Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Assigning Level of Evidence for Included Studies
2.5. Categorization of Cell Interventions
2.6. Categorization of Instruments (into Outcome Domains)
2.7. Outcome Instrument Properties
2.8. Calculating Total Number of Participants per Outcome Sub/Category
3. Results
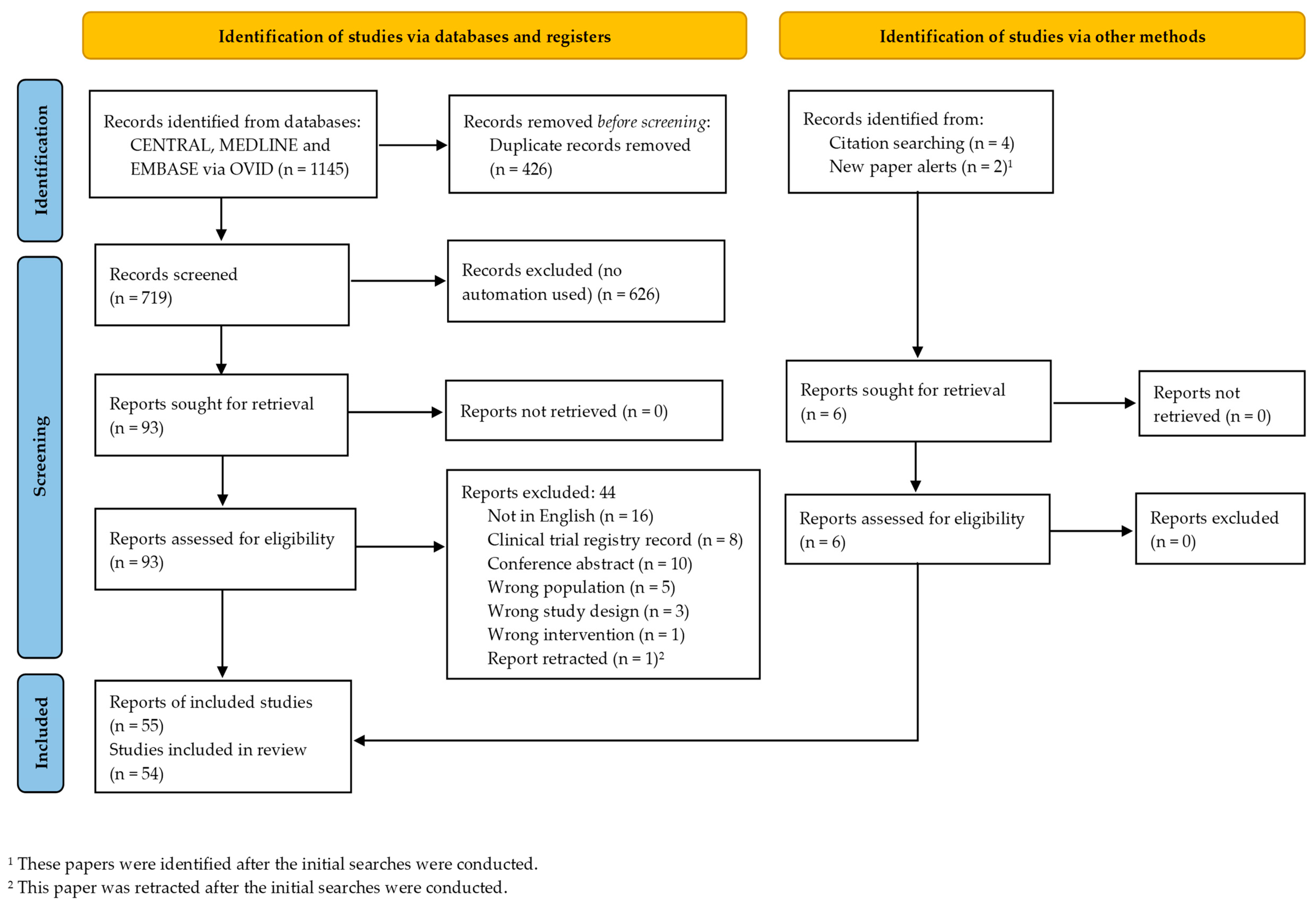
3.1. Search Results
3.2. Study Characteristics
3.3. Types of Studies
3.4. Types of Participants
3.5. Types of Interventions
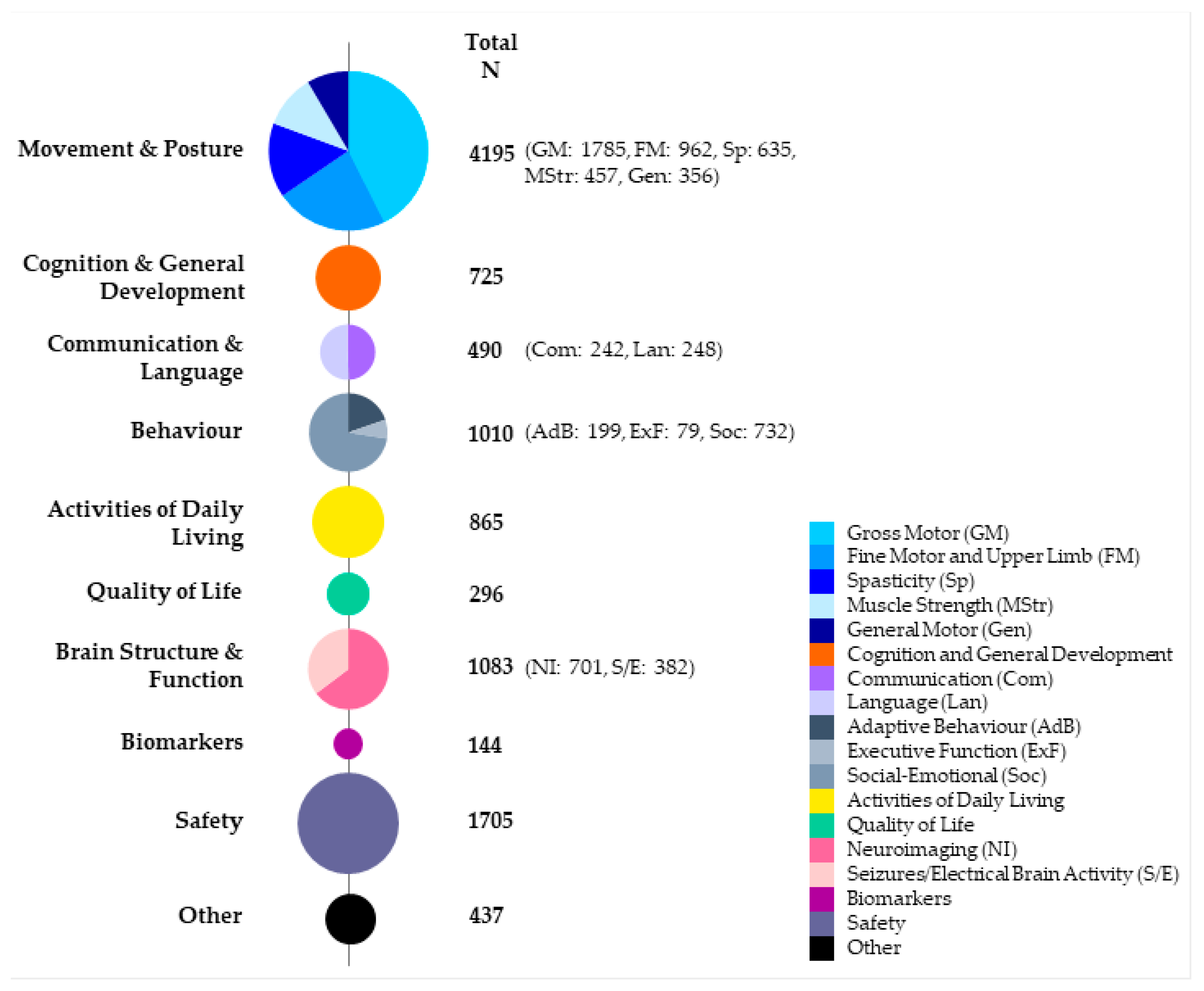
3.6. Types of Outcome Measures
Descriptive Outcomes
3.7. Outcome Instrument Properties
4. Discussion
4.1. Alignment of Reported Outcomes with Symptoms, Comorbidities and Complications of CP
4.2. Appropriate Outcome Instrument Selection in Cell Therapy Clinical Studies for CP
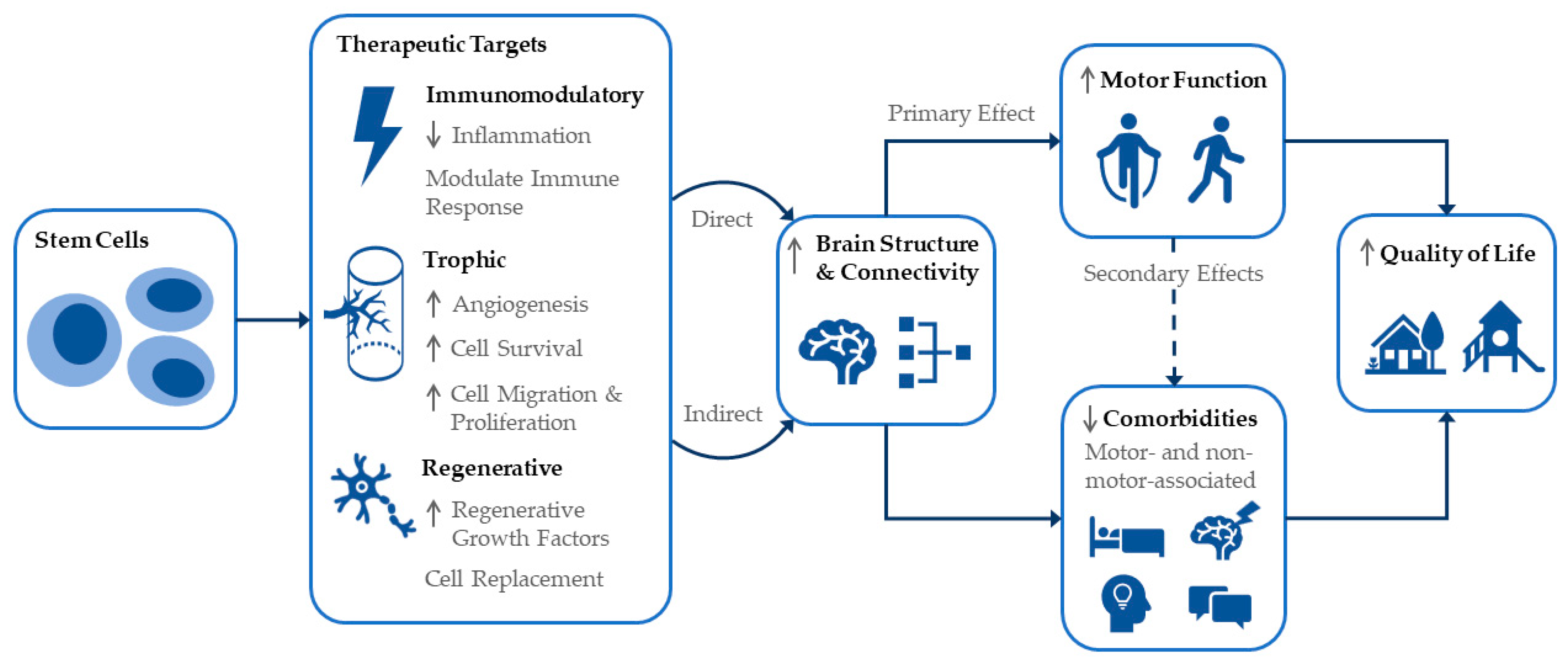
4.3. Mechanisms of Cell Therapies and Ensuing Effects
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paton, M.C.B.; Finch-Edmondson, M.; Fahey, M.C.; London, J.; Badawi, N.; Novak, I. Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J. Paediatr. Child Health 2021, 57, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Kurtzberg, J. Stem cell therapies in cerebral palsy and autism spectrum disorder. Dev. Med. Child Neurol. 2021, 63, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved Scaling of the Gross Motor Function Measure for Children with Cerebral Palsy: Evidence of Reliability and Validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, S.; Boucard, C.; Schoeberlein, A.; Guzman, R.; Limacher, A.; Surbek, D.; Mueller, M. Stem cell treatment and cerebral palsy: Systemic review and meta-analysis. World J. Stem Cells 2019, 11, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Walker, K.; Hunt, R.W.; Wallace, E.M.; Fahey, M.; Badawi, N. Concise Review: Stem Cell Interventions for People with Cerebral Palsy: Systematic Review with Meta-Analysis. Stem Cells Transl. Med. 2016, 5, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Hines, M.; Goldsmith, S.; Barclay, R. Clinical Prognostic Messages from a Systematic Review on Cerebral Palsy. Pediatrics 2012, 130, e1285–e1312. [Google Scholar] [CrossRef]
- Smith, M.J.; Finch-Edmondson, M.; Miller, S.L.; Webb, A.; Fahey, M.C.; Jenkin, G.; Paton, M.C.B.; McDonald, C.A. Acceptability of neural stem cell therapy for cerebral palsy: Survey of the Australian cerebral palsy community. Stem Cell Res. Ther. 2022; provisionally accepted. [Google Scholar] [CrossRef]
- Almoajil, H.; Toye, F.; Dawes, H.; Pierce, J.; Meaney, A.; Baklouti, A.; Poverini, L.; Hopewell, S.; Theologis, T. Outcomes of importance to children and young adults with cerebral palsy, their parents and health professionals following lower limb orthopaedic surgery: A qualitative study to inform a Core Outcome Set. Health Expect. 2022, 25, 925–935. [Google Scholar] [CrossRef]
- Vargus-Adams, J.N.; Martin, L.K. Measuring what matters in cerebral palsy: A breadth of important domains and outcome measures. Arch. Phys. Med. Rehabil. 2009, 90, 2089–2095. [Google Scholar] [CrossRef]
- Schiariti, V.; Fowler, E.; Brandenburg, J.E.; Levey, E.; McIntyre, S.; Sukal-Moulton, T.; Ramey, S.L.; Rose, J.; Sienko, S.; Stashinko, E.; et al. A common data language for clinical research studies: The National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 1.0 recommendations. Dev. Med. Child Neurol. 2018, 60, 976–986. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 24 October 2022).
- U.S. Food and Drug Administration. Clinical Outcome Assessment. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338448/def-item/glossary.clinical-outcome-assessment/ (accessed on 27 October 2022).
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, H.; Su, Y.; Yang, H.; Zhang, J. Study on reliability and unidimension of the Fine Motor Function Measure Scale for children with cerebral palsy. Chin. J. Evid.-Based Pediatr. 2008, 3, 110–118. [Google Scholar]
- Abi Chahine, N.H.; Wehbe, T.W.; Hilal, R.A.; Zoghbi, V.V.; Melki, A.E.; Habib, E.B.B. Treatment of Cerebral Palsy with Stem Cells: A Report of 17 Cases. Int. J. Stem Cells 2016, 9, 90–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amanat, M.; Majmaa, A.; Zarrabi, M.; Nouri, M.; Akbari, M.G.; Moaiedi, A.R.; Ghaemi, O.; Zamani, F.; Najafi, S.; Badv, R.S.; et al. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: A randomized double-blind sham-controlled clinical trial. Stem Cell Res. Ther. 2021, 12, 439. [Google Scholar] [CrossRef]
- Bansal, H.; Singh, L.; Verma, P.; Agrawal, A.; Leon, J.; Sundell, I.B.; Koka, P.S. Administration of autologous bone marrow-derived stem cells for treatment of cerebral palsy patients: A proof of concept. J. Stem Cells 2016, 11, 37–49. [Google Scholar]
- Boruczkowski, D.; Zdolinska-Malinowska, I. Wharton’s jelly mesenchymal stem cell administration improves quality of life and self-sufficiency in children with cerebral palsy: Results from a retrospective study. Stem Cells Int. 2019, 2019, 7402151. [Google Scholar] [CrossRef]
- Chen, L.; Huang, H.; Xi, H.; Xie, Z.; Liu, R.; Jiang, Z.; Zhang, F.; Liu, Y.; Chen, D.; Wang, Q.; et al. Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: A randomized controlled clinical trial. Cell Transplant. 2010, 19, 185–191. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.; Fang, F.; Xu, R.; Wang, Y.; Hu, X.; Fan, L.; Liu, H. Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J. Transl. Med. 2013, 11, 21. [Google Scholar] [CrossRef]
