Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Quality
2.5. Data Analysis and Synthesis
3. Results
3.1. Study Selection
3.2. Patient Characteristics
3.3. Procedural Data
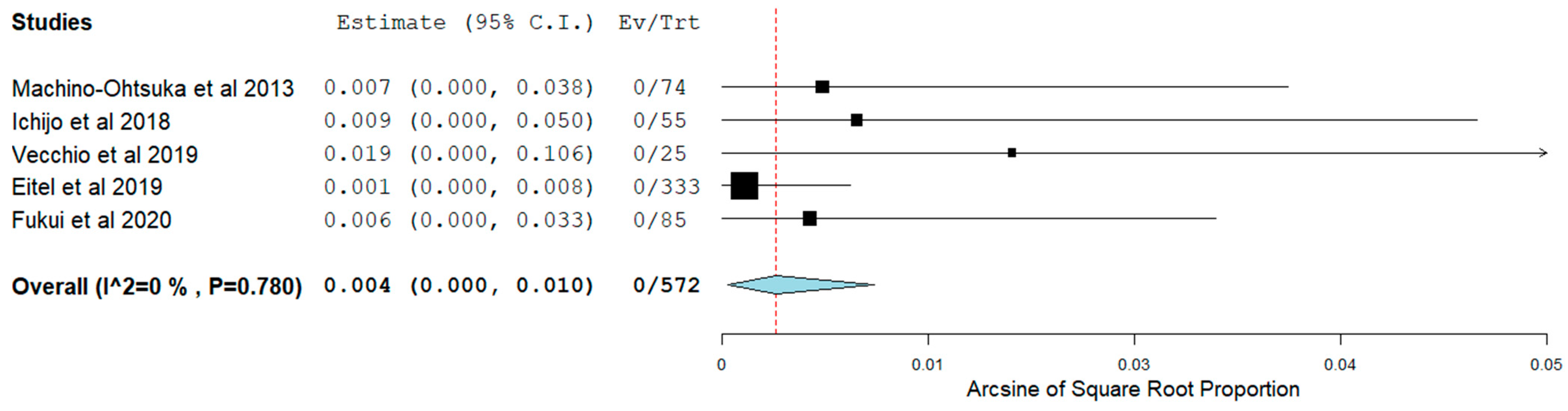
3.4. Short-Term Outcomes
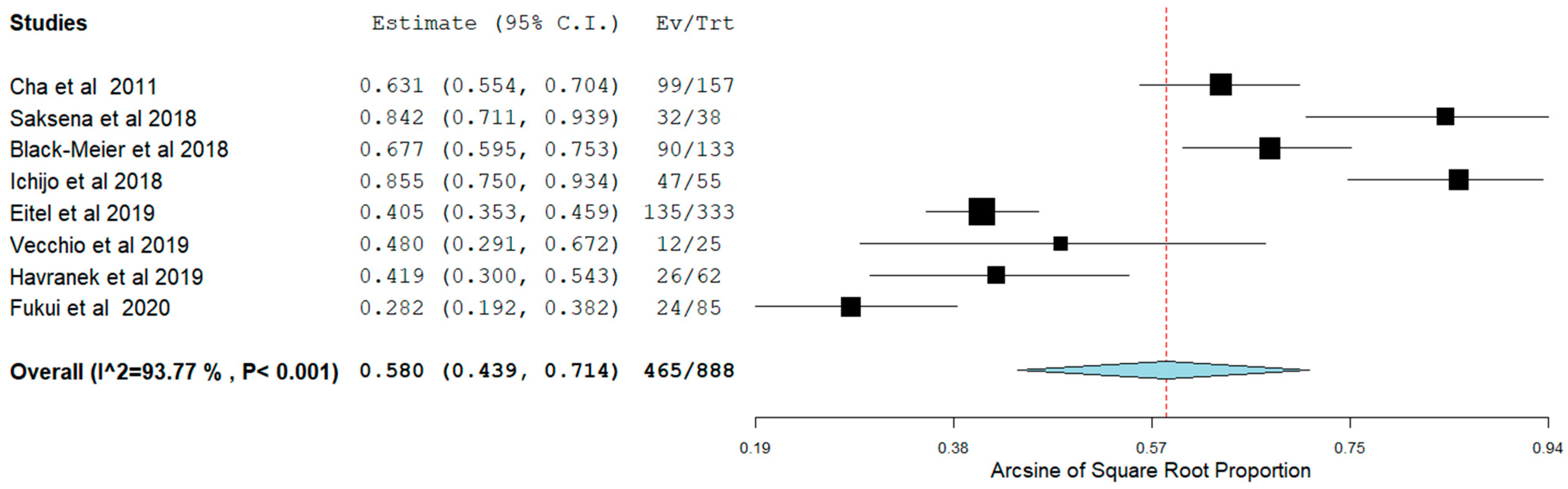
3.5. Long-Term Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Son, M.K.; Park, J.J.; Lim, N.-K.; Kim, W.-H.; Choi, D.-J. Impact of atrial fibrillation in patients with heart failure and reduced, mid-range or preserved ejection fraction. Heart 2020, 106, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.R.; Moosa, P.G.; Fatima, M.; Ochani, R.K.; Shahnawaz, W.; Jangda, M.A.; Shah, S.A. Atrial fibrillation and heart failure-results of the CASTLE-AF trial. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Brozovich, F.V. HFpEF, a disease of the vasculature: A closer look at the other half. Mayo Clin. Proc. 2018, 93, 1305–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Merino-Merino, A.; Saez-Maleta, R.; Salgado-Aranda, R.; AlKassam-Martinez, D.; Pascual-Tejerina, V.; Martin-Gonzalez, J.; Garcia-Fernandez, J.; Perez-Rivera, J.A. Biomarkers in atrial fibrillation and heart failure with non-reduced ejection fraction: Diagnostic application and new cut-off points. Heart Lung 2020, 49, 388–392. [Google Scholar] [CrossRef]
- DeVore, A.D.; Piccini, J.P. Mineralocorticoid receptor antagonism for the treatment of AF and HFpEF. JACC Heart Fail. 2018, 6, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Hoffmanns, P.; Enayati, W.; Meltendorf, U.; Geller, J.C.C.; Klein, H.U. Effect of successful electrical cardioversion on serum aldosterone in patients with persistent atrial fibrillation. Am. J. Cardiol. 2001, 88, 906–909. [Google Scholar] [CrossRef]
- Abe, Y.; Akamatsu, K.; Ito, K.; Matsumura, Y.; Shimeno, K.; Naruko, T.; Takahashi, Y.; Shibata, T.; Yoshiyama, M. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ. J. 2018, 82, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Kishima, H.; Mine, T.; Takahasi, S.; Ashida, K.; Ishihara, M.; Masuyama, T. Left atrial ejection force predicts the outcome after catheter ablation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 264–271. [Google Scholar] [CrossRef]
- Zolotarova, T.V.; Brynza, M.S.; Volkov, D.Y.; Shevchuk, M.I.; Bilchenko, O.V. Predictors of atrial fibrillation recurrence after radiofrequency ablation in patients with chronic heart failure. Wiad. Lek. 2021, 74, 1850–1855. [Google Scholar] [CrossRef]
