Abstract
The use of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with type 2 diabetes mellitus (T2DM) has been associated with decreased skeletal muscle mass but remains unclear in patients with cardiovascular disease (CVD) undergoing comprehensive outpatient cardiac rehabilitation (CR). Therefore, this study investigates the effect of SGLT2 inhibitors on the outcomes of patients with CVD and T2DM undergoing comprehensive outpatient CR. The study included 402 patients with CVD and T2DM who participated in comprehensive outpatient CR. Physical functions (grip strength, maximal quadriceps isometric strength, usual gait speed, and 6-minute walking distance) were measured at discharge as baseline and 5 months thereafter, and the association between physical functions and SGLT2 inhibitor use was reviewed. Physical functions improved regardless of SGLT2 inhibitor use. Multiple regression analysis showed that SGLT2 inhibitor use was not associated with improvement or decline in physical functions (p ≥ 0.05). The use of SGLT2 inhibitors in patients with CVD and T2DM undergoing outpatient CR did not impair improvement in physical functions.
1. Introduction
Exercise-based outpatient cardiac rehabilitation (CR), including exercise therapy, is an established non-pharmacological therapy that has shown improved outcomes in patients with cardiovascular disease (CVD) and heart failure (HF) in previous studies []. CR guidelines recommend it as equivalent (Class 1, Level A) to pharmacotherapy [,,]. Recently, sodium-glucose cotransporter 2 (SGLT2) inhibitors, insulin-independent hypoglycemic agents developed for the treatment of diabetes, have attracted much attention []. The effects of SGLT2 inhibitors have been reported in patients with CVD [,] and SGLT2 inhibitors have been added to class I standard medical therapy for patients with HF [,].
However, previous studies on type 2 diabetes mellitus (T2DM) have shown a decrease in skeletal muscle mass with SGLT2 inhibitors [,,]. In addition to reduced skeletal muscle mass, mice with adjusted dietary intake have also shown muscle weakness []. However, it remains unclear as to how SGLT2 inhibitors affect changes in skeletal muscle mass and physical function, while there is concern that SGLT2 inhibitors may reduce physical function. Although several patients with CVD undergoing CR are increasingly prescribed SGLT2 inhibitors, the impact of SGLT2 inhibitor administration on the physical outcomes of outpatient CR remains nebulous.
Therefore, this study determines the association between SGLT2 inhibitor use and changes in physical function in patients with CVD and T2DM who participated in outpatient CR.
2. Materials and Methods
In this single-center retrospective observational study, a cohort of consecutive patients with CVD admitted to Kitasato University Hospital for CVD treatment from April 2007 to August 2020 were reviewed. Among them, those who also had T2DM and for whom at least one physical function (grip strength, maximal quadriceps isometric strength [QIS], usual gait speed, or 6-minute walking distance [6 MWD]) could be measured longitudinally twice were studied. Patients who were taking SGLT2 inhibitors prior to admission were excluded. This research protocol was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Kitasato Institute Clinical Research Review Committee (KMEO B18-075).
2.1. Patient Characteristics
We obtained data on age, sex, body weight, height, body mass index (BMI), left ventricular ejection fraction (LVEF), number of outpatient rehabilitation participations, HF severity according to the New York Heart Association functional classification (NYHA class) and history of HF and acute myocardial infarction from medical records at admission. Hemoglobin (Hb), B-type natriuretic peptide, and serum creatinine (sCr) levels were analyzed as routine tests. The estimated glomerular filtration rate (eGFR) was determined from the sCr value using 194 × sCr − 1.094 × age (years) − 0.287 × 0.739 (for women) developed by the Japanese Society of Nephrology []. Further, 2-dimensional method was used to measure LVEF on echocardiogram.
2.2. SGLT2 Inhibitors
The patients in our study using SGLT2 inhibitors were prescribed any of the following four drugs: empagliflozin, dapagliflozin, canagliflozin, and luseogliflozin. Dosage and drug withdrawal were performed according to the relevant documentation.
2.3. Physical Function
Physical functions including grip strength, QIS, usual gait speed, and 6 MWD were assessed at discharge as baseline, at the end of CR, and 5 months after the start of CR.