- Chernykh, E.R.; Kafanova, M.Y.; Shevela, E.Y.; Sirota, S.I.; Adonina, E.I.; Sakhno, L.V.; Ostanin, A.A.; Kozlov, V.V. Clinical experience with autologous M2 macrophages in children with severe cerebral palsy. Cell Transplant. 2014, 23, S97–S104. [Google Scholar] [CrossRef]
- Chernykh, E.; Shevela, E.; Kafanova, M.; Sakhno, L.; Polovnikov, E.; Ostanin, A. Monocyte-derived macrophages for treatment of cerebral palsy: A study of 57 cases. J. Neurorestoratol. 2018, 6, 41–47. [Google Scholar] [CrossRef]
- Crompton, K.; Novak, I.; Fahey, M.; Badawi, N.; Lee, K.J.; Mechinaud-Heloury, F.; Edwards, P.; Colditz, P.; Soosay Raj, T.; Hough, J.; et al. Safety of sibling cord blood cell infusion for children with cerebral palsy. Cytotherapy 2022, 24, 931–939. [Google Scholar] [CrossRef]
- Dong, H.; Li, G.; Shang, C.; Yin, H.; Luo, Y.; Meng, H.; Li, X.; Wang, Y.; Lin, L.; Zhao, M. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am. J. Transl. Res. 2018, 10, 901–906. [Google Scholar]
- Feng, M.; Lu, A.; Gao, H.; Qian, C.; Zhang, J.; Lin, T.; Zhao, Y. Safety of Allogeneic Umbilical Cord Blood Stem Cells Therapy in Patients with Severe Cerebral Palsy: A Retrospective Study. Stem Cells Int. 2015, 2015, 325652. [Google Scholar] [CrossRef]
- Fu, X.; Hua, R.; Wang, X.; Wang, P.; Yi, L.; Yu, A.; Yang, J.; Li, Y.; An, Y. Synergistic Improvement in Children with Cerebral Palsy Who Underwent Double-Course Human Wharton’s Jelly Stem Cell Transplantation. Stem Cells Int. 2019, 2019, 7481069. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.; Zhang, C.; Wang, Y.; Zhang, R.; Tu, Z.; Wang, H.; Zhou, X.; Xiao, Z.; Liu, Z.; et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Res. Ther. 2020, 11, 43. [Google Scholar] [CrossRef]
- Hassan, M.A.; Gabr, H.; Fathi, S.; Ramzy, G.M.; El-Hassany, A.H.; Abd El-Ghaffar, N.A. Stem cell transplantation in Egyptian patients with cerebral palsy. Egypt. J. Neurol. Psychiatry Neurosurg. 2012, 49, 117–122. [Google Scholar]
- He, S.; Luan, Z.; Qu, S.; Qiu, X.; Xin, D.; Jia, W.; Shen, Y.; Yu, Z.; Xu, T. Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children. Neural Regen. Res. 2012, 7, 2529–2535. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C.; Gu, J.; Wu, W.; Shen, Z.; Zhou, X.; Lu, H. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children with Cerebral Palsy. Cell Transplant. 2018, 27, 325–334. [Google Scholar] [CrossRef]
- Jensen, A.; Hamelmann, E. First Autologous Cord Blood Therapy for Pediatric Ischemic Stroke and Cerebral Palsy Caused by Cephalic Molding during Birth: Individual Treatment with Mononuclear Cells. Case Rep. Transplant. 2016, 2016, 1717426. [Google Scholar] [CrossRef]
- Kang, M.; Min, K.; Jang, J.; Kim, S.C.; Kang, M.S.; Jang, S.J.; Lee, J.Y.; Kim, S.H.; Kim, M.K.; An, S.A.; et al. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem Cells Dev. 2015, 24, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, K.V.; Moon, J.H.; Jun, H.J.; Kang, H.R.; Oh, S.I.; Kim, H.S.; Um, J.S.; Kim, M.J.; Choi, Y.Y.; et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J. Transl. Med. 2012, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, A.; Zhang, F.; Dai, G.; Cheng, H.; Wang, X.; An, Y. Treatment of one case of cerebral palsy combined with posterior visual pathway injury using autologous bone marrow mesenchymal stem cells. J. Transl. Med. 2012, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, X.; Dai, G.; Wang, X.; Zhang, Z.; Cheng, H.; Zheng, P.; An, Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Liu, W.; Qu, S.; Du, K.; He, S.; Wang, Z.; Yang, Y.; Wang, C.; Gong, X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012, 21, S91–S98. [Google Scholar] [CrossRef]
- Mancias-Guerra, C.; Marroquin-Escamilla, A.R.; Gonzalez-Llano, O.; Villarreal-Martinez, L.; Jaime-Perez, J.C.; Garcia-Rodriguez, F.; Valdes-Burnes, S.L.; Rodriguez-Romo, L.N.; Barrera-Morales, D.C.; Sanchez-Hernandez, J.J.; et al. Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: An open-label phase I trial. Cytotherapy 2014, 16, 810–820. [Google Scholar] [CrossRef]
- Maric, D.M.; Radomir, M.; Milankov, Z.; Stanojevic, I.; Vojvodic, D.; Velikic, G.; Susnjevic, S.; Maric, D.L.; Abazovic, D. Encouraging effect of autologous bone marrow aspirate concentrate in rehabilitation of children with cerebral palsy. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2330–2342. [Google Scholar] [CrossRef]
- Min, K.; Song, J.; Kang, J.Y.; Ko, J.; Ryu, J.S.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Oh, D.; Kim, M.K.; et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double-blind, randomized, placebo-controlled trial. Stem Cells 2013, 31, 581–591. [Google Scholar] [CrossRef]
- Min, K.; Suh, M.R.; Cho, K.H.; Park, W.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Rhie, S.; Choi, J.I.; Kim, H.J.; et al. Potentiation of cord blood cell therapy with erythropoietin for children with CP: A 2 × 2 factorial randomized placebo-controlled trial. Stem Cell Res. Ther. 2020, 11, 509. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, A.T.; Vu, C.D.; Ngo, D.V.; Bui, A.V. Outcomes of autologous bone marrow mononuclear cells for cerebral palsy: An open label uncontrolled clinical trial. BMC Pediatr. 2017, 17, 104. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, H.P.; Nguyen, T.K. The effects of bone marrow mononuclear cell transplantation on the quality of life of children with cerebral palsy. Health Qual. Life Outcomes 2018, 16, 164. [Google Scholar] [CrossRef]