- Mitter, S.S.; Shah, S.J. Spironolactone for management of heart failure with preserved ejection fraction: Whither to after TOPCAT? Curr. Atheroscler. Rep. 2015, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Temma, T.; Nagai, T.; Watanabe, M.; Kamada, R.; Takahashi, Y.; Hagiwara, H.; Koya, T.; Nakao, M.; Omote, K.; Kamiya, K.; et al. Differential prognostic impact of atrial fibrillation in hospitalized heart failure patients with preserved ejection fraction according to coronary artery disease status—Report from the Japanese Nationwide Multicenter Registry. Circ. J. 2020, 84, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D. Systematic reviews and meta-analyses. Int. J. Tuberculol. Lung Dis. 2011, 15, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; DeVore, A.D.; Wu, J.; Hammill, B.G.; Sharma, A.; Cooper, L.B.; Felker, G.M.; Piccini, J.P.; Allen, L.A.; Heidenreich, P.A.; et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: Insights from get with the guidelines-heart failure. J. Am. Heart Assoc. 2019, 8, e011560. [Google Scholar] [CrossRef] [PubMed]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Yamamoto, M.; Hamada-Harimura, Y.; Machino, T.; Yamasaki, H.; Sekiguchi, Y.; Nogami, A.; Aonuma, K.; et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J. Cardiol. 2019, 74, 235–244. [Google Scholar] [CrossRef]
- Rahman, A.; Hasani, A.; Moussa, O.; Kumar, S.; Jahufar, F.; Saeed, O.; Murthy, S.; Vukelic, S.; Patel, S.; Shin, J.J.; et al. Efficacy of catheter ablation of atrial fibrillation in heart failure with preserved ejection fraction. J. Card. Fail. 2019, 25, S84–S85. [Google Scholar] [CrossRef] [Green Version]
- Fukui, A.; Tanino, T.; Yamaguchi, T.; Hirota, K.; Saito, S.; Okada, N.; Akioka, H.; Shinohara, T.; Yufu, K.; Takahashi, N. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J. Cardiovasc. Electrophysiol. 2020, 31, 682–688. [Google Scholar] [CrossRef]
- National Heart LaBI. Study Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 September 2020).
- Vecchio, N.; Ripa, L.; Orosco, A.; Tomas, L.; Mondragón, I.; Acosta, A.; Talavera, L.; Rivera, S.; Albina, G.; Diez, M.; et al. Atrial fibrillation in heart failure patients with preserved or reduced ejection fraction—Prognostic significance of rhythm control strategy with catheter ablation. J. Atr. Fibrillation 2019, 11, 2128. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Sugano, A.; Atsumi, A.; Yamamoto, M.; Kawamura, R.; Machino, T.; Kuroki, K.; Yamasaki, H.; et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013, 62, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Black-Maier, E.; Ren, X.; Steinberg, B.A.; Green, C.L.; Barnett, A.S.; Rosa, N.S.; Al-Khatib, S.M.; Atwater, B.D.; Daubert, J.P.; Frazier-Mills, C.; et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018, 15, 651–657. [Google Scholar] [CrossRef]
