Abstract
(1) Background: We aimed to systematically review the current literature to evaluate if in patients with postoperative endophthalmitis treated with pars plana vitrectomy, silicone oil tamponade could provide a useful contribution to the control and eradication of infection and if it could influence anatomical recovery and functional outcome. (2) Randomized controlled trials, cross-sectional studies, case series, and case reports published in the English language in peer-reviewed journals were included. No restriction was placed based on the study location. We used medical subject headings (MeSH) and text words. We searched MEDLINE (OVID and PubMed), Google Scholar, ISI Web of Science (Thom-on-Reuters), and the Cochrane Library (Wiley) from January 1995 to the present. To ensure literature saturation, we scanned the reference lists of included studies or relevant reviews identified through the search. Risk of Bias was assessed using the Newcastle-Ottawa scale for longitudinal studies and Cochrane risk-of-bias tool for randomized trials. (3) Results: abstracts of 75 articles were selected for full-text reading; after full-text reading, 44 articles were taken into consideration in the systematic review. 5 out of 7 in vitro experimental studies demonstrated antimicrobial activity against different species of bacteria and fungi. The use of SO as endotamponade associated with PPV led to better visual acuity and a lower rate of retinal detachment and the need for additional surgery. (4) Conclusions: Silicone oil reduces the risk of postoperative retinal detachment, especially in case of undetected retinal breaks, produces compartmentalization of the eye, may lead to early visual recovery, allows laser photocoagulation, prevents severe postoperative hypotony and has antimicrobic activity due to an inhibitory effect for several species of pathogens. Concerns regarding possible toxic effects on the retina and optic disc, compartmentalization and impaired washout of pathogen toxins have been reported. It may also influence intravitreal antibiotic distribution and clearance.
1. Introduction
Endophthalmitis represents one of the biggest diagnostic and therapeutic challenges in modern ophthalmology. It threatens all forms of intraocular surgery from intravitreal injections to corneal transplants and vitrectomies and it is discussed on all surgical consent forms.
The Endophthalmitis Vitrectomy Study (EVS) was a randomized clinical trial conducted in the United States between 1990 and 1995 [1]. The purpose of this landmark study was to investigate the role of initial pars plana vitrectomy (PPV) and the benefit of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis.
The EVS concluded that early vitrectomy should be recommended only for cases with visual acuity of light perception or lower on presentation; however, with the technical advances in vitrectomy, in the following years, many authors reported better results using such a technique in patients with visual acuity higher than light perception [2].
The 2013 European society of cataract and refractive surgeons’ guidelines for the prevention and treatment of endophthalmitis after cataract surgery considered a complete vitrectomy and subsequent injection of intravitreal antibiotics as the “gold standard” treatment in case of acute postoperative endophthalmitis [3].
Another debated topic is whether a complete PPV should be followed with a silicone oil (SO) injection given the higher risk of development of retinal breaks and consecutive retinal detachment after vitreous removal.
Interestingly, according to some authors, SO could exert a bacteriostatic activity helping therefore in infection control and preventing the development of reinfection.
The authors aimed to review the current literature concerning the role of SO injection after PPV for the management of postoperative endophthalmitis.
2. Materials and Methods
In this work, we adhered to the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [4]. The protocol was registered on OSF Registries (Registration DOI: 10.17605/OSF.IO/8ZTQJ).
2.1. Eligibility Criteria: Participants, Interventions, Comparators, and Outcomes (PICO)
Randomized controlled trials, cross-sectional studies, case series, in vitro experimental studies and case reports published in the English language in peer-reviewed journals, discussing the treatment of endophthalmitis with PPV and SO injection was included. Articles published before 1995 were excluded. No restriction was placed based on the study location.
The objectives of our study were to systematically review the current literature to evaluate the role of silicone oil in patients with postoperative endophthalmitis treated with pars plana vitrectomy and silicone oil tamponade. The proposed review answered the following questions: does silicon oil provide a useful contribution to the control and eradication of infection? Does the use of silicone oil influence the visual recovery and the final visual outcome?
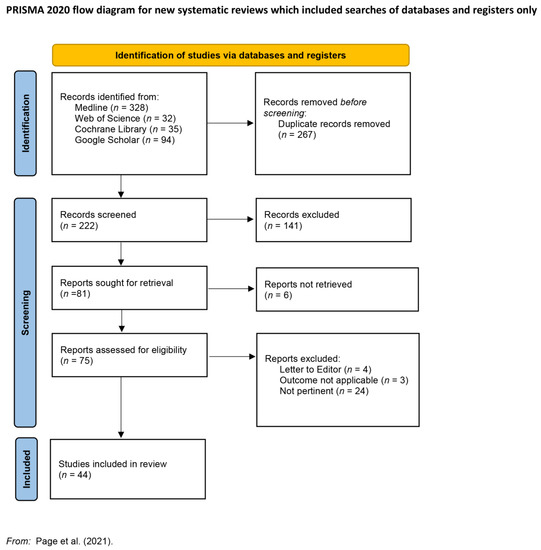
Figure 1.
Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram; adapted from Reference [4].
2.2. Search Strategy
Literature search strategies were developed using medical subject headings (MeSH) and text words. We searched MEDLINE (OVID and PubMed), Google Scholar, ISI Web of Science (Thom-on-Reuters), and the Cochrane Library (Wiley) from January 1995 to March 2022. We used various combinations of key terms related to “endophthalmitis”, “silicone oil” and “pars plana vitrectomy.” Two of the authors (F.S. and M.D.S.) independently performed the research, screening for eligibility. They independently extracted the data using pre-determined forms. Research records were compared to eliminate duplicates. Discrepancies were resolved by agreement between the reviewers or with a third, experienced reviewer (M.F.). Data extracted from every study will include the last name of the first author, year of publication, study design, sample size, purpose, anatomical outcome, visual outcome, and pathogens studied. Outcomes will be the following: antimicrobial activity of silicone oil, anatomical outcome, and visual outcome.
2.3. Risk of Bias and Quality of Evidence Assessment
The risk of bias was assessed using the Newcastle-Ottawa scale for longitudinal studies, the Cochrane risk-of-bias tool for randomized trials and the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series/case reports [5,6]. The Quality of Evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [7]. Thresholds for converting the Newcastle-Ottawa scales are as follows: good quality = above 6 stars; fair quality = 4 to 6 stars; poor quality = 0 to 3 stars [8].
3. Results of Searches
In total, 489 records were retrieved from multiple databases including MEDLINE (328), ISI Web of Science (32) Cochrane Library (35), and Google Scholar (94). After removing duplicates (267 records), 222 records were included in the screening process.
Abstracts of 75 articles published in the English language in peer-reviewed journals from January 1995 (conclusion of the Endophthalmitis vitrectomy study—EVS) to March 2022 whose topic was inherent to the surgical treatment of Endophthalmitis with vitrectomy and silicone oil injection were selected for full-text reading. Finally, after full-text reading, 44 articles were taken into consideration in the systematic review.