- Okur, S.C.; Erdogan, S.; Demir, C.S.; Gunel, G.; Karaoz, E. The effect of Umbilical Cord-derived Mesenchymal stem cell transplantation in a patient with cerebral palsy: A case report. Int. J. Stem Cells 2018, 11, 141–147. [Google Scholar] [CrossRef]
- Padma, M.V.; Bhasin, A.; Mohanty, S.; Sharma, S.; Kiran, U.; Bal, C.S.; Gaikwad, S.; Singh, M.B.; Bhatia, R.; Tripathi, M.; et al. Restorative therapy using autologous bone marrow derived mononuclear cells infusion intra-arterially in patients with cerebral palsy: An open label feasibility study. Neurol. Asia 2011, 16, 231–239. [Google Scholar]
- Papadopoulos, K.I.; Low, S.S.S.; Aw, T.C.; Chantarojanasiri, T. Safety and feasibility of autologous umbilical cord blood transfusion in 2 toddlers with cerebral palsy and the role of low dose granulocyte-colony stimulating factor injections. Restor. Neurol. Neurosci. 2011, 29, 17–22. [Google Scholar] [CrossRef]
- Purandare, C.; Shitole, D.G.; Belle, V.; Kedari, A.; Bora, N.; Joshi, M. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep. Transplant. 2012, 2012, 825289. [Google Scholar] [CrossRef]
- Purwati; Fauzi, A.A.; Gunawan, P.I.; Susilo, I.; Rini, D.P. The role of autologous adipose derived neural progenitor cells with cognitive and motoric function in cerebral palsy. J. Glob. Pharma Technol. 2019, 11, 163–169. [Google Scholar]
- Rah, W.J.; Lee, Y.H.; Moon, J.H.; Jun, H.J.; Kang, H.R.; Koh, H.; Eom, H.J.; Lee, J.Y.; Lee, Y.J.; Kim, J.Y.; et al. Neuroregenerative potential of intravenous G-CSF and autologous peripheral blood stem cells in children with cerebral palsy: A randomized, double-blind, cross-over study. J. Transl. Med. 2017, 15, 16. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Tarakanov, O.P.; Radaev, S.M.; Dugina, T.N.; Ryaskina, S.S.; Darevskaya, A.N.; Morozova, Y.V.; Khachatryan, W.A.; Lebedev, K.E.; Zotova, N.S.; et al. Human allogeneic AB0/Rh-identical umbilical cord blood cells in the treatment of juvenile patients with cerebral palsy. Cytotherapy 2015, 17, 969–978. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Kafanova, M.Y.; Rabinovich, S.S.; Poveshchenko, O.V.; Kashchenko, E.A.; Fel’de, M.A.; Samarin, D.M.; Seledtsova, G.V.; Kozlov, V.A. Cell therapy of cerebral palsy. Bull. Exp. Biol. Med. 2005, 139, 499–503. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Paranjape, A.; Gokulchandran, N.; Kulkarni, P.; Nagrajan, A.; Badhe, P. Positron emission tomography-computer tomography scan used as a monitoring tool following cellular therapy in cerebral palsy and mental retardation-a case report. Case Rep. Neurol. Med. 2013, 2013, 141983. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Kulkarni, P.; Gandhi, S.; Sundaram, J.; Paranjape, A.; Shetty, A.; Bhagwanani, K.; Biju, H.; et al. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: A new frontier. Stem Cells Int. 2015, 2015, 905874. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sane, H.; Kulkarni, P.; D’sa, M.; Gokulchandran, N.; Badhe, P. Improved quality of life in a case of cerebral palsy after bone marrow mononuclear cell transplantation. Cell J. 2015, 17, 389–394. [Google Scholar]
- Sharma, A.; Gokulchandran, N.; Kulkarni, P.; Kiran Mullangi, S.; Bhagawanani, K.; Ganar, V.; Sane, H.; Badhe, P. Multiple cellular therapies along with neurorehabilitation in spastic diplegic cerebral palsy: A case report. Innov. Clin. Neurosci. 2020, 17, 31–34. [Google Scholar] [PubMed]
- Shroff, G.; Gupta, A.; Barthakur, J.K. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J. Transl. Med. 2014, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Song, A.W.; Case, L.E.; Mikati, M.A.; Gustafson, K.E.; Simmons, R.; Goldstein, R.; Petry, J.; McLaughlin, C.; Waters-Pick, B.; et al. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial. Stem Cells Transl. Med. 2017, 6, 2071–2078. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; Mikati, M.A.; Jasien, J.M.; McLaughlin, C.; Waters-Pick, B.; Worley, G.; Troy, J.; Kurtzberg, J. Sibling umbilical cord blood infusion is safe in young children with cerebral palsy. Stem Cells Transl. Med. 2021, 10, 1258–1265. [Google Scholar] [CrossRef]
- Thanh, L.N.; Trung, K.N.; Duy, C.V.; Van, D.N.; Hoang, P.N.; Phuong, A.N.T.; Ngo, M.D.; Thi, T.N.; Viet, A.B. Improvement in gross motor function and muscle tone in children with cerebral palsy related to neonatal icterus: An open-label, uncontrolled clinical trial. BMC Pediatr. 2019, 19, 290. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Zhou, J.; Xie, J.; Zhong, Z.; Li, M.; Bai, W.; Li, N.; Zhang, Z.; Wang, X.; et al. Therapeutic potential of umbilical cord mesenchymal stromal cells transplantation for cerebral palsy: A case report. Case Rep. Transplant. 2013, 2013, 146347. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Hua, R.; Yang, J.; Dai, G.; Zhang, Z.; Wang, R.; Qin, C.; An, Y. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: A preliminary clinical study. Cytotherapy 2013, 15, 1549–1562. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Hua, R.; Yang, J.; Zheng, P.; Niu, X.; Cheng, H.; Dai, G.; Liu, X.; Zhang, Z.; et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: Pilot study on the correlation of efficacy and hereditary factors. Cytotherapy 2015, 17, 224–231. [Google Scholar] [CrossRef]