- Cha, Y.M.; Wokhlu, A.; Asirvatham, S.J.; Shen, W.K.; Friedman, P.A.; Munger, T.M.; Oh, J.K.; Monahan, K.H.; Haroldson, J.M.; Hodge, D.O.; et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: A comparison to systolic dysfunction and normal ventricular function. Circ. Arrhythmia Electrophysiol. 2011, 4, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Elkaryoni, A.; Al Badarin, F.; Spertus, J.A.; Kennedy, K.F.; Wimmer, A.P. Comparison of the Effect of Catheter Ablation for Atrial Fibrillation on All-Cause Hospitalization in Patients With Versus Without Heart Failure (from the Nationwide Readmission Database). Am. J. Cardiol. 2020, 125, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, S.; Miyazaki, S.; Kusa, S.; Nakamura, H.; Hachiya, H.; Kajiyama, T.; Iesaka, Y. Impact of catheter ablation of atrial fibrillation on long-term clinical outcomes in patients with heart failure. J. Cardiol. 2018, 72, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitel, C.; Ince, H.; Brachmann, J.; Kuck, K.H.; Willems, S.; Gerds-Li, J.H.; Tebbenjohanns, J.; Richardt, G.; Hochadel, M.; Senges, J.; et al. Atrial fibrillation ablation strategies and outcome in patients with heart failure: Insights from the German ablation registry. Clin. Res. Cardiol. 2019, 108, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Slee, A.; Saad, M. Atrial resynchronization therapy in patients with atrial fibrillation and heart failure with and without systolic left ventricular dysfunction: A pilot study. J. Interv. Cardiol. Electrophysiol. 2018, 53, 9–17. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef] [Green Version]
- Uk, N.A.A.; Atherton, J.J.; Bauersachs, J.; Uk, A.J.C.; Carerj, S.; Ceconi, C.; Coca, A.; Uk, P.E.; Erol, Ç.; Ezekowitz, J.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart failure with preserved, borderline, and reduced ejection fraction: 5-Year outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Anter, E.; Jessup, M.; Callans, D.J. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation 2009, 119, 2516–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceño, D.F.; Markman, T.M.; Lupercio, F.; Romero, J.; Liang, J.J.; Villablanca, P.A.; Birati, E.Y.; Garcia, F.C.; Di Biase, L.; Natale, A.; et al. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis of randomized controlled trials. J. Interv. Card. Electrophysiol. 2018, 53, 19–29. [Google Scholar] [CrossRef] [PubMed]




