; Bhasin, A.; Mohanty, S.; Sharma, S.; Kiran, U.; Bal, C.S.; Gaikwad, S.; Singh, M.B.; Bhatia, R.; Tripathi, M.; et al. Restorative therapy using autologous bone marrow derived mononuclear cells infusion intra-arterially in patients with cerebral palsy: An open label feasibility study. Neurol. Asia 2011, 16, 231–239. [Google Scholar]
- Papadopoulos, K.I.; Low, S.S.S.; Aw, T.C.; Chantarojanasiri, T. Safety and feasibility of autologous umbilical cord blood transfusion in 2 toddlers with cerebral palsy and the role of low dose granulocyte-colony stimulating factor injections. Restor. Neurol. Neurosci. 2011, 29, 17–22. [Google Scholar] [CrossRef]
- Purandare, C.; Shitole, D.G.; Belle, V.; Kedari, A.; Bora, N.; Joshi, M. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep. Transplant. 2012, 2012, 825289. [Google Scholar] [CrossRef]
- Purwati; Fauzi, A.A.; Gunawan, P.I.; Susilo, I.; Rini, D.P. The role of autologous adipose derived neural progenitor cells with cognitive and motoric function in cerebral palsy. J. Glob. Pharma Technol. 2019, 11, 163–169. [Google Scholar]
- Rah, W.J.; Lee, Y.H.; Moon, J.H.; Jun, H.J.; Kang, H.R.; Koh, H.; Eom, H.J.; Lee, J.Y.; Lee, Y.J.; Kim, J.Y.; et al. Neuroregenerative potential of intravenous G-CSF and autologous peripheral blood stem cells in children with cerebral palsy: A randomized, double-blind, cross-over study. J. Transl. Med. 2017, 15, 16. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Tarakanov, O.P.; Radaev, S.M.; Dugina, T.N.; Ryaskina, S.S.; Darevskaya, A.N.; Morozova, Y.V.; Khachatryan, W.A.; Lebedev, K.E.; Zotova, N.S.; et al. Human allogeneic AB0/Rh-identical umbilical cord blood cells in the treatment of juvenile patients with cerebral palsy. Cytotherapy 2015, 17, 969–978. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Kafanova, M.Y.; Rabinovich, S.S.; Poveshchenko, O.V.; Kashchenko, E.A.; Fel’de, M.A.; Samarin, D.M.; Seledtsova, G.V.; Kozlov, V.A. Cell therapy of cerebral palsy. Bull. Exp. Biol. Med. 2005, 139, 499–503. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Paranjape, A.; Gokulchandran, N.; Kulkarni, P.; Nagrajan, A.; Badhe, P. Positron emission tomography-computer tomography scan used as a monitoring tool following cellular therapy in cerebral palsy and mental retardation-a case report. Case Rep. Neurol. Med. 2013, 2013, 141983. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Kulkarni, P.; Gandhi, S.; Sundaram, J.; Paranjape, A.; Shetty, A.; Bhagwanani, K.; Biju, H.; et al. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: A new frontier. Stem Cells Int. 2015, 2015, 905874. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sane, H.; Kulkarni, P.; D’sa, M.; Gokulchandran, N.; Badhe, P. Improved quality of life in a case of cerebral palsy after bone marrow mononuclear cell transplantation. Cell J. 2015, 17, 389–394. [Google Scholar]
- Sharma, A.; Gokulchandran, N.; Kulkarni, P.; Kiran Mullangi, S.; Bhagawanani, K.; Ganar, V.; Sane, H.; Badhe, P. Multiple cellular therapies along with neurorehabilitation in spastic diplegic cerebral palsy: A case report. Innov. Clin. Neurosci. 2020, 17, 31–34. [Google Scholar] [PubMed]
- Shroff, G.; Gupta, A.; Barthakur, J.K. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J. Transl. Med. 2014, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Song, A.W.; Case, L.E.; Mikati, M.A.; Gustafson, K.E.; Simmons, R.; Goldstein, R.; Petry, J.; McLaughlin, C.; Waters-Pick, B.; et al. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial. Stem Cells Transl. Med. 2017, 6, 2071–2078. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; Mikati, M.A.; Jasien, J.M.; McLaughlin, C.; Waters-Pick, B.; Worley, G.; Troy, J.; Kurtzberg, J. Sibling umbilical cord blood infusion is safe in young children with cerebral palsy. Stem Cells Transl. Med. 2021, 10, 1258–1265. [Google Scholar] [CrossRef]