Of these, 7 were in vitro experimental studies [9,10,11,12,13,14,15], 10 were case reports [16,17,18,19,20,21,22,23,24,25], 17 were case series [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], 4 were randomized trials [43,44,45,46] and 6 were retrospective interventional studies [47,48,49,50,51,52].
In in vitro experimental studies, bacterial growth was considered in terms of Colony-forming unit (CFU) or with the onset of inhibition halo. 5 out of 7 studies demonstrated antimicrobial activity against different species of bacteria and fungi.
The use of SO as endotamponade associated with PPV led to better visual outcomes in 3 of the 4 randomized clinical trials. These functional improvements appeared more significant in traumatic subgroups [43]. In all the randomized studies, post-operative retinal detachment rate was chosen as an anatomical outcome. In patients who received SOI, the incidence of retinal detachment ranged between 0% and 6.2% and was significantly lower than in control groups (25.5–76.6%).
In retrospective studies, case series and case reports, functional outcomes were homogenously defined as best-corrected visual acuity improvements whereas anatomical success was heterogeneously identified by different Authors as postoperative retinal detachment rate, post-operative hypotony, the appearance of retinal atrophy, intravitreal injection rate, inflammation control and evisceration rate.
Given the low incidence of the disease, most of the evidence was derived from case reports and case series. The Authors chose to include these types of studies and evaluated them according to the JBI critical evaluation tool (Tables S5 and S6). All case series did not report the demographic information relative to the centres where the research was conducted; this parameter was not considered relevant to the quality of evidence considering that this review mainly focused on treatment strategies of endophthalmitis independently from the demographic area where the pathology occurs.
Two out of 4 RCTs had a low risk of bias [43,45]; higher scores of quality of evidence (GRADE system) were observed in prospective randomized trials [43,44,45,53], and retrospective studies with clearly defined control groups [49,50,54].

Table 1.
Does silicone oil have antimicrobial activity?

Table 2.
Does Silicone oil injection after vitrectomy improve visual and anatomical outcomes?
Considering the type and quality of evidence, the Authors chose to report a qualitative analysis, detailed issue-by-issue in a narrative fashion for the heterogeneity of available data and the design of the studies.
4. Definition
Endophthalmitis is an inflammatory process involving the internal structures of the eye mainly caused by exogenous agents such as bacteria, mycetes and occasionally parasites which may penetrate the eye in the intraoperative or postoperative phase, after eye injuries or may spread from ocular surface infections.
Postoperative endophthalmitis can be classified as acute, whether the infection is developed within 6 weeks from the intraocular procedure, or chronic.
Rarely, in endogenous endophthalmitis, the infectious agent reaches the eye through the bloodstream; this usually occurs in patients with risk factors such as immunosuppression or intravenous drug abuse.
Clinical Features
Endophthalmitis is usually defined by severe inflammation of the ocular tissues and fluids characterized clinically by a combination of signs and symptoms including ocular pain, decreased vision, eyelid oedema, conjunctival congestion, chemosis, anterior segment inflammation, hypopyon, vitritis, and decreased red reflex.
5. Epidemiology and Causative Agents
The incidence of postoperative endophthalmitis has sensibly decreased over the years. Most cases of endophthalmitis are exogenous, and organisms are introduced into the eye via trauma, surgery, or ocular surface infections. Endogenous endophthalmitis occurs when the eye is seeded via the bloodstream [57].
The rate of occurrence ranges between 0.13% and 0.7% following cataract surgery [58,59,60] and between 0.03 and 0.13% after PPV [61]. The large diffusion of intravitreal injections has drastically increased the volume of intraocular procedures in the population, with the result of performing some of these outside the operating room setting [62]. Nevertheless, recent studies reported a relatively low rate of post-injection infections ranging between 0.02% and 0.082% [63,64,65,66,67,68].
Post-traumatic endophthalmitis accounts for 25–31% of cases and the reported incidence rate of endophthalmitis following open-globe injury varied from 0% to 16.5% with evidence of reduction over the past 70 years [69].
Depending on the causative agent, two main categories are recognized: bacterial and fungal endophthalmitis. The predominant organism causing the infection depends on the source (vegetable matter or retained intraocular foreign body), route of spread (post-surgery, trauma, delayed onset or hematogenous dissemination), geographic location, and patient characteristics [70]. Among exogenous endophthalmitis, Gentile and coauthors observed that 85.1% were due to gram-positive bacteria, 10.3% were due to gram-negative bacteria, and 4.6% were due to fungi. The most common bacterial pathogens isolated are Staphylococcus epidermidis (30.3%), different species of coagulase-negative Staphylococcus (9.1%), Streptococcus viridans (12.1%), Staphylococcus aureus (11.1%), Enterobacteriaceae (3.4%), and Pseudomonas aeruginosa (2.5%) [71].
6. Therapy
The key points in endophthalmitis treatment are infection control and eradication, inflammation management and re-infection prevention.
6.1. Medical Therapy
6.1.1. Systemic Antibiotics
Systemic antibiotics in postoperative endophthalmitis are adopted as adjuvant therapy in some units despite little published evidence on their clinical efficacy [72]. As early as 1995, the EVS study concluded that a systemic treatment with ceftazidime 2 g every 8 h, amikacin 7.5 mg/kg initial dose followed by 6 mg/kg every 12 h did not affect the final visual outcome. Moreover, the authors hypothesized that the omission of systemic antibiotic therapy could reduce toxic effects, costs and length of hospital stay [1].
However, given the lack of univocal guidelines, adjunctive systemic antibiotic therapy is still commonly used resulting in various antibiotic regimens. The best-documented systemic drugs achieving therapeutic levels in vitreous appear to be fourth-generation fluoroquinolones [73], meropenem, and linezolid [74]. Conversely, patients with endogenous endophthalmitis seem to benefit from combined intravitreal and systemic antimicrobial therapy with lower rates of evisceration or enucleation [75].
6.1.2. Topical Antibiotics
Broad-spectrum antibiotic combinations are usually started before a specific pathogen is identified and antibiotic sensitivity is determined. One of the drugs should be effective against Gram-positive organisms and the other one against Gram-negative organisms. Once antibiotic sensitivity is determined, targeted intervention should be undertaken. In the presence of corneal ulcer or wound abscess, fortified drops (including cefazolin 5%, tobramycin 1.4% and Vancomycin) should be used.
In the EVS study, the therapeutic regimen consisted of vancomycin (25 mg/0.5 mL) and ceftazidime (100 mg/0.5 mL) for subconjunctival injection, and topical vancomycin (50 mg/mL) and amikacin (20 mg/mL) every 4 hourly for routine cases or every hour if wound leak was present [1].
6.1.3. Intravitreal Antibiotics
Intravitreal antibiotics are a mainstay for the management of endophthalmitis. The intravitreal route represents the only way the highest safe level of antibiotic can be rapidly delivered in the vitreous chamber.
An early injection is crucial because pathogens continue to replicate over time, produce toxins and dramatically damage the surrounding environment leading to irreversible visual loss. High doses of antibiotics are needed to keep their levels above the bacterial minimal inhibitory concentration as long as possible, given the variable speed of antibiotic clearance depending on the surgical and inflammatory status of the eye.