| Authors | Patients (N) | Study Design | HFpEF Inclusion Criteria | Age (Mean ± SD) | Female N (%) | BMI (Mean ± SD) | HTN, N (%) | DM, N (%) | IHD, N (%) | Stroke, N (%) | B-Blockers N (%) | CCB, N (%) | Digoxin, N (%) | AAD, N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cha (2011) | 157 | Prospective, single-centre | LVEF ≥ 50% and abnormal diastolic function | 62.2 (54.4, 70.5) | 50 (31.8) | N/A | 75 (47.8) | 15 (9.6) | 27 (17.2) | 8 (5.1) | 102 (65.0) | 31 (19.7) | N/A | 85 (54.1) |
| Machino-Ohtsuka (2013) | 74 | Prospective, single-centre | LVEF > 50% and fulfilled criteria for HFpEF according to the European Society of Cardiology recommendations [27] | 65.0 ± 7.0 | 19 (25.7) | 26.7 ± 14.7 | 57 (77.0) | 21 (28.4) | 14 (18.9) | 10 (13.5) | 53 (71.6) | 34 (45.9) | 5 (6.8) | Class I = 57 (77.0) Class III = 37 (50.0) Class IV = 12 (16.2) |
| Black-Maier (2018) | 133 | Retrospective, single-centre | LVEF ≥ 50% | 68.0 (60.0, 74.0) | 56 (42.1) | 32.0 (28.0, 38.0) | 113 (85.0) | 38 (28.6) | N/A | N/A | 97 (72.9) | N/A | 20 (15.0) | Class 1C = 10 (7.5) Class III = 73 (54.9) Amiodarone = 16 (12.0%) |
| Ichijo (2018) | 55 | Prospective, single-centre | LVEF > 45% [28] | 64.0 ± 10.0 | 11 (20.0) | 25.5 ± 4.7 | 33 (60.0) | 13 (23.6) | 10 (18.2) | 5 (9.1) | 33 (60.0) | 15 (27.3) | N/A | 24 (43.6) |
| Kelly (2019) | 15,682 (1857 patients in the rhythm control group) | Retrospective, multi-centre | LVEF ≥ 50% or normally/mildly impaired systolic function classified as HFpEF as characterised in the GWTG-HF analyses [30] | 81.0 * | 1222 (65.8) | N/A | 1556 (83.8) | 669 (36.0) | 904 (48.7) | 325 (17.5) | N/A | N/A | N/A | N/A |
| Machino-Ohtsuka (2019) | 158 (79 patients in the rhythm control group) | Retrospective, multi-centre | Fulfilled criteria for HFpEF according to guidelines [24,25] | 68.0 ± 7.0 | 32 (40.5) | 24.6 ± 4.2 | 59 (74.7) | 27 (34.1) | 13 (16.5) | 10 (12.7) | 53 (67.1) | 34 (43.0) | N/A | Class Ia = 5 (6.3) Class Ic = 31 (39.2) Amiodarone = 44 (55.7) Aprindine = 8 (10.1) |
| Eitel (2019) | 333 | Prospective, multi-centre | LVEF ≥ 50% [28] | 65.4 ± 9.6 | 113 (33.9) | N/A | 255 (76.7) | 36 (10.8) | 151 (45.3) | 24 (7.1) | 240 (72.1) | N/A | N/A | Classes I, III, IV = 177 (53.2) |
| Fukui (2020) | 85 (35 patients in the catheter ablation group) | Retrospective, single-centre | LVEF ≥ 50% with LV diastolic dysfunction | 70.0 ± 8.0 | 12 (34.3) | N/A | 21 (55.0) | 8 (21) | N/A | N/A | 20 (57.0) | N/A | N/A | Amiodarone = 14 (40) |
