Early Postoperative Pain Trajectories after Posterolateral and Axillary Approaches to Thoracic Surgery: A Prospective Monocentric Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Subjects
2.3. Procedures
2.3.1. Surgical Management
2.3.2. Anesthetic and Pain Management
2.4. Data Collection and Outcomes
2.5. Statistical Analysis
2.5.1. Number of Patients to Be Included
2.5.2. Statistical Methods
3. Results
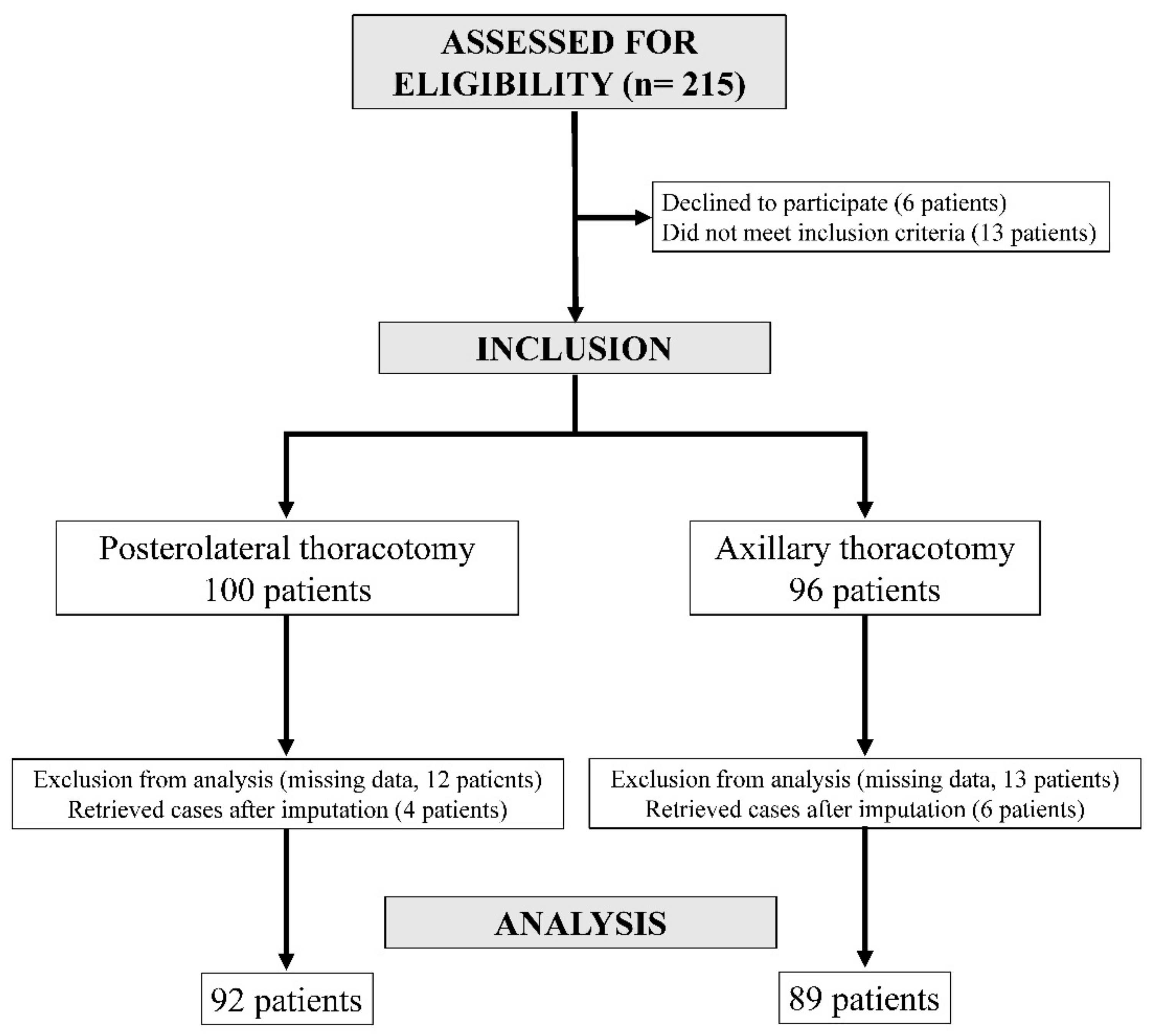
3.1. Participants
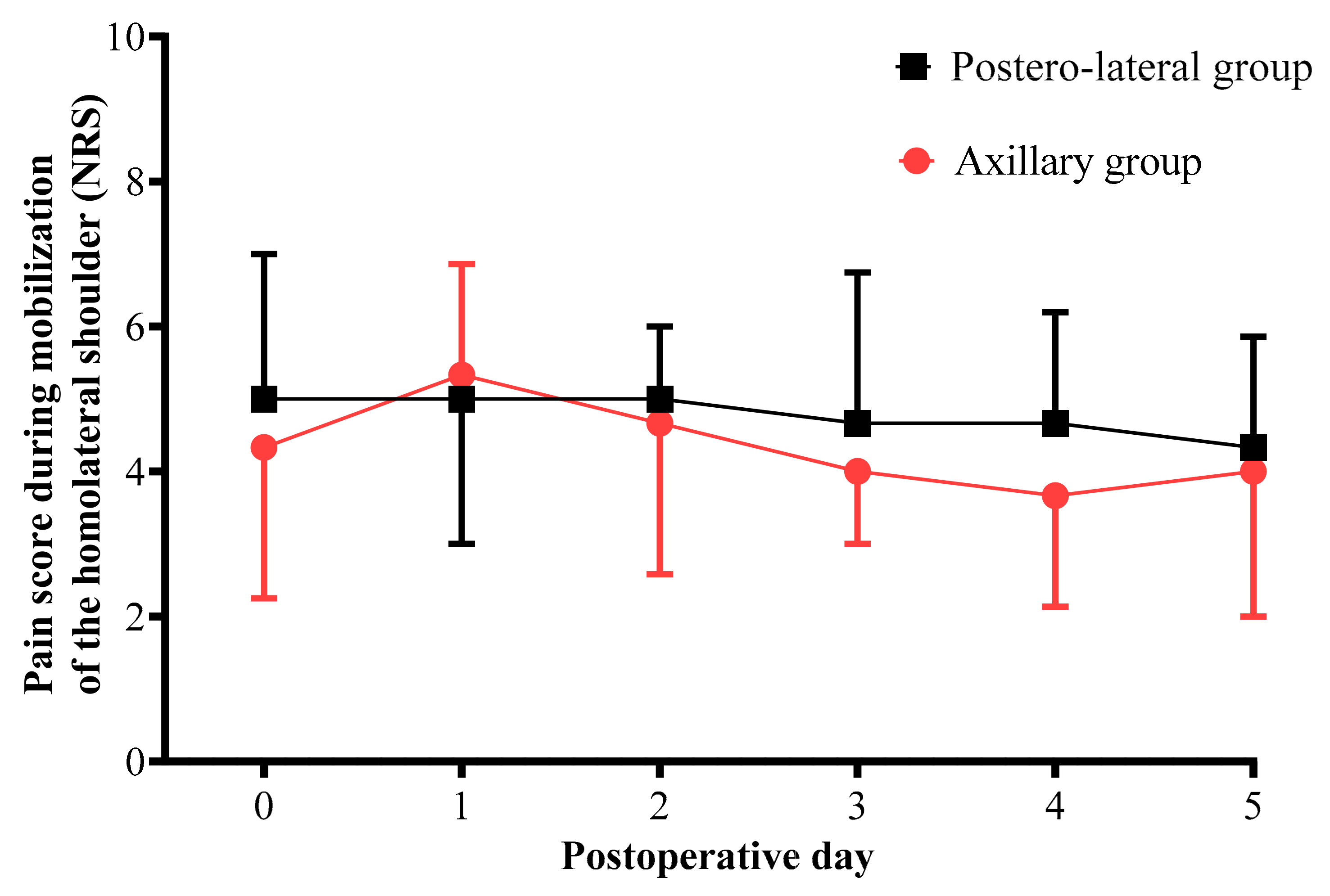
3.2. Comparison of Variables between the PL Group and the AX Group
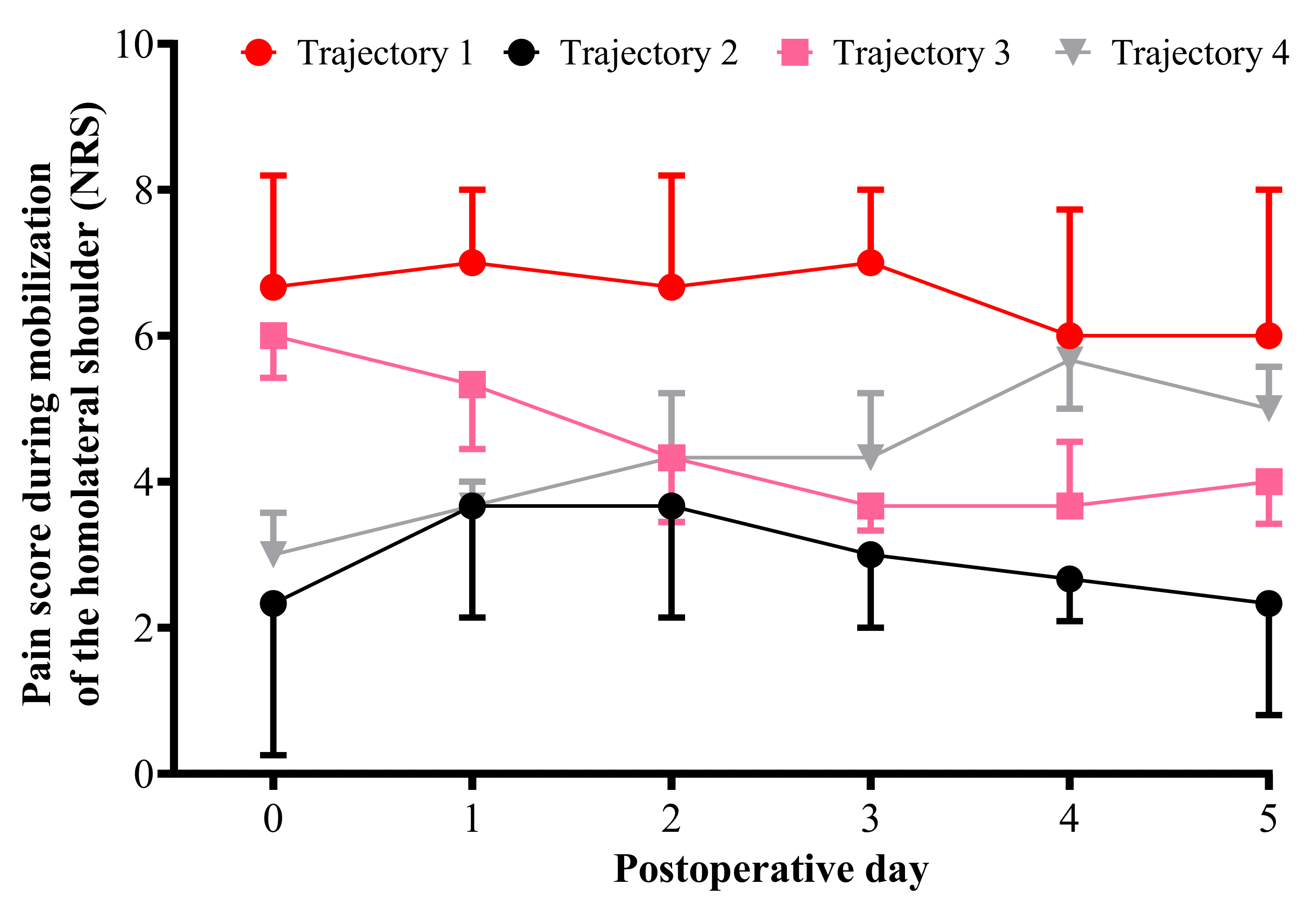
3.3. Pain Trajectories
3.4. Comparison of Variables between the Trajectory Groups
3.5. Predictive Factors of Pain Trajectories
4. Discussion
4.1. Main Results of Our Study
4.2. Interpretation
4.3. Strength and Limitations of the Study
4.4. Generalizability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pöpping, D.M.; Elia, N.; Van Aken, H.K.; Marret, E.; Schug, S.A.; Kranke, P.; Wenk, M.; Tramèr, M.R. Impact of epidural analgesia on mortality and morbidity after surgery: Systematic review and meta-analysis of randomized controlled trials. Ann. Surg. 2014, 259, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Agostini, P.J.; Naidu, B.; Rajesh, P.; Steyn, R.; Bishay, E.; Kalkat, M.; Singh, S. Potentially modifiable factors contribute to limitation in physical activity following thoracotomy and lung resection: A prospective observational study. J. Cardiothorac. Surg. 2014, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Elsaegh, M.; Dunning, J. Novel Techniques in Video-assisted Thoracic Surgery (VATS) Lobectomy. Surg. Technol. Online 2015, 26, 206–209. [Google Scholar]