Grip strength was measured using a grip strength meter (TKK 5101 Grip-D; Takei, Tokyo, Japan) to evaluate the upper extremity muscle strength. In the grip strength measurement, the grip width was adjusted so that the proximal interphalangeal joint of the index finger was at 90°, the patient was placed in a sitting position with the elbow joint angle flexed to 90°, and the grip strength meter was held outward. A 3 s strong grip was performed twice, alternately on the left and right sides. The examiner lightly supported the patient’s elbow and the tip of the grip strength tester alternately on the left and right sides and instructed the patient not to hold their breath during the measurement to avoid the Valsalva maneuver. The highest values of the left and right grip strength were expressed as the mean value (kg) [].
To assess lower limb muscle strength, QIS was measured using a hand-held dynamometer (μ-Tas; ANIMA, Tokyo, Japan). For the QIS measurements, the patient was seated with the hip and knee joints in 90° flexion and the thigh was fixed to a chair with a band. A dynamometer was placed on the anterior surface of the lower leg and above the two transverse fingers of the external tibia and maximal isometric contractions of the quadriceps were performed twice for 5 s, on alternating left and right sides. To avoid the Valsalva maneuver, patients were instructed not to hold their breath during contraction and to avoid compensatory movements as much as possible. The highest value of strength on each side was expressed as an average (kgf) [].
For usual gait speed, patients were asked to walk 16 m at usual gait speed, then walk from 3 to 13 m at 10 m intervals, and the time required was measured excluding the 3 m acceleration and deceleration times before and after the walk. A stopwatch was used for the measurements and the timing for starting and stopping the stopwatch was when the subject stepped on or over the 3 and 13 m lines, respectively. Measurements were taken twice and the faster walking time was adopted to calculate the gait speed (m/s) [].
The functional capacity was assessed using the 6 MWD. The walking distance was calculated with the help of a stopwatch by counting the number of times the subject walked back and forth along a 20 m hallway in 6 min. The patients were instructed to walk as much distance as possible in 6 min and could take as many breaks as needed during the test [].
2.4. Endpoints
The primary endpoint was the amount of change from baseline to 5 months (Δ physical function) in physical function (grip strength, QIS, usual gait speed, and 6 MWD). The secondary endpoint was the change in body weight (Δ body weight), calculated as difference between physical function at 5 months after baseline and baseline physical function for Δ physical function and difference between body weight at 5 months after baseline and baseline body weight for Δ body weight.
2.5. Cardiac Rehabilitation Program
Comprehensive outpatient CR was implemented according to the statement of the Japanese Cardiovascular Society and education on self-management of medication, nutrition, and exercise was provided by a cardiologist and medical staff prior to discharge []. Participation in outpatient CR at least once a week and self-exercise 3–5 times a week were recommended. Exercise training in the outpatient CR consisted of a 5 min warm-up, 20–40 min of aerobic exercise at an intensity of 12–14 on the Borg scale on a treadmill or bicycle ergometer, and a 3 min cool-down []. All in-hospital exercise therapies were supervised by a trained nurse or a physical therapist and included heart rate and electrocardiographic monitoring with a monitored electrocardiogram. In addition, blood pressure and Borg scale assessments were performed every 5–10 min. The total number of outpatient CR attended by each participant was collected from the medical records and analyzed.
2.6. Statistical Analysis
Statistical analyses were performed using Stata software (version 17, StataCorp LLC, College Station, TX, USA) and EZR version 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). Categorical and continuous data of the baseline characteristics were presented as median (interquartile range), n (%). Fisher’s exact test was used to compare categorical variables between the patients using and not using SGLT2 inhibitors. Mann–Whitney U test was used to compare continuous variables between the two groups. We used a mixed-effect model and multiple regression model to identify the relationship between SGLT2 inhibitor use and the amount of change in physical function (Δ grip strength, Δ QIS, Δ usual gait speed, and Δ 6 MWD). In addition, we used a linear mixed-effect model and a multiple regression model to identify the relationship between SGLT2 inhibitor use and the amount of change in body weight. In the multiple regression analyses, we used SGLT2 inhibitors, age, sex (male), BMI, NYHA class ≥ III, LVEF, HF, Hb, year of hospitalization (≥2014 or not), and baseline of each physical function as a covariate. In Japan, SGLT2 inhibitors were approved for the treatment of T2DM in 2014. Considering this, we included whether the year of admission was 2014 or later as a covariate. A p-value of <0.05 was considered statistically significant [].