- Zali, A.; Arab, L.; Ashrafi, F.; Mardpour, S.; Niknejhadi, M.; Hedayati-Asl, A.A.; Halimi-Asl, A.; Ommi, D.; Hosseini, S.E.; Baharvand, H.; et al. Intrathecal injection of CD133-positive enriched bone marrow progenitor cells in children with cerebral palsy: Feasibility and safety. Cytotherapy 2015, 17, 232–241. [Google Scholar] [CrossRef]
- Zarrabi, M.; Akbari, M.G.; Amanat, M.; Majmaa, A.; Moaiedi, A.R.; Montazerlotfelahi, H.; Nouri, M.; Hamidieh, A.A.; Badv, R.S.; Karimi, H.; et al. The safety and efficacy of umbilical cord blood mononuclear cells in individuals with spastic cerebral palsy: A randomized double-blind sham-controlled clinical trial. BMC Neurol. 2022, 22, 123. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Gu, J.; Zhou, X. Therapy for Cerebral Palsy by Human Umbilical Cord Blood Mesenchymal Stem Cells Transplantation Combined With Basic Rehabilitation Treatment: A Case Report. Glob. Pediatr. Health 2015, 2, 2333794X15574091. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Juranek, J.; Kosmach, S.; Pedroza, C.; Thakur, N.; Dempsey, A.; Rennie, K.; Scott, M.C.; Jackson, M.; Kumar, A.; et al. Autologous cellular therapy for cerebral palsy: A randomized, crossover trial. Brain Commun. 2022, 4, fcac131. [Google Scholar] [CrossRef]
- Gabr, H.; El-Kheir, W.A.; Ghannam, O.; El-Fiki, M.E.; Salah, Y. Intrathecal Autologous Bone Marrow Derived MSC Therapy in Cerebral Palsy: Safety and Short Term Efficacy. Am. J. Biosci. Bioeng. 2015, 3, 24–29. [Google Scholar] [CrossRef]
- Hirano, A.; Sano, M.; Urushihata, N.; Tanemura, H.; Oki, K.; Suzaki, E. Assessment of safety and feasibility of human allogeneic adipose-derived mesenchymal stem cells in a pediatric patient. Pediatr. Res. 2018, 84, 575–577. [Google Scholar] [CrossRef]
- Ramirez, F.; Steenblock, D.A.; Payne, A.G.; Darnall, L. Umbilical Cord Stem Cell Therapy for Cerebral Palsy. Med. Hypothesis Res. 2006, 3, 679–686. [Google Scholar]
- Kikuchi, H.; Saitoh, S.; Tsuno, T.; Hosoda, R.; Baba, N.; Wang, F.; Mitsuda, N.; Tsuda, M.; Maeda, N.; Sagara, Y.; et al. Safety and feasibility of autologous cord blood infusion for improving motor function in young children with cerebral palsy in Japan: A single-center study. Brain Dev. 2022, 44, 681–689. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; McLaughlin, C.; Burgess, A.; Skergan, N.; Crane, S.; Jasien, J.M.; Mikati, M.A.; Troy, J.; Kurtzberg, J. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev. Med. Child Neurol. 2022, 64, 1477–1486. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Shroff, G.; Gupta, A.; Barthakur, J.K. Expression of Concern to: Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J. Transl. Med. 2017, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Gioia, G.A.; Isquith, P.K.; Guy, S.C.; Kenworthy, L. Behavior Rating Inventory of Executive Function: BRIEF; Psychological Assessment Resources: Odessa, FL, USA, 2000. [Google Scholar]
- Haley, S.M.; Coster, W.; Dumas, H.M.; Fragala-Pinkham, M.A.; Moed, R. PEDI-CAT: Pediatric Evaluation of Disability Inventory Computer Adaptive Test Manual 1-3-6; Trustees of Boston University: Boston, MA, USA, 2011; under license to CREcare, LLC. [Google Scholar]
- Frankenburg, W.K.; Dodds, J.B. Denver Developmental Screening Test II (DDST-II); Denver Developmental Materials: Denver, CO, USA, 1990. [Google Scholar]
- Gracies, J.M.; Burke, K.; Clegg, N.J.; Browne, R.; Rushing, C.; Fehlings, D.; Matthews, D.; Tilton, A.; Delgado, M.R. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch. Phys. Med. Rehabil. 2010, 91, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Pearson Clinical: San Antonio, TX, USA, 2005. [Google Scholar]
- Wechsler, D. The Wechsler Intelligence Scale for Children, 5th ed.; Pearson: Bloomington, MN, USA, 2014. [Google Scholar]
- Waters, E.; Davis, E.; Mackinnon, A.; Boyd, R.; Graham, H.K.; Kai Lo, S.; Wolfe, R.; Stevenson, R.; Bjornson, K.; Blair, E.; et al. Psychometric properties of the quality of life questionnaire for children with CP. Dev. Med. Child Neurol. 2007, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Tatla, S.; Sauve, K.; O’Donnell, M. Toolbox of multiple-item measures aligning with the ICF Core Sets for children and youth with cerebral palsy. Eur. J. Paediatr. Neurol. 2017, 21, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Parkinson, K.N.; Ravens-Sieberer, U.; Schirripa, G.; Thyen, U.; Arnaud, C.; Beckung, E.; Fauconnier, J.; McManus, V.; Michelsen, S.I.; et al. Self-reported quality of life of 8-12-year-old children with cerebral palsy: A cross-sectional European study. Lancet 2007, 369, 2171–2178. [Google Scholar] [CrossRef]
- Narayanan, U.G.; Fehlings, D.; Weir, S.; Knights, S.; Kiran, S.; Campbell, K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Dev. Med. Child Neurol. 2006, 48, 804–812. [Google Scholar] [CrossRef]
- Difazio, R.L.; Vessey, J.A.; Zurakowski, D.; Snyder, B.D. Differences in health-related quality of life and caregiver burden after hip and spine surgery in non-ambulatory children with severe cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 298–305. [Google Scholar] [CrossRef]
- Montague, J. The ‘Unwarranted Hype’ of Stem Cell Therapies. Available online: https://www.bbc.com/future/article/20190819-the-unwarranted-hype-of-stem-cell-therapies-for-autism-ms (accessed on 1 November 2022).