In vitrectomized eyes, if on one hand there is a higher risk of transient neurotoxicity due to macular pooling of the drug after injection, on the other hand, the absence of the vitreous body entails a quicker antibiotic clearance and drug concentration may sooner decrease to subtherapeutic levels [76].
As intravitreal injection usually takes place before or simultaneously to tap biopsy, broad-spectrum antibiotics are used to cover both gram-positive and gram-negative organisms. The most injected antibiotics are Vancomycin 1 mg/0.1 mL (for coverage of gram-positive organisms), Ceftazidime 2.25 mg/0.1 mL or Amikacin 0.4 mg/0.1 mL (for gram-negative organisms).
Amikacin seems to have a worse side-effect profile than Ceftazidime given the risk of macular infarction. Reddy and colleagues found similar bacterial resistance percentages for both antibiotics (18% for Ceftazidime and 13% for Amikacin) concluding that Ceftazidime still represented a first-line anti-gram-negative choice [77]. However, if nonresponse is observed, Amikacin could be an effective second-line choice.
6.2. Surgical Therapy
The EVS concluded that early vitrectomy in endophthalmitis was only beneficial to patients with vision of light perception or worse; however, this was a secondary finding as the study had not been designed for such subgroup analysis. Also, in EVS only a “core” vitrectomy was performed [78].
Already in 2005 Kuhn and Gini questioned the EVS indications on vitrectomy showing their results in a case series of 47 patients who had undergone early vitrectomy for endophthalmitis, with a ‘complete’ surgical vitrectomy as opposed to “core”: 91% of the cases achieved final acuity of 20/40 or better, as opposed to 53% in the EVS group [2]. The authors suggested that removing the posterior vitreous was advantageous in clearing the toxic load away from the macula whereas the EVS approach may have led to ‘macular hypopyon’, resulting in long-term dysfunction.
In recent years, with increased experience and surgical technique refinement, a more proactive stance in favour of vitrectomy has been adopted by many surgeons, outdating the EVS indications [72].
Consequently, new questions arose: Does SO tamponade after vitrectomy lead to better results? How does it affect microbial replication? Which patients would profit more from silicone oil tamponade?
The use of SO tamponade after completion (PPV) for endophthalmitis is debated. Several studies reported better visual outcomes in endophthalmitis patients treated with vitrectomy and SO tamponade, however other authors expressed concerns regarding the effectiveness and the true utility of this procedure.
7. Antimicrobial Activity of Silicon Oil
Among SO properties, antimicrobial activity has been extensively investigated. It has been suggested that the high surface tension and low permeability of SO could limit the freedom of movement of the pathogens, concentrating them in the ciliary body or close to the retinal blood vessels where the defence mechanisms could act more effectively [79]. Moreover, the space-occupying action of a long-standing tamponade may play an important role in pathogens’ and toxins’ wash-out, preventing the damage of the delicate retinal structures [17,80].
In 1999 Ozdamar et al. evaluated SO (1300 centistokes, medical grade, autoclave sterilized) in vitro antimicrobial activity against the most common endophthalmitis pathogenetic agents (S. aureus, S. epidermidis, P. aeruginosa, C. albicans, Aspergillus spp.) [9]. The authors observed that bacterial replication was significantly reduced in SO medium in comparison with a balanced salt solution. The authors explained their results both with a reduced nutrient concentration in SO and with a direct toxic activity exerted by the low molecular weight silicone oil particles. These findings have been confirmed and extended in the following years: in 2012 the antimicrobial activity of three different silicone oils—Arciolane 1300 cSt (Arcadophta, Toulouse, France), Arciolane 5500 cSt (Arcadophta, Toulouse, France) and OxaneR Hd (Bausch & Lomb Inc., Waterford, Ireland)—on different pathogens (Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Bacillus sp., Pseudomonas aeruginosa, Candida albicans and Aspergillus fumigatus) was tested. The authors found that OxaneR Hd had the highest antibacterial and antimycotic activity and after two weeks Candida and Aspergillus growth was inhibited as well. The antimicrobial activity could therefore depend on SO chemical composition [14].
Bactericidal and antimycotic activity of 1000 cSt and 5000 cSt silicone oil against multi-resistant pathogens has been reported as well (Methicillin-Resistant Staphylococcus Aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa multiresistant, Klebsiella pneumoniae multiresistant, Escherichia coli, Candida albicans, and Aspergillus). The time to exert bactericidal activity was different between the two oils (20 days for 1000 cSt oil and 30 days for 5000 cSt oil), whereas fungistatic activity was similar [15].
In contrast, Adams and colleagues, in 2013, explored the in vitro antimicrobial properties of a 1000 cSt SO (Óleo de Silicone®, Ophthalmos, São Paulo, Brazil) against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Candida albicans, Klebsiella pneumoniae and Streptococcus pneumoniae; in all cases, no inhibition halo was observed [12]. An explanation for the different results of this study relies on the method: while other researchers used bacterial suspensions and observed the bacterial growth on different mediums in terms of Colony-forming unit, in the latter study, filter paper discs containing SO were placed in seeded plates and inhibition halo was observed. The Authors finally concluded that the SO at 1000 cSt did not have any effect on in vitro bacterial growth. They hypothesized that the substitution of the vitreous with SO could expose intraocular pathogens to nutritional deprivation leading them to death by starvation. This mechanism could explain the previously observed in vivo efficacy of silicone oil in the control of the infectious process [12].
The in vitro antimicrobial activity of SO against anaerobic agents, specifically Propionibacterium acnes, Peptostreptococcus spp., Peptostreptococcus anaerobius, Bacteroides fragilis, Fusobacterium spp., and Clostridium tertium has been investigated [10]. After a prolonged incubation of 7 days, 9.2 × 106 colonies were observed in the silicone oil for Propionibacterium acnes, which may have been due to its biofilm formation capabilities. Additionally, Propionibacterium acnes produces propionic acid as a metabolic product, and the chemical effect of propionic acid on SO is not known. This chemical interaction may have contributed to the retention of bacterial viability in the SO [10].
Another evidence of biofilm-producing capabilities causing SO resistance has been reported in a case of endophthalmitis following retinal detachment surgery with SO injection at the end of the procedure. M. abscessus, a notorious organism, was isolated after gene sequencing; it tends to form a biofilm, hence becoming highly resistant to antibiotics. In this case, the antibiogram sensitivity chart showed sensitivity only to piperacillin-tazobactam [81].
Finally, SO seems to lack effectiveness against Fusarium spp., the most frequently isolated fungus after Aspergillum. In these cases, a central vitrectomy may be preferred to a complete one due to lower visibility and consequently higher risk of iatrogenic damage. SO tamponade is not recommended given the absence of proven fungicidal activity, moreover, antimycotic drug concentration may change in silicone oil [37,47].
In cases of fungal endophthalmitis associated with retinal detachment, it could be appropriate to perform retinal detachment surgery as soon as the infection resolves [82].