3. Results
Patients with CVD and T2DM who participated in comprehensive outpatient CR and were taking SGLT2 inhibitors prior to admission (n = 14) were excluded from the study. As a result, 402 patients were included in the analysis (Figure 1). The median age of patients in this study was 69 years, 274 (68.2%) were males, and 32 (8.0%) were SGLT2 inhibitor users (Table 1).

Figure 1.
Flow chart in this study. SGLT2, Sodium-Glucose Cotransporter 2.

Table 1.
Baseline characteristics of patients using and not using SGLT2 inhibitors.
3.1. Association between SGLT2 Inhibitor Use and Change in Physical Function
In the mixed-effects model, a significant main effect of time showed an increase in physical functions (all p < 0.001), and no significant difference in change in physical function with and without SGLT2 inhibitor use was observed (time × SGLT2 inhibitor; all p ≥ 0.05) (Figure 2). Multiple regression analysis showed that SGLT2 inhibitor use was not significantly associated with the change in physical functions (Table 2).
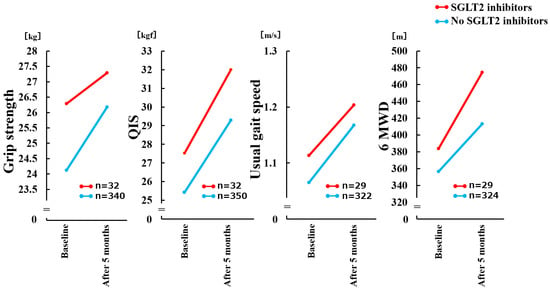
Figure 2.
Comparison of Δ physical functions with and without SGLT2 inhibitor use in a mixed-effects model. There was no significant time × group interaction (p = 0.178) for change in Grip strength. There was no significant time × group interaction (p = 0.575) for change in QIS. There was no significant time × group interaction (p = 0.757) for change in Usual gait speed. There was no significant time × group interaction (p = 0.151) for change in 6 MWD. QIS, maximal quadriceps isometric strength; 6 MWD, 6-minute walking distance; SGLT2, Sodium-Glucose Cotransporter 2.

Table 2.
Association of physical functions with SGLT2 inhibitors use.
3.2. Association between SGLT2 Inhibitor Use and Change in Body Weight
In the mixed-effects model, no significant main effect of time showed an increase in body weight (p = 0.188), and no significant difference in change in body weight with and without SGLT2 inhibitor use was observed (time × SGLT2 inhibitor; p = 0.493) (Supplementary Figure S1). Multiple regression analysis showed that SGLT2 inhibitor use was not significantly associated with change in body weight (Supplementary Table S1).
4. Discussion
The primary findings of our study were as follows: (1) improvement in physical function was observed in CVD and T2DM patients who participated in an exercise-based CR with or without SGLT2 inhibitor use and (2) there was no significant difference in the amount of change in physical function between the patients using and not using SGLT2 inhibitors. These findings suggest that the favorable effect of exercise-based CR on physical function was equally manifested in patients with CVD and T2DM, with or without SGLT2 inhibitor use. Our study is consistent with a previous study, which reported that exercise-based CR contributed to the recovery of physical function in HF patients, with or without T2DM []. Previous studies examining the effect of SGLT2 inhibitor use on exercise tolerance in nondiabetic patients with HF have shown that the prescription of SGLT2 inhibitors contributed to the improvement in exercise tolerance (peak VO2, 6 MWD) [,]. However, it remains unclear whether prescribing SGLT2 inhibitors affected the functional recovery associated with CR. To the best of our knowledge, this is the first study to show that the use of SGLT2 inhibitors in patients with CVD and T2DM does not interfere with the improvement in outcomes regarding skeletal muscle function in outpatient CR.