| Authors | Duration of AF Prior to Intervention (Years ± SD) | AF Type N (%) | Pre-LVEF (%, Mean ± SD) | LA Volume (Mean ± SD) | E/E’ (Mean ± SD) | Treated Using Catheter Ablation N (%) | First Procedure, N (%) | Radiofrequency N (%) | Circumferential PVI, N (%) | 3D Mapping System | Procedure Time (min, Mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cha (2011) | 4.2 (1.7, 8.5) | Paroxysmal = 78 (49.7) Non-paroxysmal = 79 (50.3) | 62.0 [60.0, 65.0] | 40 cm3/m2 [35, 50] | 12.0 [8.6, 15.7] | 157 (100) | 138 (88.0) | 157 (100) | 157 (100) (PVI and WACA) | N/A | 94.0 (57.0, 133.0) |
| Machino-Ohtsuka (2013) | 7.3 ± 7.2 | Paroxysmal = 23 (31.0) Persistent = 7 (9.5) Long-standing = 44 (59.5) | 66.7 ± 7.2 | Baseline = 45.2 ± 17.5 mL/m2 Follow-up = 42.6 ± 20.2 mL/m2 | Baseline = 11.8 ± 4.7 Follow-up = 10.3 ± 3.7 | 74 (100) | 24 (32.4) | N/A | N/A | N/A | N/A |
| Black-Maier (2018) | N/A | Paroxysmal = 45 (37.2) Non-paroxysmal = 76 (62.8) | 55.0 (55.0, 55.0) | N/A | N/A | 133 (100) | 127 (95.5) | 133 (100) | 133 (100) | CARTO (Biosense-Webster Inc, Diamond Bar, CA) or NavX (St Jude Medical, Inc, Minneapolis, MN) | 233.0 (192.0, 290.0) |
| Ichijo (2018) | N/A | Paroxysmal = 23 (41.8) Non-paroxysmal = 32 (58.2) | 57.0 ± 8.0 | N/A | N/A | 55 (100) | N/A | 55 (100) | N/A | CARTO 3 (Biosense-Webster, Irvine, CA, USA) | N/A |
| Kelly (2019) | N/A | N/A | 58.0 * | N/A | N/A | 19 (1) | N/A | N/A | N/A | N/A | N/A |
| Machino-Ohtsuka (2019) | 5.0 ± 5.3 | Paroxysmal = 34 (43.0) Non-paroxysmal = 45 (57.0) | 65.0 ± 8.0 | 51.0 ± 21.0 mL/m2 | 12.0 ± 4.6 | 66 (83.5) | N/A | N/A | N/A | N/A | N/A |
| Eitel (2019) | N/A | Paroxysmal = 153 (45.8) Persistent = 136 (41.0) Permanent = 44 (13.3) | N/A | N/A | N/A | 333 (100) | 271 (80.2) | 294 (87.0) | 282 (83.4) | N/A | 175.8 ± 77.8 |
| Fukui (2020) | N/A | Paroxysmal = 14 (40) Non-paroxysmal = 21 (60.0) | 62.0 ± 8.0 | N/A | 16.0 ± 7.0 | 35 (100) | N/A | 35 (100) | N/A | CARTO 3 (Biosense Webster, Diamond Bar, CA) or EnSite NavX (Abbott Medical, St. Paul, MN) | 168.0 ± 45.0 |
| Authors | Follow-Up (Months) | Major Bleeding N (%) | Vascular Complications, N (%) | Stroke, N (%) | Total Complications N (%) | AF Recurrence N (%) | Patients in SR N (%) | Change in Symptoms | HF Admission N (%) | All-Cause Admission, N (%) | Death/All-Cause Mortality, N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black-Maier (2018) | 10.3 (7.3, 12.1) | Peri-procedural = 4 (3.0) (access site bleeding) | Peri-procedural = 0 (0) | Peri-procedural = 0 (0) | Peri-procedural = 9 (6.8) | 43 (33.9) | 90 (67.7) | MAFSI symptom severity = −0.23 MAFSI symptom frequency = −1.05 | 8 (6.0) | 35 (26.3) | N/A |