8. Anatomical and Visual Outcomes
Does substituting the vitreous humor with SO affect anatomical and visual outcomes?
Back in 2009, SO injection after complete PPV proved to lead to earlier infection control, better anatomical and visual outcomes, and a lower re-intervention rate in comparison with only intravitreal antibiotic injection [49]. In 2012 Patel et al. confronted the results obtained in 129 endophthalmitis patients treated with and without SO injection PPV in a prospective randomized clinical trial. The use of SO was related to better anatomical outcomes and a reduced number of reinterventions, the latter related to better visual outcomes. These results were even better in the post-traumatic endophthalmitis subgroup. The authors explained that in the silicone oil group, complete PPV with a surgically induced posterior vitreous detachment and a thorough examination of the peripheral retina led to overall better visual improvement. SO also prevents retinal detachments due to missed peripheral retinal breaks [53]. The results are in line with previous studies assessing the overall enhanced outcomes in patients treated with silicone oil injection [31,32,44,48,49,55].
The protective action exerted by SO has been confirmed in eyes at high risk for infections: the 2-year cumulative incidence of endophthalmitis was 31.2% in patients who received Boston Type 1 keratoprosthesis alone versus 0% in the group who received PPV and SO injection [83].
9. Retinal Detachment and Endophthalmitis
Retinal detachment is a serious condition that can dramatically undermine a patient’s visual prognosis; its occurrence in the context of endophthalmitis (concurrent or delayed onset) has variable incidence rates reported in literature ranging from 4.6% to 16% [29,84,85,86,87].
A retrospective case series of patients suffering from endophthalmitis, and retinal detachment treated with PPV and SO injection analyzed anatomical and visual outcomes among two groups. Group 1 included patients with concurrent endophthalmitis and retinal detachment, Group 2 included patients with delayed onset retinal detachment. The retinal reattachment rate was higher in the delayed onset group; however, the final visual outcome did not show statistically significant differences. The authors concluded that SO was effective in the management of retinal detachment related to endophthalmitis although low visual outcomes are likely to be expected [29].
Farouk and colleagues observed better infection control and lower postoperative retinal detachment rates in post-cataract endophthalmitis patients treated with PPV and SO injection [34]. Although postoperative visual acuity was not significantly better in eyes treated with SO injection, in a subgroup analysis, the number of patients with worsened visual acuity after the intervention was lower in the SO group. The authors, therefore, concluded that SO may play a role in preventing visual deterioration [34].
Similarly, Lin and colleagues observed lower postoperative retinal detachment rates in eyes with endophthalmitis treated with PPV and SO injection [52].
10. Traumatic Endophthalmitis
Several studies observed better anatomical and functional results after SO tamponade in patients with endophthalmitis following eye trauma; the explanations given have been multiple.
A complete vitrectomy (and not just a core vitrectomy) is needed to inject SO; this may help to remove more pathogens and to prevent post-operative vitreoretinal proliferation. When a complete vitrectomy cannot be performed because of low visibility, residual pathogens may indeed cause proliferative damage and hinder visual recovery [39].
Moreover, the use of SO creates compartmentalization of the eye allowing systemic antibiotics to concentrate between the retina and the oil bubble. Finally, SO prevents postoperative hypotony and ciliary body shock [44].
In 2019, a large study involving 98 eyes with post-traumatic endophthalmitis observed that low preoperative visual acuity, intraocular foreign bodies, and repeated intravitreal antibiotic injections were associated with worse visual outcomes. Patients who underwent SO tamponade received fewer intravitreal injections, ending up with better visual results [56].
Zhou and colleagues, on the other hand, observed worse results in patients who received primary SO tamponade compared with those who received Octafluoropropane (C3F8); these results, however, may be biased by the worse condition at presentation in the SO group [51].
Finally, in cases of traumatic endophthalmitis with an intraocular foreign body, different approaches have been proposed, according to the grade of retinal damage. If no retinal damage was intraoperatively detected, a balanced salt solution could be the tamponade of choice, reserving gas tamponade to cases with limited retinal damage and employing SO in eyes with large retinal breaks or proliferative disease. This strategy could spare unnecessary procedures and could be more cost-effective [38,50].
11. Endogenous Endophthalmitis
In 2014, Do and colleagues conducted a Vietnamese randomized clinical trial to compare two different treatment pathways in 108 patients with severe bacterial endogenous endophthalmitis [45]. A first group was treated with intravitreal antibiotic injections + PPV and a second group with intravitreal antibiotic injection + PPV + SO tamponade.
Nine months after surgery the second group showed better visual acuity and overall better results (anatomical and reintervention rates); therefore, according to the authors, in cases of severe bacterial endophthalmitis, PPV should be followed by SO injection.
12. Endophthalmitis in Silicone Oil Filled Eyes
There are few reported cases of endophthalmitis after SO injection, the causative pathogens being Pseudomonas Aeruginosa [21,88,89], coagulase-negative Staphilococcus [90], S. pneumoniae [89] and Mucorales spp. Mucormycosi [91].
In most cases, the chosen approach was to remove SO, inject antibiotics in the vitreous chamber and then fill the eye again with SO. In a case of Mucorales spp. endophthalmitis, the patient refused further surgical procedures leading to phthisis bulbi and eye loss [91].
Steinmetz and colleagues describe their experience in two cases of endophthalmitis in SO-filled eyes after vitrectomy treated with an antibiotic injection directly in a SO-filled vitreous cavity [27]. Only in one patient anterior chamber puncture was performed with negative bacterial and leukocyte counts. In one case half of the usual antibiotic (vancomycin and Ceftazidime) dose was used whereas in the other full dose was administered; in neither case, retinal toxicity was observed, and the infection was resolved in all cases.
The antibiotic might have injected occupied the space between silicone oil and the retina where bacteria are more likely to replicate. This approach could be advantageous in eyes that cannot be operated on straight away and spare additional surgeries in multi-operative eyes.
13. Limitation of Silicone Oil Use
Performing a complete vitrectomy before SO injection is not always possible in eyes with severe endophthalmitis; firm adherence of inflammatory debris to the retina and fundus visualization problems due to corneal oedema and pupil membranes may complicate the surgical procedures. Moreover, the use of SO during the surgical treatment of endophthalmitis has some disadvantages, including the retinotoxic potential and the observation that not all pathogens are inhibited by this substance.
SO low-molecular weight compounds may diffuse out of the vitreous cavity, resulting in retinal toxicity and affecting eye physiology by absorption of lipophilic molecules from the ocular environment [92].
It has been suggested that substances like cellular debris, inflammatory substances and blood products may enhance early emulsification [31].
Vitreous humor plays a crucial role in eye physiology and must not be considered an inert optical medium; it is central in intravitreal pharmacodynamics and its buffering capacities have been demonstrated [93].