- Guo, F.; Ma, D.; Li, S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine 2019, 98, e17089. [Google Scholar] [CrossRef]
- Gupta, R.; Van de Ven, T.; Pyati, S. Post-Thoracotomy Pain: Current Strategies for Prevention and Treatment. Drugs 2020, 80, 1677–1684. [Google Scholar] [CrossRef]
- Humble, S.R.; Dalton, A.J.; Li, L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur. J. Pain 2015, 19, 451–465. [Google Scholar] [CrossRef]
- Willingham, M.D.; Vila, M.R.; Ben Abdallah, A.; Avidan, M.S.; Haroutounian, S. Factors Contributing to Lingering Pain after Surgery: The Role of Patient Expectations. Anesthesiology 2021, 134, 915–924. [Google Scholar] [CrossRef]
- Chapman, C.R.; Donaldson, G.W.; Davis, J.J.; Bradshaw, D.H. Improving Individual Measurement of Postoperative Pain: The Pain Trajectory. J. Pain 2011, 12, 257–262. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamasaki, M.; Yokota, I.; Mori, M.; Matsuda, M.; Yamaguchi, Y.; Yamakita, S.; Ueno, H.; Sawa, T.; Taguchi, T.; et al. Classification of acute pain trajectory after breast cancer surgery identifies patients at risk for persistent pain: A prospective observational study. J. Pain Res. 2018, 11, 2197–2206. [Google Scholar] [CrossRef]
- Vasilopoulos, T.; Wardhan, R.; Rashidi, P.; Fillingim, R.B.; Wallace, M.R.; Crispen, P.L.; Parvataneni, H.K.; Prieto, H.A.; Machuca, T.N.; Hughes, S.J.; et al. Patient and Procedural Determinants of Postoperative Pain Trajectories. Anesthesiology 2021, 134, 421–434. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Hurtig-Wennlöf, A.; Ahlsson, A.; Vidlund, M.; Cao, Y.; Westerdahl, E. In-hospital physiotherapy improves physical activity level after lung cancer surgery: A randomized controlled trial. Physiotherapy 2019, 105, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mongardon, N.; Pinton-Gonnet, C.; Szekely, B.; Michel-Cherqui, M.; Dreyfus, J.F.; Fischler, M. Assessment of chronic pain after thoracotomy: A 1-year prevalence study. Clin. J. Pain 2011, 27, 677–681. [Google Scholar] [CrossRef]
- Erus, S.; Tanju, S.; Kapdağlı, M.; Özkan, B.; Dilege, Ş.; Toker, A. The comparison of complication, pain, quality of life and performance after lung resections with thoracoscopy and axillary thoracotomy. Eur. J. Cardio-Thorac. Surg. 2014, 46, 614–619. [Google Scholar] [CrossRef]
- Bayman, E.O.; Oleson, J.J.; Rabbitts, J.A. AAAPT: Assessment of the Acute Pain Trajectory. Pain Med. 2021, 22, 533–547. [Google Scholar] [CrossRef]
- Althaus, A.; Becker, O.A.; Moser, K.-H.; Lux, E.A.; Weber, F.; Neugebauer, E.; Simanski, C. Postoperative Pain Trajectories and Pain Chronification—an Empirical Typology of Pain Patients. Pain Med. 2018, 19, 2536–2545. [Google Scholar] [CrossRef]
- Kehlet, H.; Foss, N.B. Acute Postoperative Pain Trajectory Groups: Comment. Anesthesiology 2021, 135, 547. [Google Scholar] [CrossRef]
- Ip, H.Y.; Abrishami, A.; Peng, P.W.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009, 111, 657–677. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Pogatzki-Zahn, E.; Aduckathil, S.; Peelen, L.M.; Kappen, T.; van Wijck, A.J.M.; Kalkman, C.; Meissner, W. Procedure-specific Risk Factor Analysis for the Development of Severe Postoperative Pain. Anesthesiology 2014, 120, 1237–1245. [Google Scholar] [CrossRef]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.; Ronksley, P.E.; Jetté, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9, e025091. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.T.; Zhao, L.; Reddy, R.M.; Chang, A.C.; Orringer, M.B.; Brummett, C.M.; Lin, J. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J. Thorac. Cardiovasc. Surg. 2017, 154, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tighe, P.J.; Riley, J.L., III; Fillingim, R.B. Sex differences in the incidence of severe pain events following surgery: A review of 333,000 pain scores. Pain Med. 2014, 15, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.F.; Zaslansky, R.; van Boekel, R.L.; Cheuk-Alam, J.M.; Baart, S.J.; Huygen, F.J.; Rijsdijk, M. Postoperative pain and age: A retrospective cohort association study. Anesthesiology 2021, 135, 1104–1119. [Google Scholar] [CrossRef]
| Posterolateral Thoracotomy n = 92 | Axillary Thoracotomy n = 89 | p Value | |
|---|---|---|---|
| Age, years | 66 (57–72) | 62 (53–69) | 0.041 |
| Sex, Male/Female | 50 (54.3)/42 (45.7) | 42 (47.2)/47 (52.8) | 0.374 |
| Body mass index, kg/m2 | 25 (22–28) | 24 (22–27) | 0.227 |
| ASA | 0.129 | ||
| I | 5 (5.4) | 13 (14.6%) | |
| II | 68 (73.9) | 59 (66.3%) | |
| III | 19 (20.6) | 17 (19.1%) | |
| Cancer | 74 (80.4) | 71 (79.8) | 1 |
| History of tobacco use | 63 (75.0) {84} | 60 (76.9) {78} | 0.855 |
| Current anxiety * | 64 (69.6) | 67 (81.7) {82} | 0.079 |
| Painful patients | 42 (45.6) | 33 (37.1) | 0.291 |
| Pain on inclusion day ** | 1 (0–3) {41} | 2 (0–3) {29} | 0.997 |
| Mean pain during the prior week ** | 3 (2–6) {39} | 4 (2–5) {28} | 0.639 |
| Thoracic location of pain | 10 (25.0) {40} | 4 (12.9) {31} | 0.242 |
| Neuropathic pain *** | 8 (12.5) {64} | 5 (9.1) {55} | 0.769 |
| Analgesic used by all patients **** | {66} | {59} | |
| At least one | 27 (40.9) | 17 (28.8%) | 0.191 |