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef]
- Klinck, M.P.; Mogil, J.S.; Moreau, M.; Lascelles, B.D.X.; Flecknell, P.A.; Poitte, T.; Troncy, E. Translational pain assessment: Could natural animal models be the missing link? Pain 2017, 158, 1633–1646. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Hidecker, M.J.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, K., Jr.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian occupational performance measure: An outcome measure for occupational therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef]
| Study Reference | Study Design | Participant Details at Baseline | Intervention/s and Comparator/s | Cell Therapy, Donor Type and Route | Last Follow Up Post-Cell Treatment 1 | Outcome Sub-Categories: Instrument/s Reported | Level of Evidence 2 |
|---|---|---|---|---|---|---|---|
| AbiChahine 2016 [16] | Case series n = 17 n = 2 LTFU | Subtype: Various Severity: GMFCS I-II, IV-V Age: 1.5–17 years | BM-MNCs (n = 17) | Autologous, intrathecal | Not reported | Gross Motor: GMFCS Spasticity; Cognition & General Development; Activities of Daily Living; Adaptive Behavior: Descriptive Safety | 4 |
| Amanat 2021 [17] 3 | RCT n = 72 n = 5 LTFU | Subtype: Spastic quadriplegia and diplegia Severity: GMFCS II-V Age: Mean 8.5 years | Group 1: UC-MSCs + rehab (n = 36) Group 2: Sham procedure + rehab (n = 36) | Allogeneic, intrathecal | 1 year | Gross Motor: GMFM-66, PEDI Spasticity: MAS Activities of Daily Living: PEDI Social-Emotional: PEDI Quality of Life: CP-QoL Neuroimaging: MRI-DTI Safety | 2 |
| Bansal 2016 [18] | Single-arm n = 10 | Subtype: Not reported Severity: GMFCS II-IV Age: 2–10 years | BM-MNCs + rehab (n = 10) | Autologous, intrathecal | 2 years | Gross Motor: GMFCS Fine Motor & Upper Limb: MACS Communication: CFCS Spasticity: Descriptive Neuroimaging: MRI Safety | 4 |
| Boruczkowski 2019 [19] | Case series (Retrospective) n = 107 n = 17 LTFU + n = 36–67 missing data (outcome dependent) | Subtype: Not reported Severity: Not reported Age: 1.4–17 years | UC-MSCs (n = 107) | Allogeneic, intravenous | Not reported | Gross Motor; Fine Motor & Upper Limb; Spasticity; Muscle Strength; Quality of Life; Activities of Daily Living; Cognition & General Development; Adaptive Behavior; Executive Function; Social-Emotional; Communication; Seizures/Electrical Brain Activity: Descriptive Other: Descriptive (sensory, sleep, circulation, medications) Safety | 4 |
| Chen 2010 [20] | RCT n = 33 n = 19 LTFU | Subtype: Not reported Severity: Not reported Age: 1–12 years | Group 1: Fetal OECs + rehab (n = 18) Group 2: Rehab alone (n = 15) | Allogeneic, intracerebral | 6 months | Gross Motor: GMFM-66 Other: Caregiver Questionnaire Scale Safety | 2 |
| Chen 2013 [21] | Non-randomised controlled n = 60 | Subtype: Not reported Severity: GMFCS III-V Age: 1–35 years | Group 1: BM-MSC-derived NSC-like cells + rehab (n = 30) Group 2: Rehab alone (n = 30) | Autologous, intrathecal | 6 months | Gross Motor: GMFM-88 Language: Gesell Developmental Schedules Safety | 3 |
| Chernykh 2014 [22] | Single-arm n = 21 | Subtype: Various Severity: GMFCS IV-V Age: 2–8 years | Peripheral blood expanded M2-like macrophages (n = 21) | Autologous, intrathecal | 5 years | Gross Motor: GMFM-66 Fine Motor & Upper Limb: PDMS-FM Spasticity: Ashworth Scale Muscle Strength: MRC Scale Cognition & General Development; Seizures/Electrical Brain Activity: Descriptive Other: Descriptive (infections, temperatures) Biomarkers Safety | 4 |
| Chernykh 2018 [23] | Single-arm n = 57 | Subtype: Various Severity: GMFCS III-V Age: 1–10 years | Peripheral blood expanded M2-like macrophages (n = 57) | Autologous, intrathecal | 5 years | Gross Motor: GMFM-66 Fine Motor & Upper Limb: PDMS-FM Spasticity: Ashworth Scale Muscle Strength: MRC Scale Cognition & General Development; Seizures/Electrical Brain Activity: Descriptive Biomarkers Safety | 4 |
| Cox 2022 [66] | RCT: Cross-over n = 20 n = 2 LTFU (longer term endpoint only) | Subtype: Various Severity: GMFCS II-V Age: 2.4–10.9 years | Group 1: UCB then placebo (n = 3) Group 2: BM-MNCs then placebo (n = 10) Group 3: Placebo then UCB (n = 2) Group 4: Placebo then BM-MNCs (n = 5) | Autologous, intravenous | 2 years (1 year post cross-over) | Gross Motor: GMFM-66/-88 General Motor: VABS-2 Communication: VABS-2 Activities of Daily Living: VABS-2 Adaptive Behavior: VABS-2 Social-Emotional: VABS-2 Neuroimaging: MRI/MRI-DTI Safety | 2 |
| Crompton 2022 [24] | Single-arm n = 12 4 n = 1 withdrew before treatment | Subtype: Various Severity: GMFCS I-V Age: 2.7–11.6 years | UCB (n = 12) | Allogeneic, intravenous | 1 year | Gross Motor: GMFM-66 Fine Motor & Upper Limb: QUEST General Motor: VABS-2 Communication: VABS-2 Activities of Daily Living: VABS-2 Cognition & General Development: BSID-3, WPPSI-IV or WISC-V Adaptive Behavior: VABS-2 Executive Function: BRIEF Social-Emotional: SDQ, VABS-2 Quality of Life: CP-QoL-Child Safety | 4 |
| Dong 2018 [25] | Case report n = 1 | Subtype: Not reported Severity: Not reported Age: 4 years | UC-MSCs (n = 1) | Donor type not specified, intravenous and intrathecal | Not reported | Seizures/Electrical Brain Activity: EEG Muscle Strength; General Motor; Language; Cognition & General Development: Descriptive | 4 |
| Feng 2015 [26] | Case series (Retrospective) n = 47 | Subtype: Not reported Severity: “Severe” Age: 1–29 years | UCB (n = 47) | Allogeneic, intravenous then intrathecal | 6 months | Safety | 4 |
| Fu 2019 [27] | Non-randomised dose comparison n = 60 n = 3 LTFU | Subtype: Spastic, topography not reported Severity: GMFCS IV-V Age: Not reported | Group 1: UC-MSCs 1 course (n = 30) Group 2: UC-MSCs 2 courses (n = 27) | Allogeneic, intrathecal | 1 year | Gross Motor: GMFM-88 Fine Motor & Upper Limb: FMFM Safety | 4 |
| Gabr 2015 [67] | RCT n = 100 n = 6 withdrew before treatment | Subtype: Various Severity: GMFCS II-V Age: Mean 4.8 years | Group 1: BM-MSCs (n = 44) Group 2: Standard care (n = 50) | Autologous, intrathecal | 1 year | Gross Motor: GMFCS, PEDI Quality of Life: CHQ Activities of Daily Living: PEDI Social-Emotional: PEDI Safety | 2 |
| Gu 2020 [28] | RCT n = 40 n = 1 withdrew before treatment | Subtype: Not reported Severity: Not reported Age: Mean 4.3 years | Group 1: UC-MSCs + rehab (n = 19) Group 2: Placebo + rehab (n = 20) | Allogeneic, intravenous | 1 year | Gross Motor: GMFM-88 Activities of Daily Living: ADL Neuroimaging: PET-CT Other: CFA Safety | 2 |
| Hassan 2012 [29] | Non-randomised controlled n = 52 | Subtype: Athetoid and spastic, various topography Severity: GMFCS unclear 5 Age: 1–8 years | Group 1: BM-MSC (n = 26) Group 2: No treatment (n = 26) | Autologous, intrathecal | 1 year | Gross Motor: GMFCS, BDPS Activities of Daily Living: BDPS Communication: BDPS Other: Descriptive (‘100 points scale’) | 3 |
| Hirano 2018 [68] | Case report n = 1 | Subtype: Hemiplegia, type not reported Severity: GMFCS II Age: 7 years | Adipose-MSCs (n = 1) | Allogeneic, intravenous, intramuscular, subcutaneous and intra-articular | 1 year | Gross Motor: GMFCS Quality of Life: SF-8 Other: Descriptive (clinical condition) Safety | 4 |
| Huang 2018 [31] | RCT n = 56 n = 2 LTFU | Subtype: Not reported Severity: Not reported Age: 3–12 years | Group 1: UCB-MSCs + rehab (n = 27) Group 2: Placebo + rehab (n = 27) | Allogeneic, intravenous | 2 years | Gross Motor: GMFM-88 Neuroimaging: MRI Seizures/Electrical Brain Activity: EEG Other: CFA Safety | 2 |
| Jensen 2016 [32] | Case report n = 1 | Subtype: Spastic hemiplegia Severity: GMFCS I equivalent Age: 5 years | UCB + rehab (n = 1) | Autologous, intravenous | 5.5 years | Gross Motor; Fine Motor & Upper Limb; Spasticity; Muscle Strength; Cognition & General Development: Descriptive Safety | 4 |