Replacement of vitreous humor with SO may interfere with toxin washout. Several defence mechanisms within the retina such as the blood-retinal barrier, cytochrome p450 activity and antioxidants protect the retina against a variety of insults. Since the waste products of the retina diffuse into the vitreous, the effective volume of the vitreous space is also important in detoxification as the amount of this space is inversely related to the concentration of toxic materials. Therefore, with the reduction of effective vitreous space, the tolerable number of acids or toxins is likely to be reduced. In the context of endophthalmitis with a high retinal metabolic rate, increased production of waste products, and acidification of the retinal environment, replacing vitreous with SO may expose the retina to more rapid decompensation and damage [94].
Dosing intravitreal antibiotics in SO-filled eyes can be challenging. The physician will struggle both with lower retinal toxicity thresholds and with a higher antibiotic clearance; the medication becomes more dangerous and stays in the vitreous chamber for less time [95].
SO causes compartmentalization in the eye that may trap inflammatory debris between the SO and retina, leading to the development of pre-retinal membranes that could cause retinal tears. The presence of SO and consequent compartmentalization may also influence the distributions of intravitreal antibiotics that can reach toxic concentrations. In addition, the toxicity of SO for the optic disc should be seriously considered [96].
Many authors, therefore, concluded that SO following PPV for endophthalmitis should be reserved only for cases with trauma history and/or associated retinal detachment.
14. Discussion
In this review, we examined 44 articles aiming to answer the main questions that we considered for this work. Firstly, we focused on the antimicrobial activity of SO and its contribution to controlling the infection (Table 1). 5 out of 7 in vitro experimental studies demonstrated antimicrobial activity against different species of bacteria (e.g., S. aureus, S. epidermidids, P. aeruginosa, C. albicans) and fungi (Asperigillus spp., C. albicans) [9,11,13,14,15]. They employed different types of SO, observing a greater antimicrobial effect with heavy SOs. In contrast, 2 studies [10,12] did not observe inhibition on growth against the species examined (e.g., Propionibacterium acnes, Pseudomonas aeruginosa; Escherichia coli; Staphylococcus aureus; Staphylococcus epidermidis; Candida albicans; Klebsiella pneumoniae; Streptococcus pneumoniae). A possible explanation for the different results among studies could rely on different methodologies to assess antimicrobial activity. Some authors inoculated bacterial suspensions on different SO mediums; others used filter paper discs containing SO, placed in seeded plates. In addition, we found 3 case series [26,27,28] and one case report [21] of postoperative endophthalmitis in SO-filled eyes.
The use of SO as endotamponade associated with PPV lead to better outcomes and provided better control of the infection in all 4 randomized clinical trials, in 15 out of 18 case series and in 3 out 5 retrospective interventional studies. In 3 case series [38,41,42] and 2 retrospective studies [51,56], there was no difference in visual acuity between eyes that received SO tamponade and controls (Table 2). However, in some studies, patients that received SO had worst visual acuity and more severe ocular damage compared with eyes that received other tamponades. The use of SO contributed to a better visual and anatomical outcome, reduced the need for additional surgery [30,32,48,49,53], decreased the risk of retinal detachment [29,30,32,49] and was considered an eligible option for intravitreal injection of antibiotics [35]. Early PPV with endotamponade should be preferred to PPV without tamponade [46,55]. Some Authors reserved PPV and endotamponade only in complicated cases associated with corneal involvement, fungal endophthalmitis and cases requiring intraocular lens removal [36].
A limitation of this review was related to the typology of the studies included since most of them were case reports and case series that implicated a lack of a control group. Furthermore, we excluded editorials, letters to editors and articles that were not in English.
In conclusion, injections of SO after PPV in cases of endophthalmitis may entail several advantages. SO reduces the risk of postoperative retinal detachment, especially in case of undetected retinal breaks, produces compartmentalization of the eye, may lead to early visual recovery due to an optically clear medium, allows laser photocoagulation in case of missed retinal breaks, prevents the occurrence of severe hypotony in the early postoperative period and has antimicrobic activity due to an inhibitory effect for several species of pathogens. However, concerns regarding possible toxic effects on the retina and optic disc, compartmentalization and impaired washout of pathogen toxins have been reported. SO may also influence intravitreal antibiotic distribution and clearance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11185445/s1, Table S1: Does silicone oil have antimicrobial activity? The quality of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. Table S2: Does Silicone oil injection after vitrectomy improve visual and anatomical outcomes? The quality of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. Table S3: Risk of Bias (ROB) according to Revised Cochrane risk-of-bias tool for randomized clinical trials. Table S4: A detailed Newcastle-Ottawa Scale of each included retrospective study. Table S5: Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case series. Table S6: Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Repots. File S1: PRISMA 2020 checklist.
Author Contributions
Conceptualization, P.L.; writing—original draft preparation, F.S. and M.D.S.; data curation, M.F.; writing—review and editing, P.L.; supervision, G.C. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting this work are included in the manuscript and/or Supplementary Materials. The protocol was prepared and can be requested from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forster, R.K. The Endophthalmitis Vitrectomy Study. Arch. Ophthalmol. 1995, 113, 1555–1557. [Google Scholar] [CrossRef]