| Step 1 | 24 (36.4) | 17 (28.8%) | 0.446 |
| Step 2 | 9 (13.6) | 2 (3.4%) | 0.058 |
| Step 3 | 2 (3.0) | 0 (0.0%) | 0.497 |
| Posterolateral Thoracotomy n = 92 | Axillary Thoracotomy n = 89 | p Value | |
|---|---|---|---|
| Duration of anesthesia, minutes | 158 (136–195) {85} | 166 (139–195) {88} | 0.551 |
| Duration of surgery, minutes | 103 (78–129) {84} | 109 (82–133) {88} | 0.623 |
| Surgical procedure | 0.153 | ||
| Lobectomy | 57 (62.0) | 64 (71.9%) | 0.160 |
| Wedge resection | 23 (25.0) | 19 (21.3%) | 0.600 |
| Pneumonectomy | 7 (7.6) | 1 (1.1%) | 0.065 |
| Other procedure | 5 (5.4) | 5 (5.6%) | 1 |
| Right side of surgery | 46 (50.0) | 52 (58.4) | 0.297 |
| Surgical retractors | 92 (100.0) | 59 (67.8) {87} | <0.0001 |
| Rib fracture | 22 (24.7) {89} | 7 (8.2) {85} | 0.004 |
| Number of pleural drains | 0.600 | ||
| One | 23 (25.0) | 19 (21.3%) | |
| Two | 69 (75.0) | 70 (78.6%) | |
| Serratus preservation | 83 (92.2) {90} | 73 (85.9) {85} | 0.226 |
| Latissimus dorsi preservation | 6 (6.8) {88} | 65 (76.5) {85} | <0.0001 |
| Transcostal suture | 3 (3.4) {88} | 48 (55.8) {86} | <0.0001 |
| Extrapleural detachment | 16 (18.0) {89} | 7 (8.4) {83} | 0.076 |
| Posterolateral Thoracotomy n = 92 | Axillary Thoracotomy n = 89 | p Value | |
|---|---|---|---|
| Type of postoperative analgesia | 0.101 | ||
| Thoracic epidural analgesia | 82 (89.1) | 77 (86.5) | 0.591 |
| Paravertebral block | 7 (7.6) | 3 (3.4) | 0.330 |
| No locoregional procedure | 3 (3.3) | 9 (10.1) | 0.078 |
| Chest tube in place at day 6 (±1) | 18 (19.6) | 21 (23.6) | 0.589 |
| Pain at day 6 (±1 day) * | |||
| Pain score at rest | 1 (0–2) | 1 (0–2) | 0.867 |
| Pain score for cough | 4 (2–6) {90} | 3 (2–5) {87} | 0.019 |