| Kang 2015 [33] | RCT n = 36 n = 2 withdrew before treatment | Subtype: Not reported Severity: GMFCS I-V Age: 0.5–18 years | Group 1: UCB + rehab (n = 17) Group 2: Placebo + rehab (n = 17) | Allogeneic, intravenous or intra-arterial | 6 months | Gross Motor: GMFM, GMPM, WeeFIM, PEDI Muscle Strength: MMT score General Motor: BSID-2 6 Cognition & General Development: WeeFIM Activities of Daily Living: WeeFIM, PEDI Social-Emotional: PEDI Neuroimaging: PET-CT Biomarkers Safety | 2 |
| Kikuchi 2022 [70] | Single-arm n = 6 | Subtype: Spastic hemiplegia, diplegia and quadriplegia Severity: GMFCS I, III-V Age: 1.7–6.7 years | UCB (n = 6) | Autologous, intravenous | 3 years | Gross Motor: GMFM-66, GMFCS General Motor: KSPD Cognition & General Development: KSPD, WISC-IV Social-emotional: KSPD Neuroimaging: MRI-DTI Seizures/Electrical Brain Activity: EEG Safety | 4 |
| Lee 2012 [34] | Single-arm n = 20 | Subtype: Various topography, type not reported Severity: Not reported Age: 1.9–7.6 years | UCB (n = 20) | Autologous, intravenous | 6 months | Gross Motor: GMFM-88, GMFCS, PEDI, DDST-2 Fine Motor & Upper Limb: QUEST, MACS, DDST-2 Activities of Daily Living: PEDI Social-Emotional: PEDI, DDST-2 Language: DDST-2 Neuroimaging: MRI-DTI, SPECT | 4 |
| Li 2012 [35] | Case report n = 1 | Subtype: Not reported Severity: Ambulant Age: 11 years | BM-MSCs (n = 1) | Autologous, intravenous | 1 year | Spasticity: Descriptive Other: Descriptive (vision) Safety | 4 |
| Liu 2017 [36] | RCT n = 105 n = 3 LTFU | Subtype: Spastic, topography not reported Severity: GMFCS II-V Age: 0.5–12.5 years | Group 1: BM-MSCs (n = 35) Group 2: BM-MNCs (n = 35) Group 3: Rehab (n = 35) | Autologous, intrathecal | 1 year | Gross Motor: GMFM Fine Motor & Upper Limb: FMFM | 2 |
| Luan 2012 [37] | RCT n = 94 | Subtype: Various Severity: “Severe” Age: Mean 1.3 years | Group 1: Fetal NPCs + rehab (n = 45) Group 2: Rehab alone (n = 49) | Allogeneic, intra-cerebroventricular | 1 year | Gross Motor: GMFM Fine Motor & Upper Limb: PDMS-FM Cognition & General Development: Descriptive Other: Descriptive (sleep) Safety | 2 |
| Mancias-Guerra 2014 [38] | Single-arm n = 18 n = 5 LTFU | Subtype: Various Severity: Not reported Age: 2.2–5.5 years | BM-TNCs (n = 18) | Autologous, intrathecal and intravenous | 6 months | General Motor: BDI Cognition & General Development: BDI Communication: BDI Adaptive Behavior: BDI Social-Emotional: BDI Neuroimaging: MRI Safety | 4 |
| Maric 2022 [39] | Single-arm n = 42 | Subtype: Various types, topography not reported Severity: GMFCS I-V Age: 1–12 years | BM-MNCs (n = 42) | Autologous, intrathecal | 1 year | Gross Motor: GMFCS, S-D Fine Motor & Upper Limb: LAP-D Spasticity: MAS Cognition & General Development: LAP-D Language: LAP-D Neuroimaging: MRI Seizures/Electrical Brain Activity: EEG Safety | 4 |
| Min 2013 [40] | RCT n = 105 n = 9 LTFU | Subtype: Various Severity: GMFCS I-V Age: 0.6–9.8 years | Group 1: UCB + EPO + rehab (n = 35) Group 2: Placebo UCB + EPO + rehab (n = 36) Group 3: Placebo UCB + Placebo EPO + rehab (n = 34) | Allogeneic, intravenous | 6 months | Gross Motor: GMFM, GMPM, PEDI, WeeFIM Fine Motor & Upper Limb: QUEST Muscle Strength: MMST General Motor: BSID-2 Cognition & General Development: BSID-2, WeeFIM Activities of Daily Living: PEDI, WeeFIM Social-Emotional: PEDI Neuroimaging: MRI-DTI, PET-CT Safety | 2 |
| Min 2020 [41] | RCT n = 92 n = 4 LTFU | Subtype: Various Severity: GMFCS I-V Age: 1–6.3 years | Group 1: UCB + EPO (n = 22) Group 2: UCB + Placebo EPO (n = 24) Group 3: Placebo UCB + EPO (n = 20) Group 4: Placebo UCB + Placebo EPO (n = 24) | Allogeneic, intravenous | 1 year | Gross Motor: GMFM, GMPM, GMFCS, PEDI, SCALE Fine Motor & Upper Limb: QUEST Spasticity: MAS, Modified Tardieu Scale Muscle Strength: MRC Scale General Motor: BSID-2 Cognition & General Development: BSID-2, FIM Activities of Daily Living: FIM, PEDI Social-Emotional: PEDI Neuroimaging: MRI-DTI, PET-CT Seizures/Electrical Brain Activity: EEG Other: Descriptive (parent satisfaction), Beery VMI Biomarkers Safety | 2 |
| Nguyen 2017 [42] | Single-arm n = 40 | Subtype: Spastic bilateral and unilateral Severity: GMFCS III-V Age: 1–12 years | BM-MNCs (n = 40) | Autologous, intrathecal | 6 months | Gross Motor: GMFM-66/-88 Spasticity: MAS Safety | 4 |
| Nguyen 2018 [43] | Single-arm n = 30 | Subtype: Quadriplegia and hemiplegia, type not reported Severity: GMFCS II-V Age: 2–15.5 years | BM-MNCs + rehab (n = 30) | Autologous, intrathecal | 6 months | Gross Motor: GMFM-66/-88 Spasticity: MAS Quality of Life: CP-QoL-Child | 4 |
| Okur 2018 [44] | Case report n = 1 | Subtype: Dystonic Severity: GMFCS V Age: 6 years | UC-MSCs + rehab (n = 1) | Allogeneic, intrathecal, intramuscular and intravenous | 1.5 years | Gross Motor: GMFCS, TCMS Fine Motor & Upper Limb: MACS Spasticity: Modified Tardieu Scale Communication: CFCS Cognition & General Development: FIM Activities of Daily Living: FIM Safety | 4 |
| Padma Srivastava 2011 [45] | Case series n = 30 | Subtype: Dystonic and spastic 7, topography not reported Severity: “Moderate to severe” Age: 5–25 years | BM-MNCs (n = 30) | Autologous, intra-arterial | 1 year | Spasticity: Ashworth Scale Muscle Strength: MRC Scale Activities of Daily Living: mBI Other: mRS Safety | 4 |
| Papadopoulos 2011 [46] | Case report n = 2 | Subtype: Spastic diplegia Severity: GMFCS III Age: 1.6 and 2.7 years | Case 1: UCB + G-CSF 12 months post-infusion Case 2: UCB + G-CSF pre- and post-infusion | Autologous, intravenous | Case 1: 2.3 years Case 2: 7 months | Gross Motor: GMFCS Neuroimaging: MRI Spasticity: Descriptive Safety | 4 |
| Purandare 2012 [47] | Case report n = 1 | Subtype: Not reported Severity: GMFCS III Age: 6 years | BM-MNCs (n = 1) | Autologous, intrathecal | 2 years | Gross Motor: GMFCS Neuroimaging: PET-CT Seizures/Electrical Brain Activity: EEG Fine Motor & Upper Limb; Cognition & General Development; Executive Function; Language: Descriptive Other: Descriptive (sensory) | 4 |
| Purwati 2019 [48] | Single-arm n = 14 n = 2 LTFU 8 | Subtype: Not reported Severity: GMFCS III-IV Age: 1–11 years | Adipose-derived NPCs (n = 12) | Autologous, intra-cerebroventricular | 1 year | Gross Motor: GMFCS Spasticity; Cognition & General Development; Communication: Descriptive Safety | 4 |
| Rah 2017 [49] | RCT: Cross-over n = 57 n = 10 LTFU | Subtype: Various Severity: “Non-severe” Age: 2–10 years | Group 1: Peripheral blood-MNCs then placebo (n = 28) Group 2: Placebo then peripheral blood-MNCs (n = 29) | Autologous, intravenous | 1 year (6 months post cross-over) | Gross Motor: GMFM-88, GMFCS, PEDI, DDST-2 9 Fine Motor & Upper Limb: MACS, QUEST Activities of Daily Living: PEDI Social-Emotional: PEDI Neuroimaging: MRI-DTI, PET-CT General Motor; Cognition & General Development: Descriptive Safety | 2 |
| Ramirez 2006 [69] | Single-arm n = 8 | Subtype: Various types, topography not reported Severity: Not reported Age: 3–12 years | Expanded UCB CD133+ cells (n = 8) | Allogeneic, subcutaneous intramuscular | 6 months | Gross Motor; Fine Motor & Upper Limb; Spasticity; Cognition & General Development; Communication; Language: Descriptive Other: Descriptive (infections, vision) Safety | 4 |
| Romanov 2015 [50] | Case series (Retrospective) n = 80 n = 25 LTFU/excluded + n = 17–19 missing data (outcome dependent) | Subtype: Various Severity: GMFCS IV-V Age: 1–12 years | UCB (n = 80) | Allogeneic, intravenous | 3 years post first treatment | Gross Motor: GMFCS Spasticity: MAS Muscle strength: Hand dynamometry Safety | 4 |
| Seledtsov 2005 [51] | Non-randomised controlled n = 60 | Subtype: Double hemiplegia, spastic diplegia and atonic-astatia Severity: “Severe” Age: 1.5–7 years | Group 1: Fetal nervous and hematopoietic cells (n = 30) Group 2: Standard care (n = 30) | Allogeneic, intrathecal | 1 year | Seizures/Electrical Brain Activity: EEG Gross Motor; Fine Motor & Upper Limb; Cognition & General Development; Communication; Language: Descriptive Other: Descriptive (‘100 points scale’, vision) Safety | 3 |