- Kuhn, F.; Gini, G. Ten years after… are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 1197–1199. [Google Scholar] [CrossRef]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilemmas and Conclusions. Available online: http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/ESCRS-Guidelines-for-use-of-prophylactic-Antibiotics-post-cataract-sugery.pdf (accessed on 14 April 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 15 August 2022).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Piggott, T.; Morgan, R.L.; Cuello-Garcia, C.A.; Santesso, N.; Mustafa, R.A.; Meerpohl, J.J.; Schünemann, H.J. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: Extremely serious, GRADE’s terminology for rating down by three levels. J. Clin. Epidemiol. 2020, 120, 116–120. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers ‘to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Ozdamar, A.; Aras, C.; Ozturk, R.; Akin, E.; Karacorlu, M.; Ercikan, C. In vitro antimicrobial activity of silicone oil against endophthalmitis-causing agents. Retina 1999, 19, 122–126. [Google Scholar] [CrossRef]
- Arici, C.; Aras, C.; Tokman, H.B.; Torun, M.M. An in Vitro Experimental Study on the Antimicrobial Activity of Silicone Oil against Anaerobic Bacteria. Ocul. Immunol. Inflamm. 2016, 24, 173–177. [Google Scholar] [CrossRef]
- Economou-Stamatelopoulou, C.; Roussopoulos, G.P.; Prouskas, J.C.; Apostolopoulos, M. Antifungal activity of intraocularly used liquids against Aspergillus. Ophthalmologica 2004, 218, 323–327. [Google Scholar] [CrossRef]
- Adams, F.; Romero, I.L.; Silva, C.B.; Manzano, R.P. Evaluation of silicon oil on bacterial growth. Arq. Bras. Oftalmol. 2012, 75, 89–91. [Google Scholar] [CrossRef]
- Ornek, N.; Apan, T.; Oğurel, R.; Ornek, K. Comparison of the antimicrobial effect of heavy silicone oil and conventional silicone oil against endophthalmitis-causing agents. Indian J. Ophthalmol. 2014, 62, 388–391. [Google Scholar] [CrossRef]
- Chrapek, O.; Vecerova, R.; Koukalova, D.; Maresova, K.; Jirkova, B.; Sin, M.; Rehak, J. The in vitro antimicrobial activity of silicone oils used in ophthalmic surgery. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2012, 156, 7–13. [Google Scholar] [CrossRef]
- Dave, V.P.; Joseph, J.; Jayabhasker, P.; Pappuru, R.R.; Pathengay, A.; Das, T. Does ophthalmic-grade silicone oil possess antimicrobial properties? J. Ophthalmic. Inflamm. Infect. 2019, 9, 20. [Google Scholar] [CrossRef]
- Olgun, A.; Imamoğlu, S.; Karapapak, M.; Düzgün, E.; Kaçar, H. Endophthalmitis After XEN Gel Stent Implantation: 2 Cases. J. Glaucoma 2018, 27, e191–e194. [Google Scholar] [CrossRef]
- Baxter, K.R.; Robinson, J.E.; Ruby, A.J. Occlusive vasculitis due to hyperacute Streptococcus mitis endophthalmitis after intravitreal ranibizumab. Retin. Cases Brief Rep. 2015, 9, 201–204. [Google Scholar] [CrossRef]
- Mohd-Ilham, I.; Zulkifli, M.; Yaakub, M.; Muda, R.; Shatriah, I. A Case of a Large Sub-retinal Abscess Secondary to Klebsiella pneumoniae Endophthalmitis in a Pyelonephritis Patient. Cureus 2019, 11, e4656. [Google Scholar] [CrossRef]
- Krėpštė, L.; Žemaitienė, R.; Barzdžiukas, V.; Miliauskas, A. Bilateral endogenous bacterial panophthalmitis. Medicina 2013, 49, 143–147. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Lee, S.U.; Sohn, J.H.; Lee, S.E. Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis. Retina 2003, 23, 366–370. [Google Scholar] [CrossRef]
- Goel, N.; Bhambhwani, V.; Ghosh, B. Multidrug-resistant Pseudomonas aeruginosa endophthalmitis in a silicone oil-filled eye treated with piperacillin/tazobactam: Report of a case and review of literature. Int. Ophthalmol. 2015, 35, 599–602. [Google Scholar] [CrossRef]
- Siu, G.D.; Lo, E.C.; Young, A. Endogenous endophthalmitis with a visual acuity of 6/6. BMJ Case Rep. 2015, 2015, bcr2014205048. [Google Scholar] [CrossRef]
- Chon, J.; Kim, M. Successful management of late-onset Streptococcus mitis endophthalmitis. Ther. Clin. Risk Manag. 2017, 13, 1439–1442. [Google Scholar] [CrossRef]
- Mackiewicz, J.; Haszcz, D.; Zagórski, Z. Exogenous Candida endophthalmitis in a hop grower—A case report. Ann. Agric. Environ. Med. 2000, 7, 131–132. [Google Scholar]
- Won, J.Y.; Kim, M. Vancomycin-resistant Staphylococcus hominis endophthalmitis following cataract surgery. Clin. Ophthalmol. 2013, 7, 1193–1195. [Google Scholar] [CrossRef]
- Okonkwo, O.N.; Hassan, A.O.; Oderinlo, O.; Gyasi, M.E. Burkholderia cepacia, a cause of post pars plana vitrectomy silicone oil related endophthalmitis: Clinico-pathological presentation and outcome of management. Int. J. Retina Vitr. 2018, 4, 35. [Google Scholar] [CrossRef]
- Steinmetz, R.L.; Vyas, S.; Ashmore, E.; Brooks, H.L. Acute-Onset Postoperative Endophthalmitis in Silicone Oil–Filled Eyes Managed With Intravitreal Antibiotics Alone. J. Vitr. Dis. 2018, 2, 107–110. [Google Scholar] [CrossRef]
- Tayyib, M. Endophthalmitis After Vitrectomy An Silicone Oil Implant. Ann. King Edw. Med. Univ. 1997, 3, 60–61. [Google Scholar]
- Dave, V.P.; Pathengay, A.; Relhan, N.; Sharma, P.; Jalali, S.; Pappuru, R.R.; Tyagi, M.; Narayanan, R.; Chhablani, J.; Das, T.; et al. Endophthalmitis and Concurrent or Delayed-Onset Rhegmatogenous Retinal Detachment Managed With Pars Plana Vitrectomy, Intravitreal Antibiotics, and Silicone Oil. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 546–551. [Google Scholar] [CrossRef]
- Hudieb, A. Early pars plana vitrectomy and silicon oil Endotamponade in the treatment of Acute infectious Endophthalmitis. Azhar Assyot Med. J. 2012, 10, 3. [Google Scholar]
- Aras, C.; Ozdamar, A.; Karacorlu, M.; Ozkan, S. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int. Ophthalmol. 2001, 24, 147–150. [Google Scholar] [CrossRef]
- Bali, E.; Huyghe, P.; Caspers, L.; Libert, J. Vitrectomy and silicone oil in the treatment of acute endophthalmitis. Preliminary results. Bull. Soc. Belge Ophtalmol. 2003, 288, 9–14. [Google Scholar]
- Pinarci, E.Y.; Yesilirmak, N.; Bayar, S.A.; Sizmaz, S.; Akkoyun, I.; Yilmaz, G. The results of pars plana vitrectomy and silicone oil tamponade for endophthalmitis after intravitreal injections. Int. Ophthalmol. 2013, 33, 361–365. [Google Scholar] [CrossRef]
- Farouk, M.M.; Mounir, A.; Elagouz, M. The Role of Silicone Oil in Management of Postoperative Endophthalmitis. Sohag Med. J. 2017, 21, 575–581. [Google Scholar] [CrossRef]