- Thanh, L.N.; Trung, K.N.; Duy, C.V.; Van, D.N.; Hoang, P.N.; Phuong, A.N.T.; Ngo, M.D.; Thi, T.N.; Viet, A.B. Improvement in gross motor function and muscle tone in children with cerebral palsy related to neonatal icterus: An open-label, uncontrolled clinical trial. BMC Pediatr. 2019, 19, 290. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Zhou, J.; Xie, J.; Zhong, Z.; Li, M.; Bai, W.; Li, N.; Zhang, Z.; Wang, X.; et al. Therapeutic potential of umbilical cord mesenchymal stromal cells transplantation for cerebral palsy: A case report. Case Rep. Transplant. 2013, 2013, 146347. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Hua, R.; Yang, J.; Dai, G.; Zhang, Z.; Wang, R.; Qin, C.; An, Y. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: A preliminary clinical study. Cytotherapy 2013, 15, 1549–1562. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Hua, R.; Yang, J.; Zheng, P.; Niu, X.; Cheng, H.; Dai, G.; Liu, X.; Zhang, Z.; et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: Pilot study on the correlation of efficacy and hereditary factors. Cytotherapy 2015, 17, 224–231. [Google Scholar] [CrossRef]
- Zali, A.; Arab, L.; Ashrafi, F.; Mardpour, S.; Niknejhadi, M.; Hedayati-Asl, A.A.; Halimi-Asl, A.; Ommi, D.; Hosseini, S.E.; Baharvand, H.; et al. Intrathecal injection of CD133-positive enriched bone marrow progenitor cells in children with cerebral palsy: Feasibility and safety. Cytotherapy 2015, 17, 232–241. [Google Scholar] [CrossRef]
- Zarrabi, M.; Akbari, M.G.; Amanat, M.; Majmaa, A.; Moaiedi, A.R.; Montazerlotfelahi, H.; Nouri, M.; Hamidieh, A.A.; Badv, R.S.; Karimi, H.; et al. The safety and efficacy of umbilical cord blood mononuclear cells in individuals with spastic cerebral palsy: A randomized double-blind sham-controlled clinical trial. BMC Neurol. 2022, 22, 123. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Gu, J.; Zhou, X. Therapy for Cerebral Palsy by Human Umbilical Cord Blood Mesenchymal Stem Cells Transplantation Combined With Basic Rehabilitation Treatment: A Case Report. Glob. Pediatr. Health 2015, 2, 2333794X15574091. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Juranek, J.; Kosmach, S.; Pedroza, C.; Thakur, N.; Dempsey, A.; Rennie, K.; Scott, M.C.; Jackson, M.; Kumar, A.; et al. Autologous cellular therapy for cerebral palsy: A randomized, crossover trial. Brain Commun. 2022, 4, fcac131. [Google Scholar] [CrossRef]
- Gabr, H.; El-Kheir, W.A.; Ghannam, O.; El-Fiki, M.E.; Salah, Y. Intrathecal Autologous Bone Marrow Derived MSC Therapy in Cerebral Palsy: Safety and Short Term Efficacy. Am. J. Biosci. Bioeng. 2015, 3, 24–29. [Google Scholar] [CrossRef]
- Hirano, A.; Sano, M.; Urushihata, N.; Tanemura, H.; Oki, K.; Suzaki, E. Assessment of safety and feasibility of human allogeneic adipose-derived mesenchymal stem cells in a pediatric patient. Pediatr. Res. 2018, 84, 575–577. [Google Scholar] [CrossRef]
- Ramirez, F.; Steenblock, D.A.; Payne, A.G.; Darnall, L. Umbilical Cord Stem Cell Therapy for Cerebral Palsy. Med. Hypothesis Res. 2006, 3, 679–686. [Google Scholar]