| Sharma 2013 [52] | Case report n = 1 | Subtype: Spastic diplegia Severity: GMFCS III equivalent Age: 20 years | BM-MNC + rehab (n = 1) | Autologous, intrathecal | 1 year | Cognition & General Development: FIM, IQ Score Activities of Daily Living: FIM Neuroimaging: PET-CT Other: Mental Status Examination, Descriptive (appetite) Gross Motor; Fine Motor & Upper Limb; Executive Function, Social-Emotional; Communication; Language: Descriptive | 4 |
| Sharma 2015 [53] | Single-arm n = 40 | Subtype: Various Severity: GMFCS I-V Age: 1.4–22 years | BM-MNCs + rehab (n = 40) | Autologous, intrathecal | 6 months | Neuroimaging: PET-CT Gross Motor; Fine Motor & Upper Limb; Spasticity; Muscle Strength; General Motor; Activities of Daily Living; Cognition & General Development; Social-Emotional; Language: Descriptive Safety | 4 |
| Sharma 2015 [54] | Case report n = 1 | Subtype: Spastic diplegia Severity: GMFCS III Age: 12 years | BM-MNCs + rehab (n = 1) | Autologous, intrathecal | 1 year | Cognition & General Development: FIM Activities of Daily Living: FIM Neuroimaging: PET-CT Gross Motor; Fine Motor & Upper Limb; Muscle Strength: Descriptive Other: Descriptive (sense of smell) | 4 |
| Sharma 2020 [55] | Case report n = 1 | Subtype: Spastic diplegia Severity: GMFCS III Age: 4 years | BM-MNCs + rehab (n = 1) | Autologous, intrathecal | 1.3 years post first treatment | Gross Motor: GMFM, GMFCS Cognition & General Development: FIM Activities of Daily Living: FIM Neuroimaging: PET-CT Fine Motor & Upper Limb; Spasticity; Muscle Strength; General Motor; Adaptive Behavior; Executive Function: Descriptive Other: Descriptive (sensory processing) Safety | 4 |
| Shroff 2014 [56] 10 | Case series (Retrospective) n = 101 n = 10 excluded from analysis n= 25−76 LTFU between treatment phases | Subtype: Not reported Severity: GMFCS I-V Age: ≤2 to 18 years | ESCs + rehab (n = 101) | Allogeneic, multiple routes 11 | 2.4 years post first treatment | Gross Motor: GMFCS Neuroimaging: SPECT Activities of Daily Living; Cognition & General Development; Executive Function; Social-Emotional; Language; Seizures/Electrical Brain Activity: Descriptive Other: Descriptive (hearing) Safety | 4 |
| Sun 2017 [57] | RCT: Cross-over n = 63 | Subtype: Various Severity: GMFCS I-IV Age: 1.1–7 years | Group 1: UCB then placebo (n = 32) Group 2: Placebo then UCB (n = 31) | Autologous, intravenous | 2 years (1 year post cross-over) | Gross Motor: GMFM-66, PDMS Fine Motor & Upper Limb: PDMS Neuroimaging: MRI-DTI Safety | 2 |
| Sun 2021 [58] | Single-arm n = 15 | Subtype: Spastic, various topography Severity: GMFCS II-IV Age: 1–6 years | UCB (n = 15) | Allogeneic, intravenous | 2 years | Gross Motor: GMFM-66, PDMS Fine Motor & Upper Limb: AHA, PDMS Safety | 4 |
| Sun 2022 [71] | RCT n = 91 n = 1 withdrew before treatment + n = 22 LTFU incl. 18 due to COVID-19 | Subtype: Hypertonic, various topography Severity: GMFCS I-IV Age: 2.1–5 years | Group 1: UCB (n = 31) Group 2: UC-MSCs (n = 28) Group 3: Control (n = 31) | Allogeneic, intravenous | 1 year | Gross Motor: GMFM-66, PDMS, PEDI-CAT Fine Motor & Upper Limb: PDMS Activities of Daily Living: PEDI-CAT Adaptive Behavior: PEDI-CAT Social-Emotional: PEDI-CAT Safety | 2 |
| Thanh 2019 [59] | Single-arm n = 25 | Subtype: Spastic bilateral Severity: GMFCS II-V Age: 2–15 years | BM-MNCs + rehab (n = 25) | Autologous, intrathecal | 1 year | Gross Motor: GMFM-66/-88 Spasticity: MAS | 4 |
| Wang 2013 [60] | Case report n = 1 | Subtype: Not reported Severity: Not reported Age: 5 years | UC-MSCs + rehab (n = 1) | Allogeneic, intravenous and intrathecal | 2.3 years | Cognition & General Development: FIM Activities of Daily Living: FIM Muscle Strength; Communication: Descriptive Other: Descriptive (immunity) | 4 |
| Wang 2013 [61] | Single-arm n = 52 n = 6 withdrew before treatment + n = 6 LTFU | Subtype: Spastic and/or athetoid, topography not reported Severity: GMFCS I-V Age: 0.5–15 years | BM-MSC (n = 46) | Autologous, intrathecal +/− intra-parenchymal | 1.5 years | Gross Motor: GMFM-66/-88 Safety | 4 |
| Wang 2015 [62] | Single-arm n = 16 | Subtype: Spastic, topography not reported Severity: Not reported Age: 3–12 years | UC-MSC (n = 16) | Allogeneic, intrathecal | 6 months | Gross Motor: GMFM-88 Fine Motor & Upper Limb: FMFM | 4 |
| Zali 2015 [63] | Single-arm n = 13 n = 1 LTFU | Subtype: Various types, topography not reported Severity: GMFCS III-V Age: 4–10 years | BM-CD133+ cells (n = 13) | Autologous, intrathecal | 6 months | Gross Motor: GMFM-66, GMFCS, BBS Spasticity: MAS Activities of Daily Living: UK FIM + FAM Cognition & General Development: UK FIM + FAM Seizures/Electrical Brain Activity: EEG Safety | 4 |
| Zarrabi 2022 [64] 3 | RCT n = 72 n = 6–9 LTFU (outcome dependent) | Subtype: Spastic quadriplegia and diplegia Severity: GMFCS II-V Age: Mean 9 years | Group 1: UCB + rehab (n = 36) Group 2: Sham procedure + rehab (n = 36) | Allogeneic, intrathecal | 1 year | Gross Motor: GMFM-66, PEDI Spasticity: MAS Activities of Daily Living: PEDI Social-Emotional: PEDI Quality of Life: CP-QoL Neuroimaging: MRI-DTI Safety | 2 |
| Zhang 2015 [65] | Case report n = 1 | Subtype: Not reported Severity: Not reported Age: 0.5 years | UCB-MSCs + rehab (n = 1) | Allogeneic, intravenous | 5 years | Gross Motor: GMFM-88 Spasticity: Ashworth Scale Neuroimaging: MRI Seizures/Electrical Brain Activity: EEG Other: CDCC Infant Mental Development Scale, CFA Safety | 4 |
| Outcome Sub-Category | Instrument [Subdomain] 1 | n 2 | Format | Primary Purpose | Population Designed for |
|---|---|---|---|---|---|
| Movement & Posture | |||||
| Gross Motor | Gross Motor Function Measure (GMFM) -66/-88 | 1163 | Performance-based | E | CP |
| Pediatric Evaluation of Disability Inventory (PEDI)/PEDI-Computer Adaptive Test (CAT) [Mobility] | 573 | Clinician Observation +/− Parent/other Interview | E, D | General | |
| Gross Motor Function Classification System (GMFCS) | 533 | Clinician Observation (or Parent/other/Self Interview) | C | CP | |
| Gross Motor Performance Measure (GMPM) | 218 | Performance-based | E | CP | |
| Peabody Developmental Motor Scales-2 (PDMS-2) [Gross Motor Quotient] | 132 | Performance-based | D (2nd E) | General | |
| The Functional Independence Measure for Children (WeeFIM) [Mobility] | 130 | Clinician Observation +/− Parent/other Interview | E | Pediatric Rehab | |
| Selective Control Assessment of Lower Extremity (SCALE) | 88 | Performance-based | D, E | CP | |
| Denver Developmental Screening Test 2 (DDST-II) [Gross Motor] | 67 | Performance-based +/− Parent/other Interview | D | General | |
| Boyd Developmental Progress Scale (BDPS) [Motor] | 52 | Performance-based + Clinician Observation +/− Parent/other Interview | D | General | |
| Learning accomplishment system diagnostic (LAP-D) Score [Sitting and Standing] | 42 | Performance-based/Parent/other Observation | D | General | |
| Berg Balance Scale (BBS) | 12 | Performance-based | P (2nd D, E) | Adult Rehab | |
| Trunk Control Measurement Scale (TCMS) | 1 | Performance-based | D, E | CP | |
| Fine Motor and Upper Limb | PDMS-2 [Fine Motor Quotient] | 304 | Performance-based | D (2nd E) | General |
| Quality of Upper Extremity Skills Test (QUEST) | 262 | Performance-based | E | CP (Spastic) | |
| Fine Motor Function Measure (FMFM) | 176 | Performance-based | E | CP | |
| Manual Ability Classification Scale (MACS) | 78 | Clinician Observation (or Parent/other/Self Interview) | C | CP | |
| LAP-D [Fine Motor Skills] | 42 | Performance-based/Parent/other Observation | D | General | |
| DDST-II [Fine Motor-Adaptive] | 20 | Performance-based +/− Parent/other Interview | D | General | |
| Assisting Hand Assessment (AHA) | 15 | Performance-based | E | CP (Hemiplegia) | |
| Muscle Strength | Medical Research Council (MRC) Scale for Muscle Strength; MRC Summed Scores | 196 | Performance-based | D, E | General |
| Manual Muscle Strength Test | 96 | Performance-based | D, E | General | |
| Manual Muscle Testing (MMT) Score | 34 | Performance-based | D, E | General | |
| Hand dynamometry | 15 | Performance-based | D, E | General | |
| Spasticity | Modified Ashworth Scale | 381 | Performance-based | D, E | CP (Spastic) |