SGLT2 inhibitors are effective for improving the prognosis of patients with HF [,]. However, there is concern regarding skeletal muscle loss caused by SGLT2 inhibitors []. SGLT2 inhibitors significantly reduced body weight and approximately two-thirds of the weight loss was from fat mass and a third from lean body mass [,]. Non-randomized observational studies found significant reductions in lean body mass and fat mass in Japanese patients with T2DM after the initiation of SGLT2 inhibitors [,]. Other previous studies have reported no effect of SGLT2 inhibitors on lean mass [,,,]. A marked decrease in lean mass was observed with high doses of canagliflozin []. Several researchers have investigated the effect of SGLT2 inhibitors on skeletal muscle mass and function in mice [,,]. Interestingly, Bamba et al. showed that the db/db young mice fed a diet with SGLT2 inhibitors had higher skeletal muscle mass [], while Nambu et al. found no significant effect in muscle mass of HF model mice []. Contrary to these studies, Otsuka et al. reported that when the amount of food in SGLT2 inhibitor-treated mice was adjusted to that in vehicle-treated mice, muscle mass and function were reduced in the treated mice []. Muscle mass reduction was found in the tibialis anterior and extensor digitorum longus muscles but not in the soleus muscle, indicating a muscle-fiber-type-specific change []. When food intake in the treated group was intentionally adjusted to the control group, protein degradation exceeded protein synthesis as a catabolic response to SGLT2 inhibition, likely causing skeletal muscle mass loss in mice []. These studies have raised concerns that SGLT2 inhibitors may induce muscle atrophy. Neither skeletal muscle mass nor lean body mass was measured in our study; however, we confirmed no difference in the amount of change in body weight between SGLT2 inhibitor users and non-users (Supplementary Figure S1). Body weight is the simplest indicator of nutritional status and significant weight loss is a criterion for frailty []. In particular, unintentional weight loss of more than 10 pounds in the previous year or more than 5% of the previous year’s weight at follow-up is suspected to be due to frailty. Considering these previous studies and the present study, it is unlikely that SGLT2 inhibitors induced significant loss of skeletal muscle during 5 months of CR.
The effect of SGLT2 inhibitor use on physical functions, such as muscle strength, gait speed, and walking capacity, is unclear. Deterioration in physical function and muscle atrophy, as well as the subsequent inhibition of recovery, has been concerns in terms of potential side effects from SGLT2 inhibitors. Our results partially addressed these concerns. In this study, we showed that the physical function of SGLT2 inhibitor users was ameliorated after 5 months of exercise-based CR and the degree of recovery was not different from that of non-prescribers. These findings suggest that SGLT2 inhibitors do not interfere with exercise-based CR-induced recovery of physical function. In addition to body weight, grip strength [,], QIS [,], gait speed [,], and 6 MWD [] are prognostic factors. Our study found as much improvement in physical function as in the SGLT2 inhibitor non-user group, even if SGLT2 inhibitor use. Taken together with the fact that body weight was not altered, our results suggest that SGLT2 inhibitors had no adverse effects on skeletal muscle. We also found significant improvements in gait speed and 6 MWD. Gait speed and 6 MWD are useful for identifying difficulties in activities of daily living (ADL) in patients with CVD []. Therefore, SGLT2 inhibitors may not inhibit improvement in ADL. Linden et al. investigated the effects of co-treatment with an SGLT2 inhibitor and exercise training on exercise capacity of T2DM model rats []. The authors showed that rats that were administered the SGLT2 inhibitor and underwent exercise training had better exercise capacity than those that underwent exercise alone []. However, in this study, we found no additional value of SGLT2 inhibitors for improving exercise tolerance with CR. Differences in results between Linden’s study and our study may be attributed to the following factors: (1) age (middle aged (12 weeks old) vs. elderly (69 years)) and (2) different animal species (rat vs. human).
Exercise-based CR has shown improvements in quality of life [], physical function [], ADL [], and reduced incidence of clinical events [] and is becoming increasingly important. Patients with CVD exhibit lower values of various physical functions, such as gait speed [], 6 MWD [], and skeletal muscle strength [], each of which is a predictor of prognosis. Therefore, this study adopted four measures of physical function outcomes: grip strength, QIS, usual gait speed, and 6 MWD. Thus, another strength of this study was its multifaceted examination of the effects of CR.
This study has some limitations. First, this was a single-center observational study and external validity could not be guaranteed. Second, there might have been a selection bias since we included patients in whom the intervention was possible for 5 months in outpatient CR. Additionally, the number of patients in the SGLT2-inhibitors-used group was small; thus, analyses to match patient backgrounds between the two groups, such as propensity matching, were difficult to perform and so was subgroup analyses in patients with sarcopenia or frailty. SGLT2 inhibitors have been shown to improve prognosis, even in frail patients [], although further studies are required to determine their association with physical function.