- Kikuchi, H.; Saitoh, S.; Tsuno, T.; Hosoda, R.; Baba, N.; Wang, F.; Mitsuda, N.; Tsuda, M.; Maeda, N.; Sagara, Y.; et al. Safety and feasibility of autologous cord blood infusion for improving motor function in young children with cerebral palsy in Japan: A single-center study. Brain Dev. 2022, 44, 681–689. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; McLaughlin, C.; Burgess, A.; Skergan, N.; Crane, S.; Jasien, J.M.; Mikati, M.A.; Troy, J.; Kurtzberg, J. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev. Med. Child Neurol. 2022, 64, 1477–1486. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Shroff, G.; Gupta, A.; Barthakur, J.K. Expression of Concern to: Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J. Transl. Med. 2017, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Gioia, G.A.; Isquith, P.K.; Guy, S.C.; Kenworthy, L. Behavior Rating Inventory of Executive Function: BRIEF; Psychological Assessment Resources: Odessa, FL, USA, 2000. [Google Scholar]
- Haley, S.M.; Coster, W.; Dumas, H.M.; Fragala-Pinkham, M.A.; Moed, R. PEDI-CAT: Pediatric Evaluation of Disability Inventory Computer Adaptive Test Manual 1-3-6; Trustees of Boston University: Boston, MA, USA, 2011; under license to CREcare, LLC. [Google Scholar]
- Frankenburg, W.K.; Dodds, J.B. Denver Developmental Screening Test II (DDST-II); Denver Developmental Materials: Denver, CO, USA, 1990. [Google Scholar]
- Gracies, J.M.; Burke, K.; Clegg, N.J.; Browne, R.; Rushing, C.; Fehlings, D.; Matthews, D.; Tilton, A.; Delgado, M.R. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2010, 91, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Pearson Clinical: San Antonio, TX, USA, 2005. [Google Scholar]
- Wechsler, D. The Wechsler Intelligence Scale for Children, 5th ed.; Pearson: Bloomington, MN, USA, 2014. [Google Scholar]
- Waters, E.; Davis, E.; Mackinnon, A.; Boyd, R.; Graham, H.K.; Kai Lo, S.; Wolfe, R.; Stevenson, R.; Bjornson, K.; Blair, E.; et al. Psychometric properties of the quality of life questionnaire for children with CP. Dev. Med. Child Neurol. 2007, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Tatla, S.; Sauve, K.; O’Donnell, M. Toolbox of multiple-item measures aligning with the ICF Core Sets for children and youth with cerebral palsy. Eur. J. Paediatr. Neurol. 2017, 21, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Parkinson, K.N.; Ravens-Sieberer, U.; Schirripa, G.; Thyen, U.; Arnaud, C.; Beckung, E.; Fauconnier, J.; McManus, V.; Michelsen, S.I.; et al. Self-reported quality of life of 8-12-year-old children with cerebral palsy: A cross-sectional European study. Lancet 2007, 369, 2171–2178. [Google Scholar] [CrossRef]
- Narayanan, U.G.; Fehlings, D.; Weir, S.; Knights, S.; Kiran, S.; Campbell, K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Dev. Med. Child Neurol. 2006, 48, 804–812. [Google Scholar] [CrossRef]
- Difazio, R.L.; Vessey, J.A.; Zurakowski, D.; Snyder, B.D. Differences in health-related quality of life and caregiver burden after hip and spine surgery in non-ambulatory children with severe cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 298–305. [Google Scholar] [CrossRef]
- Montague, J. The ‘Unwarranted Hype’ of Stem Cell Therapies. Available online: https://www.bbc.com/future/article/20190819-the-unwarranted-hype-of-stem-cell-therapies-for-autism-ms (accessed on 1 November 2022).
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef]
- Klinck, M.P.; Mogil, J.S.; Moreau, M.; Lascelles, B.D.X.; Flecknell, P.A.; Poitte, T.; Troncy, E. Translational pain assessment: Could natural animal models be the missing link? Pain 2017, 158, 1633–1646. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Hidecker, M.J.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, K., Jr.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian occupational performance measure: An outcome measure for occupational therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).