| Ashworth Scale | 109 | Performance-based | D, E | CP (Spastic) | |
| Modified Tardieu Scale | 89 | Performance-based | D, E | CP (Spastic) | |
| General Motor 3 | Bayley Scales of Infant and Toddler Development 2nd Edition (BSID-II) [Motor Scale] | 218 | Performance-based | D (2nd E) | General |
| Vineland Adaptive Behavior Scales 2nd Edition (VABS-2), parent report questionnaire [Motor Skills Domain] | 30 | Parent/other Questionnaire | D (2nd P, E) | General | |
| Battelle Developmental Inventory (BDI) [Motor] | 13 | Performance-based +/− Parent/other Observation/Interview | D (2nd P, E) | General | |
| Kyoto Scale of Psychological Development (KSPD) [Postural-Motor] | 6 | Performance-based | D, E | General | |
| Activities of Daily Living | |||||
| Activities of Daily Living | PEDI/PEDI-CAT [Self-care/Daily Activities] | 573 | Clinician Observation +/− Parent/other Interview | E, D | General |
| WeeFIM [Self Care] | 130 | Clinician Observation +/− Parent/other Interview | E | Pediatric Rehab | |
| Functional Independence Measure (FIM) [Motor Subscale] | 93 | Clinician Observation | E | General & Rehab | |
| BDPS [Independence] | 52 | Performance-based + Clinician Observation +/− Parent/other Interview | D | General | |
| Activities of Daily Living (ADL) 4 | 39 | Unknown | Unknown | Unknown | |
| Modified Barthel Index (mBI) | 30 | Performance-based/Self/Parent/other Observation/Interview/Questionnaire | E | Adult Rehab | |
| VABS-2 parent report questionnaire [Daily Living Skills Domain] | 30 | Parent/other Questionnaire | D (2nd P, E) | General | |
| UK Functional Independence Measure and Functional Assessment Measure (UK FIM + FAM) [Total Motor Subscore] | 12 | Clinician Observation | E | Rehab | |
| Behavior | |||||
| Social-Emotional | PEDI/PEDI-CAT [Social Function/Social/Cognitive] | 573 | Clinician Observation +/− Parent/other Questionnaire | E, D | General |
| VABS-2 parent report questionnaire [Socialization Domain] | 30 | Parent/other Questionnaire | D (2nd P, E) | General | |
| DDST-II [Personal-Social] | 20 | Performance-based +/− Parent/other Interview | D | General | |
| BDI [Social-Emotional] | 13 | Clinician Observation +/− Parent/other Observation/Interview | D (2nd P, E) | General | |
| Strengths and Difficulties Questionnaire (SDQ) | 8 | Parent/other Questionnaire +/− Interview | D, E | General | |
| KSPD [Language-Social] | 6 | Clinician Observation | D, E | General | |
| Adaptive Behavior | PEDI-CAT [Responsibility] | 86 | Parent/other Questionnaire | E, D | General |
| VABS-2 parent report questionnaire [Maladaptive Behavior Domain] | 30 | Parent/other Questionnaire | D (2nd P, E) | General | |
| BDI [Adaptive] | 13 | Clinician Observation +/− Parent/other Observation/Interview | D (2nd P, E) | General | |
| Executive Function | Behavior Rating Inventory of Executive Function (BRIEF) | 7 | Parent/other Questionnaire | D (2nd E) | General |
| Brain Structure & Function | |||||
| Neuroimaging | Magnetic resonance imaging (MRI); MRI with Diffusion tensor imaging (DTI) | 525 | Clinician Observation | D, P | General |
| Positron emission tomography and computed tomography scan (PET-CT) | 293 | Clinician Observation | D, P | General | |
| Single photon emission computed tomography scan (SPECT) | 111 | Clinician Observation | D, P | General | |
| Seizures/Electrical brain activity | Electroencephalogram (EEG) | 255 | Clinician Observation | D, P | General |
| Seizure burden/frequency | 128 | Clinician Observation | D, P | General | |
| Cognition & General Development | |||||
| Cognition and General Development | BSID-II [Mental Scale] | 184 | Performance-based | D (2nd E) | General |
| WeeFIM [Cognition] | 130 | Clinician Observation +/− Parent/other Interview | E | Pediatric Rehab | |
| FIM [Cognition Subscale] | 93 | Clinician Observation | E | General & Rehab | |
| LAP-D [Cognitive Skills] | 42 | Performance-based/Parent/other Observation | D | General | |
| BDI [Cognitive] | 13 | Clinician Observation +/− Parent/other Observation/Interview | D (2nd P, E) | General | |
| UK FIM + FAM [Total Cognitive Subscore] | 12 | Clinician Observation | E | Rehab | |
| Wechsler Intelligence Scale for Children 4th/5th Edition (WISC-IV/-V) | 7 | Performance-based | D (2nd P) | General | |
| KSPD [Cognitive-Adaptive] | 6 | Performance-based | D, E | General | |
| Bayley Scales of Infant and Toddler Development 3rd Edition (BSID-III) [Cognitive Scale] | 1 | Performance-based | D (2nd P, E) | General | |
| Intelligence Quotient (IQ) Score | 1 | Performance-based | D (2nd P) | General | |
| Wechsler Preschool & Primary Scale of Intelligence 4th Edition (WPPSI-IV) | 1 | Performance-based | D (2nd P) | General | |
| Quality of Life | |||||
| Quality of Life | Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL-Child), Primary Caregiver Questionnaire | 147 | Parent/other Questionnaire | D, E | CP |
| Child Health Questionnaire Parent Form 50 (CHQ) | 94 | Parent/other Questionnaire | D, E | General | |
| Short Form 8 (SF-8) Health Survey Quality of Life Questionnaire | 1 | Self/Parent/other Questionnaire | D, E | General | |
| Language & Communication | |||||
| Language | Gesell Developmental Schedules | 60 | Clinician Observation | D | General |
| LAP-D [Speech skills] | 42 | Performance-based/Parent/other Observation | D | General | |
| DDST-II [Language Skills] | 20 | Performance-based +/− Parent/other Interview | D | General | |
| Communication | BDPS [Communication] | 52 | Performance-based + Clinician Observation +/− Parent/other Interview | D | General |
| VABS-2 parent report questionnaire [Communication Domain] | 30 | Parent/other Questionnaire | D (2nd P, E) | General | |
| BDI [Communication] | 13 | Performance-based +/− Parent/other Observation/Interview | D (2nd P, E) | General | |
| Communication Function Classification System (CFCS) | 11 | Clinician Observation | C | CP | |
| Other | |||||
| Other | Comprehensive Functional Assessment (CFA) Scale | 94 | Unknown | Unknown | Unknown |
| Beery-Buktenica Developmental Test of Visual-Motor Integration 6th Edition | 88 | Performance-based | D | General | |
| Modified Rankin Scale (mRS) | 30 | Self (Clinician-led) Interview | E | Adult Rehab | |
| Caregiver Questionnaire Scale | 14 | Parent/other Questionnaire | Unknown | General | |
| CDCC Infant Mental Development Scale for general development status | 1 | Performance-based | D (2nd E) | General | |
| Mental Status Examination | 1 | Clinician Observation | D, E | General | |
| Safety | |||||
| Safety | Safety reports/AEs/Routine laboratory and clinical assessments (including neuroimaging for safety exclusively) | 1705 | N/A | D | General |
| Biomarkers | |||||
| Biomarkers | Biomarkers (various) | 144 | N/A | D | General |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finch-Edmondson, M.; Paton, M.C.B.; Honan, I.; Karlsson, P.; Stephenson, C.; Chiu, D.; Reedman, S.; Griffin, A.R.; Morgan, C.; Novak, I. Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy. J. Clin. Med. 2022, 11, 7319. https://doi.org/10.3390/jcm11247319
Finch-Edmondson M, Paton MCB, Honan I, Karlsson P, Stephenson C, Chiu D, Reedman S, Griffin AR, Morgan C, Novak I. Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy. Journal of Clinical Medicine. 2022; 11(24):7319. https://doi.org/10.3390/jcm11247319
Chicago/Turabian StyleFinch-Edmondson, Megan, Madison C. B. Paton, Ingrid Honan, Petra Karlsson, Candice Stephenson, Darryl Chiu, Sarah Reedman, Alexandra R. Griffin, Catherine Morgan, and Iona Novak. 2022. "Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy" Journal of Clinical Medicine 11, no. 24: 7319. https://doi.org/10.3390/jcm11247319
APA StyleFinch-Edmondson, M., Paton, M. C. B., Honan, I., Karlsson, P., Stephenson, C., Chiu, D., Reedman, S., Griffin, A. R., Morgan, C., & Novak, I. (2022). Are We Getting It Right? A Scoping Review of Outcomes Reported in Cell Therapy Clinical Studies for Cerebral Palsy. Journal of Clinical Medicine, 11(24), 7319. https://doi.org/10.3390/jcm11247319