5. Conclusions
The use of SGLT2 inhibitors in patients with CVD and T2DM undergoing outpatient CR did not impair improvement in physical functions. Further investigation remains warranted to determine the impact on the improvement in physical function in patients with CVD, especially those with sarcopenia and frailty.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11195956/s1, Figure S1: Comparison of Δ body weight with and without SGLT2 inhibitor use in a mixed-effects model; Table S1: Association of body weight with SGLT2 inhibitor use.
Author Contributions
Conceptualization, A.K., K.K. and K.U.; methodology, A.K., K.K. and J.A.; validation, A.K., K.K., N.H., K.U., K.N., T.I., M.Y., S.U., T.N., K.H., E.M., M.Y.-T. and A.M.; data curation, A.K., K.K., N.H., K.U., K.N., T.I., M.Y., S.U. and T.N.; writing—original draft preparation, A.K.; writing—review and editing, K.K., K.U. and K.H.; visualization, A.K.; supervision, J.A.; project administration, A.K., K.K., E.M. and J.A.; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by JSPS KAKENHI (Grant Number 21H03309).
Institutional Review Board Statement
The study protocol was designed according to the tenets of the Declaration of Helsinki and approved by the Ethics Committee of the Kitasato University Medical Ethics Organization (KMEO) (no. KMEO B18-075).
Informed Consent Statement
This was an observational study that did not involve invasive procedures or interventions and written informed consent was not required according to the principles set forth in the “Ethical Guidelines for Medical and Health Research for Subjects” by the Japanese Ministry of Health, Labor and Welfare. Therefore, the requirement for informed consent was waived by the KMEO according to the institutional guidelines for retrospective observational studies.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank all the collaborators from our team at Kitasato University Hospital for their clinical work.
Conflicts of Interest
Although Yamashita has no conflict of interest related to the conduct of this study, he holds company stock (less than 5% of the total) and receives a salary as one of the directors of an employer. All other authors have nothing to disclose.
References
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019, 1, Cd003331. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C., Jr.; Benjamin, E.J.; Bonow, R.O.; Braun, L.T.; Creager, M.A.; Franklin, B.A.; Gibbons, R.J.; Grundy, S.M.; Hiratzka, L.F.; Jones, D.W.; et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011, 124, 2458–2473. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Gliflozins in the Management of Cardiovascular Disease. N. Engl. J. Med. 2022, 386, 2024–2034. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail 2019, 21, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef]
- Koike, Y.; Shirabe, S.I.; Maeda, H.; Yoshimoto, A.; Arai, K.; Kumakura, A.; Hirao, K.; Terauchi, Y. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2019, 149, 140–146. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Yokomizo, H.; Nakamura, S.; Izumi, Y.; Takahashi, M.; Obara, S.; Nakao, M.; Ikeda, Y.; Sato, N.; Sakamoto, R.; et al. Differential effect of canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on slow and fast skeletal muscles from nondiabetic mice. Biochem. J. 2022, 479, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Ito, S.; Uemura, O.; Kato, T.; Kimura, G.; Nakao, T.; Hattori, M.; Fukagawa, M.; Horio, M.; Mitarai, T.; et al. CKD Clinical Practice Guidebook. The essence of treatment for CKD patients. Clin. Exp. Nephrol. 2009, 13, 191–248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kamiya, K.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Matsunaga, A.; Masuda, T.; et al. Incremental Value of Objective Frailty Assessment to Predict Mortality in Elderly Patients Hospitalized for Heart Failure. J. Card. Fail 2018, 24, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Masuda, T.; Tanaka, S.; Hamazaki, N.; Matsue, Y.; Mezzani, A.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; et al. Quadriceps Strength as a Predictor of Mortality in Coronary Artery Disease. Am. J. Med. 2015, 128, 1212–1219. [Google Scholar] [CrossRef]