| Pain score for mobilization of the ipsilateral shoulder | 3 (2–4) | 2 (1–4) | 0.035 |
| Neuropathic pain ** | 6 (6.5) | 4 (4.5) | 0.747 |
| Height of hypoesthesia area around the scar, cm | |||
| On the breast line | 0 (0–8) | 0 (0–5) {87} | 0.722 |
| On the axillary line | 0 (0–5) | 0 (0–4) {88} | 0.354 |
| On the tip of the scapula | 0 (0–0) {91} | 0 (0–0) {88} | 0.016 |
| Scar length, cm | 17 (14–19) {91} | 8 (6–11) {84} | <0.0001 |
| Satisfaction score *** | 8 (7–9) {91} | 9 (8–10) {87} | 0.075 |
| Postoperative complications ≥ IIIa **** | 3 (3.3) | 6 (6.7) | 0.325 |
| Postoperative hospital stay, days (n) | 8 (7–12) | 8 (7–11) | 0.132 |
| Trajectory 1 n = 54 | Trajectory 2 n = 59 | Trajectory 3 n = 41 | Trajectory 4 n = 27 | Global p Value | Intergroup p Value | |
|---|---|---|---|---|---|---|
| Age, years | 60 (51–67) | 69 (58–72) | 64 (55–71) | 64 (56–70) | 0.049 | T1 vs. T2; p = 0.031 |
| Sex, Male/Female | 22 (40.7)/32 (59.3) | 35 (59.3)/24 (40.7) | 22 (53.7)/19 (46.3) | 13 (48.1)/14 (51.9) | 0.251 | |
| Body mass index, kg/m2 | 23 (21–27) | 24 (22–27) | 25 (22–27) | 26 (22–29) | 0.442 | |
| ASA | 0.073 | |||||
| I | 8 (14.8) | 4 (6.8) | 3 (7.3) | 3 (11.1) | ||
| II | 42 (77.8) | 37 (62.7) | 29 (70.7) | 19 (70.4) | ||
| III | 4 (7.4) | 18 (30.5) | 9 (21.9) | 5 (18.5) | ||
| Cancer | 42 (77.8) | 47 (79.7) | 33 (80.5) | 23 (85.2) | 0.917 | |
| History of tobacco use | 36 (73.5) {49} | 38 (71.7) {53} | 30 (85.7) {35} | 19 (76.0) {25} | 0.479 | |
| Current anxiety * | 45 (86.5) {52} | 39 (68.4) {57} | 28 (71.8) {39} | 19 (73.1) {26} | 0.131 | |
| Painful patients | 33 (61.1) | 19 (32.2) | 15 (36.6) | 8 (29.6) | 0.006 | T1 vs. T2; p = 0.006 T1 vs. T3; p = 0.049 T1 vs. T4; p = 0.021 |