- Yan, H.; Lu, Y.; Yu, J.; Han, J.; Zhang, J.; Chen, S.; Xu, Y. Silicone oil in the surgical treatment of traumatic endophthalmitis. Eur. J. Ophthalmol. 2008, 18, 680–684. [Google Scholar] [CrossRef]
- Verma, L.; Chakravarti, A. Prevention and management of postoperative endophthalmitis: A case-based approach. Indian J. Ophthalmol. 2017, 65, 1396–1402. [Google Scholar] [CrossRef]
- Cakir, M.; Imamoğlu, S.; Cekiç, O.; Bozkurt, E.; Alagöz, N.; Oksüz, L.; Yilmaz, O.F. An outbreak of early-onset endophthalmitis caused by Fusarium species following cataract surgery. Curr. Eye Res. 2009, 34, 988–995. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, J.; Wang, R.; Lei, J.; Zhou, Y. Visual Outcomes and Prognostic Factors after Pars Plana Vitrectomy for Traumatic Endophthalmitis. Biomed. Res. Int. 2017, 2017, 5851318. [Google Scholar] [CrossRef]
- Jin, W.; Xu, Y.; Wang, W.; Xing, Y.; Yang, A. Efficacy and Safety of 23-Gauge Pars Plana Vitrectomy/Silicone Oil Tamponade Combination for Treatment of Pediatric Post-Traumatic Endophthalmitis. Curr. Eye Res. 2017, 42, 1143–1148. [Google Scholar] [CrossRef]
- Zhang, J.; Han, F.; Zhai, X. Clinical analysis of 23-gauge vitrectomy for the treatment of acute endophthalmitis after cataract surgery. Eur. J. Ophthalmol. 2015, 25, 503–506. [Google Scholar] [CrossRef]
- Yospaiboon, Y.; Intarapanich, A.; Laovirojjanakul, W.; Ratanapakorn, T.; Sinawat, S.; Sanguansak, T.; Bhoomibunchoo, C. Factors affecting visual outcomes after treatment of infectious endophthalmitis in northeastern Thailand. Clin. Ophthalmol. 2018, 12, 765–772. [Google Scholar] [CrossRef]
- Yospaiboon, Y.; Meethongkam, K.; Sinawat, S.; Laovirojjanakul, W.; Ratanapakorn, T.; Sanguansak, T.; Bhoomibunchoo, C. Predictive factors in the treatment of streptococcal endophthalmitis. Clin. Ophthalmol. 2018, 12, 859–864. [Google Scholar] [CrossRef]
- Nagpal, M.; Jain, P.; Nagpal, K. Pars Plana Vitrectomy With or Without Silicone Oil Endotamponade in Surgical Management of Endophthalmitis. Asia Pac. J. Ophthalmol. 2012, 1, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Azad, R.; Ravi, K.; Talwar, D.; Rajpal; Kumar, N. Pars plana vitrectomy with or without silicone oil endotamponade in post-traumatic endophthalmitis. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Hon, D.N.; Aung, T.; Hien, N.D.; Cowan, C.L., Jr. Bacterial endogenous endophthalmitis in Vietnam: A randomized controlled trial comparing vitrectomy with silicone oil versus vitrectomy alone. Clin. Ophthalmol. 2014, 8, 1633–1640. [Google Scholar] [CrossRef]
- Khaqan, H.A.; Imtiaz, U.; Buksh, H.M.; REHMAN, H.A.; Raheela, N.J.C.; Trauma, E.O. Outcomes of Early Pars Plana Vitrectomy for Acute Post Operative Endophthalmitis with or without Silicone Oil. Clin. Exp. Ocul. Trauma Infect. 2017, 2, 56–60. [Google Scholar]
- Kapoor, K.G.; Khurshid, G.S.J.I.O.; Science, V. Silicone Oil As An Adjunct In The Surgical Management Of Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2619. [Google Scholar]
- Kaynak, S.; Oner, F.H.; Koçak, N.; Cingil, G. Surgical management of postoperative endophthalmitis: Comparison of 2 techniques. J. Cataract Refract. Surg. 2003, 29, 966–969. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Gil, A.D.; Canamary, F.; Minari, M.; Jorge, R. Pars plana vitrectomy and silicone oil tamponade for acute endophthalmitis treatment. Arq. Bras. Oftalmol. 2009, 72, 28–32. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.L.; Wang, Y.; Chen, S.J.; Huang, X.Y.; Wu, N.; Ying, X. Endotamponades in pars plana vitrectomy for metallic intraocular foreign body associated with endophthalmitis. Int. J. Ophthalmol. 2011, 4, 95–99. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wang, Y.X.; Yao, T.T.; Yang, Y.; Wang, Z.Y. Traumatic endophthalmitis and the outcome after vitrectomy in oung children. Int. J. Ophthalmol. 2020, 13, 406–411. [Google Scholar] [CrossRef]
- Lin, H.; Ling, S.; Liu, Z.; Zhong, X.; Chen, W. Preventive scleral buckling and silicone oil tamponade are important for posttraumatic endophthalmitis successfully managed with vitrectomy. Ophthalmologica 2011, 226, 214–219. [Google Scholar] [CrossRef]
- Patel, A.; Gentile, R. Pars Plana Vitrectomy With or Without Silicone Oil Endotamponade in Surgical Management of Endophthalmitis. Asia Pac. J. Ophthalmol. 2012, 1, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [CrossRef]
- Kresloff, M.S.; Castellarin, A.A.; Zarbin, M.A. Endophthalmitis. Surv. Ophthalmol. 1998, 43, 193–224. [Google Scholar] [CrossRef]
- Du, D.T.; Wagoner, A.; Barone, S.B.; Zinderman, C.E.; Kelman, J.; MaCurdy, T.; Forshee, R.A.; Worrall, C.; Izurieta, H.S.J.O. Incidence of endophthalmitis after corneal transplant or cataract surgery in a medicare population. Ophthalmology 2014, 121, 290–298. [Google Scholar] [CrossRef]
- Nam, K.Y.; Lee, J.E.; Lee, J.E.; Jeung, W.J.; Park, J.M.; Park, J.M.; Chung, I.Y.; Han, Y.S.; Yun, I.H.; Kim, H.W.; et al. Clinical features of infectious endophthalmitis in South Korea: A five-year multicenter study. BMC Infect. Dis. 2015, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; Flynn, H.W., Jr.; Acar, N.; Dev, S.; Shaikh, S.; Mittra, R.A.; Arevalo, J.F.; Kychenthal, A.; Kunselman, A. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 377–380. [Google Scholar] [CrossRef]
- Rasul, A.; Subhi, Y.; Sørensen, T.L.; Munch, I.C. Non-physician delivered intravitreal injection service is feasible and safe–a systematic review. Dan. Med. J. 2016, 63, A5229. [Google Scholar]
- Shah, R.E.; Gupta, O. The microsurgical safety task force: Guidelines for minimizing endophthalmitis with vitrectomy surgery. Curr. Opin. Ophthalmol. 2012, 23, 189–194. [Google Scholar] [CrossRef]
- McCannel, C.A. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: Causative organisms and possible prevention strategies. Retina 2011, 31, 654–661. [Google Scholar] [CrossRef]
- Chaudhary, K.M.; Romero, J.M.; Ezon, I.; Fastenberg, D.M.; Deramo, V.A. Pars plana vitrectomy in the management of patients diagnosed with endophthalmitis following intravitreal anti-vascular endothelial growth factor injection. Retina 2013, 33, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Rayess, N.; Rahimy, E.; Shah, C.P.; Wolfe, J.D.; Chen, E.; DeCroos, F.C.; Storey, P.; Garg, S.J.; Hsu, J. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br. J. Ophthalmol. 2016, 100, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Storey, P.; Dollin, M.; Pitcher, J.; Reddy, S.; Vojtko, J.; Vander, J.; Hsu, J.; Garg, S.J. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology 2014, 121, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.J.; Dollin, M.; Storey, P.; Pitcher, J.D., 3rd; Fang-Yen, N.H.; Vander, J.; Hsu, J. Microbial spectrum and outcomes of endophthalmitis after intravitreal injection versus pars plana vitrectomy. Retina 2016, 36, 351–359. [Google Scholar] [CrossRef]