- Ueno, K.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Yamashita, M.; Uchida, S.; Noda, T.; Maekawa, E.; Yamaoka-Tojo, M.; et al. Usefulness of measuring maximal gait speed in conjunction with usual gait speed for risk stratification in patients with cardiovascular disease. Exp. Gerontol. 2022, 164, 111810. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Yoshida, T.; Ikegame, T.; Izawa, K.P.; Ito, Y.; Okamura, H.; Osada, N.; Kinugawa, S.; Kubozono, T.; Kono, Y.; et al. Standard Cardiac Rehabilitation Program for Heart Failure. Circ. J. 2019, 83, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Murray, E.M.; Whellan, D.J.; Chen, H.; Bertoni, A.G.; Duncan, P.; Pastva, A.M.; Kitzman, D.W.; Mentz, R.J. Physical Rehabilitation in Older Patients Hospitalized with Acute Heart Failure and Diabetes: Insights from REHAB-HF. Am. J. Med. 2022, 135, 82–90. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlavek, J.; Bohm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Groothof, D.; Eisenga, M.F.; Bakker, S.J.L. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Outcomes: True Renoprotection, Loss of Muscle Mass or Both? J. Clin. Med. 2020, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef]
- Nambu, H.; Takada, S.; Fukushima, A.; Matsumoto, J.; Kakutani, N.; Maekawa, S.; Shirakawa, R.; Nakano, I.; Furihata, T.; Katayama, T.; et al. Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. Eur. J. Pharmacol. 2020, 866, 172810. [Google Scholar] [CrossRef]
- Bamba, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J. Cachexia Sarcopenia Muscle 2022, 13, 574–588. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker For Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kamiya, K.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Ichikawa, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Noda, C.; et al. Quadriceps Strength and Mortality in Older Patients With Heart Failure. Can. J. Cardiol. 2021, 37, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Mezzani, A.; Hotta, K.; Shimizu, R.; Kamekawa, D.; Noda, C.; Yamaoka-Tojo, M.; Matsunaga, A.; Masuda, T. Quadriceps isometric strength as a predictor of exercise capacity in coronary artery disease patients. Eur. J. Prev. Cardiol. 2014, 21, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hamazaki, N.; Matsue, Y.; Mezzani, A.; Corrà, U.; Matsuzawa, R.; Nozaki, K.; Tanaka, S.; Maekawa, E.; Noda, C.; et al. Gait speed has comparable prognostic capability to six-minute walk distance in older patients with cardiovascular disease. Eur. J. Prev. Cardiol. 2018, 25, 212–219. [Google Scholar] [CrossRef]
- Tabata, M.; Shimizu, R.; Kamekawa, D.; Kato, M.; Kamiya, K.; Akiyama, A.; Kamada, Y.; Tanaka, S.; Noda, C.; Masuda, T. Six-minute walk distance is an independent predictor of hospital readmission in patients with chronic heart failure. Int. Heart J. 2014, 55, 331–336. [Google Scholar] [CrossRef]
- Sato, A.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Yamashita, M.; Uchida, S.; Maekawa, E.; Yamaoka-Tojo, M.; Matsunaga, A.; et al. Gait speed and 6-minute walking distance are useful for identifying difficulties in activities of daily living in patients with cardiovascular disease. Heart Lung 2022, 51, 46–51. [Google Scholar] [CrossRef]
- Linden, M.A.; Ross, T.T.; Beebe, D.A.; Gorgoglione, M.F.; Hamilton, K.L.; Miller, B.F.; Braun, B.; Esler, W.P. The combination of exercise training and sodium-glucose cotransporter-2 inhibition improves glucose tolerance and exercise capacity in a rodent model of type 2 diabetes. Metabolism 2019, 97, 68–80. [Google Scholar] [CrossRef]
- Saeidi, M.; Mostafavi, S.; Heidari, H.; Masoudi, S. Effects of a comprehensive cardiac rehabilitation program on quality of life in patients with coronary artery disease. ARYA Atheroscler. 2013, 9, 179–185. [Google Scholar] [CrossRef]
- Paneroni, M.; Scalvini, S.; Corrà, U.; Lovagnini, M.; Maestri, R.; Mazza, A.; Raimondo, R.; Agostoni, P.; La Rovere, M.T. The Impact of Cardiac Rehabilitation on Activities of Daily Life in Elderly Patients With Heart Failure. Front. Physiol. 2021, 12, 785501. [Google Scholar] [CrossRef]
- Adachi, T.; Iritani, N.; Kamiya, K.; Iwatsu, K.; Kamisaka, K.; Iida, Y.; Yamada, S. Prognostic Effects of Cardiac Rehabilitation in Patients With Heart Failure (from a Multicenter Prospective Cohort Study). Am. J. Cardiol. 2022, 164, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. Prognostic utility of dynapenia in patients with cardiovascular disease. Clin. Nutr. 2021, 40, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. Dapagliflozin Safe and Effective for Heart Failure with Frailty. JAMA 2022, 327, 1950. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).