| Ichijo (2018) | 32.8 ± 18.5 | Post-procedure = 1 (1.8) | Post-procedure = 0 (0) | Post-procedure = 0 (0) | Procedural = 3 (5.5) Post-procedure = 1 (1.8) | 8 (14.5) | 47 (85.5) | N/A | 2 (3.8) | N/A | N/A |
| Kelly (2019) | 12 * | Rhythm = 79 (4.3) Rate = 655 (4.7) | N/A | Rhythm = 29 (1.6) Rate = 318 (2.3) | Rhythm = 680 (36.6) Rate = 4858 (35.1) | N/A | N/A | N/A | Rhythm = 488 (26.3) Rate = 3830 (27.7) | Rhythm = 1151 (62.0) Rate = 8931 (64.6) | Rhythm = 572 (30.8) Rate = 5184 (37.5) |
| Machino-Ohtsuka (2019) | 24 (11–37) | 0(0) | 0 (0) | 0 (0) | 0 (0) | Rhythm = 22 (27.8) Rate = 75 (94.9) | Rhythm = 57 (72.2) Rate = 4 (5.1) | N/A | Rhythm = 5 (6.3) Rate = 18 (22.8) | N/A | Rhythm = 2 (2.5) Rate = 8 (10.1) |
| Eitel (2019) | 12 | In-hospital = 7 (2.1) Post-procedure = 1 (0.3) | In-hospital = 8 (2.4) Post-procedure = 0 (0) | In-hospital = 2 (0.6) Post-procedure = 4 (1.3) | In-hospital = 41 (12.3) Post-procedure = 7 (2.2) | 140 (47.9) | Without AADs = 135 (49.1) | N/A | N/A | 150 (50.0) | 8 (2.5) |
| Rahman (2019) | 12 | N/A | N/A | Rhythm = 2 (2.4) Rate = 9 (9.5) | Rhythm = 2 (2.4) Rate = 9 (9.5) | Rhythm = 16 (18.8) Rate = 63 (66.3) | Rhythm = 69 (81.2) Rate = 32 (33.7) | N/A | N/A | Rhythm = 51 (60.0) Rate = 52 (54.7) | Rhythm = 3 (3.5) Rate = 2 (2.1) |
| Fukui (2020) | 24 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | Rhythm = 11 (26.0) Rate = N/A | Rhythm = 24 (68.6) Rate = N/A | N/A | Rhythm = 3 (8.6) Rate = 24 (48.0) | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Androulakis, E.; Sohrabi, C.; Briasoulis, A.; Bakogiannis, C.; Saberwal, B.; Siasos, G.; Tousoulis, D.; Ahsan, S.; Papageorgiou, N. Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 288. https://doi.org/10.3390/jcm11020288
Androulakis E, Sohrabi C, Briasoulis A, Bakogiannis C, Saberwal B, Siasos G, Tousoulis D, Ahsan S, Papageorgiou N. Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(2):288. https://doi.org/10.3390/jcm11020288
Chicago/Turabian StyleAndroulakis, Emmanuel, Catrin Sohrabi, Alexandros Briasoulis, Constantinos Bakogiannis, Bunny Saberwal, Gerasimos Siasos, Dimitris Tousoulis, Syed Ahsan, and Nikolaos Papageorgiou. 2022. "Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 2: 288. https://doi.org/10.3390/jcm11020288
APA StyleAndroulakis, E., Sohrabi, C., Briasoulis, A., Bakogiannis, C., Saberwal, B., Siasos, G., Tousoulis, D., Ahsan, S., & Papageorgiou, N. (2022). Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(2), 288. https://doi.org/10.3390/jcm11020288