- Ahmed, Y.; Schimel, A.M.; Pathengay, A.; Colyer, M.H.; Flynn, H.W., Jr. Endophthalmitis following open-globe injuries. Eye 2012, 26, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Simakurthy, S.; Tripathy, K. Endophthalmitis. In StatPearls; StatPearls Publishing Copyright© 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gentile, R.C.; Shukla, S.; Shah, M.; Ritterband, D.C.; Engelbert, M.; Davis, A.; Hu, D.N. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: A 25-year review. Ophthalmology 2014, 121, 1634–1642. [Google Scholar] [CrossRef]
- Clarke, B.; Williamson, T.H.; Gini, G.; Gupta, B. Management of bacterial postoperative endophthalmitis and the role of vitrectomy. Surv. Ophthalmol. 2018, 63, 677–693. [Google Scholar] [CrossRef]
- Lott, M.N.; Fuller, J.J.; Hancock, H.A.; Singh, J.; Singh, H.; McGwin, G., Jr.; Marcus, D.M. Vitreal penetration of oral moxifloxacin in humans. Retina 2008, 28, 473–476. [Google Scholar] [CrossRef]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting systemic treatment of bacterial endophthalmitis: A review of intravitreal penetration of systemic antibiotics. Clin. Microbiol. Infect. 2019, 25, 1364–1369. [Google Scholar] [CrossRef]
- Jackson, T.L.; Eykyn, S.J.; Graham, E.M.; Stanford, M.R. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv. Ophthalmol. 2003, 48, 403–423. [Google Scholar] [CrossRef]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic Inflamm. Infect. 2014, 4, 22. [Google Scholar] [CrossRef]
- Reddy, A.K.; Reddy, R.R.; Paruvelli, M.R.; Ambatipudi, S.; Rani, A.; Lodhi, S.A.; Reddy, J.M.; Reddy, K.R.; Pandey, N.; Videkar, R.; et al. Susceptibility of bacterial isolates to vancomycin and ceftazidime from patients with endophthalmitis: Is there a need to change the empirical therapy in suspected bacterial endophthalmitis? Int. Ophthalmol. 2015, 35, 37–42. [Google Scholar] [CrossRef]
- Endophthalmitis Vitrectomy Study Group. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995, 113, 1479–1496. [Google Scholar] [CrossRef]
- Giordano, G.G.; Refojo, M.F. Silicone oils as vitreous substitutes. Prog. Polym. Sci. 1998, 23, 509–532. [Google Scholar] [CrossRef]
- Thomas, B.J.; Yonekawa, Y.; Ruby, A.J.; Capone, A., Jr. Aggressive Surgical Therapy With Early Vitrectomy, Panretinal Photocoagulation, and Silicone Oil Tamponade for Streptococcus mitis Endophthalmitis. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 893–895. [Google Scholar] [CrossRef]
- Suganeswari, G.; Shah, D.; Anand, A.R. Intravitreal piperacillin-tazobactam in endophthalmitis caused by Mycobacterium abscessus in silico ne-filled eye: A case report. Indian J. Ophthalmol. 2020, 68, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K.D.; Flynn, H.W., Jr.; Alfonso, E.C.; Miller, D. Fusarium endophthalmitis following keratitis associated with contact lenses. Ophthalmic Surg. Lasers Imaging 2006, 37, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, N.; Dong, X.G.; Yuan, G.Q.; Yu, B.; Xie, L.X. Surgical management of fungal endophthalmitis resulting from fungal keratitis. Int. J. Ophthalmol. 2016, 9, 848–853. [Google Scholar] [CrossRef]
- Abou Shousha, M.; Eleiwa, T.; Gibbons, A.; Smith, C.; Edelstein, S.; Kontadakis, G.; Schmitz, Z.; Abernathy, J.; Chod, R.; Bodnar, Z.; et al. Risk of Endophthalmitis in Boston Type 1 Keratoprosthesis Combined with Vitrectomy and Silicone Oil Insertion. J. Ophthalmol. 2019, 2019, 9648614. [Google Scholar] [CrossRef]
- Nelsen, P.T.; Marcus, D.A.; Bovino, J.A. Retinal detachment following endophthalmitis. Ophthalmology 1985, 92, 1112–1117. [Google Scholar] [CrossRef]
- Doft, B.M.; Kelsey, S.F.; Wisniewski, S.R. Retinal detachment in the endophthalmitis vitrectomy study. Arch. Ophthalmol. 2000, 118, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.E.; Rubsamen, P.E.; Joondeph, B.C.; Flynn, H.W., Jr.; Smiddy, W.S. Concurrent endophthalmitis and retinal detachment. Ophthalmology 1994, 101, 490–498. [Google Scholar] [CrossRef]
- Olson, J.C.; Flynn, H.W., Jr.; Forster, R.K.; Culbertson, W.W. Results in the treatment of postoperative endophthalmitis. Ophthalmology 1983, 90, 692–699. [Google Scholar] [CrossRef]
- Lu, X.; Xia, H.; Jin, C.; Chen, W.; Siu-Chun Ng, D.; Yan, H.; Chen, H. Prognostic factors associated with visual outcome of salvageable eyes with posttraumatic endophthalmitis. Sci. Rep. 2019, 9, 12678. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.P.; de Juan, E., Jr.; McCuen, B.W., 2nd; Landers, M.B., 3rd. Endophthalmitis in a silicone oil-filled eye. Am. J. Ophthalmol. 1986, 102, 660–661. [Google Scholar] [CrossRef]
- Al Taisan, A.; Semidey, V.A. Culture-Positive Acute Postvitrectomy Endophthalmitis in a Silicone Oil-Filled Eye. Retin Cases Brief Rep. 2022, 16, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Zimmer-Galler, I.E.; Santos, A.; Haller, J.A.; Campochiaro, P.A. Management of endophthalmitis in a silicone oil-filled eye. Retina 1997, 17, 507–509. [Google Scholar] [CrossRef]
- Dogra, M.; Bhutani, G.; Gupta, V. Mucormycosis Endophthalmitis in a Silicone Oil-Filled Eye of an Immunocompetent Patient. Ocul. Immunol. Inflamm. 2019, 27, 1293–1295. [Google Scholar] [CrossRef]
- Nakamura, K.; Refojo, M.F.; Crabtree, D.V.; Pastor, J.; Leong, F.L. Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3007–3020. [Google Scholar]
- Conway, M.D.; Jermak, C.M.; Peyman, G.A.; Swanson, H.T.; Blake, D.A. Buffering capacity of bovine vitreous. Retina 2008, 28, 150–153. [Google Scholar] [CrossRef]
- Nowroozzadeh, M. The equivocal role of silicone oil in the treatment of traumatic endophthalmitis. Eur. J. Ophthalmol. 2009, 19, 496–497, author reply 497–499. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.M.; Kivilcim, M.; Peyman, G.A.; Unal, M.H.; Liang, C.; Molinari, L.C.; Kazi, A.A. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina 1999, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Aras, C.; Yolar, M.; Sevim, O. Surgical management of postoperative endophthalmitis. J. Cataract Refract. Surg. 2004, 30, 1612. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).