| Pain on inclusion day ** | 2 (0–4) {29} | 1 (0–2) {18} | 2 (0–5) {15} | 0 (0,1) {8} | 0.306 | |
| Mean pain during the prior week ** | 4 (3–6) {29} | 3 (2–4) {17} | 4 (2–4) {13} | 4 (1–4) {8} | 0.085 | |
| Thoracic pain | 7 (23.3) {30} | 3 (16.7) {18} | 3 (20) {15} | 1 (12.5) {8} | 0.947 | |
| Neuropathic pain *** | 9 (25.0) {36} | 1 (2.5) {40} | 2 (8.0) {25} | 1 (5.6) {18} | 0.015 | T1 vs. T2; p = 0.013 |
| Analgesic used by all patients **** | {36} | {46} | {27} | {16} | ||
| At least one | 19 (52.8) | 10 (21.7) | 9 (33.3) | 6 (37.5%) | 0.035 | T1 vs. T2; p = 0.022 |
| Step 1 | 16 (44.4) | 10 (21.7) | 9 (33.3) | 6 (37.5%) | 0.166 | |
| Step 2 | 5 (13.9) | 2 (4.3) | 2 (7.4) | 2 (12.5%) | 0.409 | |
| Step 3 | 1 (2.8) | 1 (2.2) | 0 (0.0) | 0 (0.0%) | 1 |
| Trajectory 1 n = 54 | Trajectory 2 n = 59 | Trajectory 3 n = 41 | Trajectory 4 n = 27 | Global p Value | |
|---|---|---|---|---|---|
| Axillary approach | 24 (44.4) | 32 (54.2) | 20 (48.8) | 13 (48.1) | 0.781 |
| Duration of anesthesia, minutes | 162 (129–189) {52} | 156 (134–198) {57} | 166 (149–199) {39} | 168 (135–214) {25} | 0.502 |
| Duration of surgery, minutes | 102 (77–127) {52} | 109 (76–130) {57} | 107 (89–134) {38} | 111 (79–152) {25} | 0.825 |
| Surgical procedure | |||||
| Lobectomy | 35 (64.8) | 39 (66.1) | 29 (70.7) | 18 (66.7) | 0.940 |
| Wedge resection | 11 (20.4) | 17 (28.8) | 8 (19.5) | 6 (22.2) | 0.679 |
| Pneumonectomy | 3 (5.6) | 1 (1.7) | 3 (7.3) | 1 (3.7) | 0.513 |
| Other procedure | 5 (9.3) | 2 (3.4) | 1 (2.4) | 2 (7.4) | 0.444 |
| Right side of surgery | 29 (53.7) | 32 (54.2) | 23 (56.1) | 14 (51.8) | 0.993 |
| Surgical retractors | 48 (88.9) | 46 (80.0) | 33 (82.5) {40} | 24 (92.3) {26} | 0.283 |
| Rib fracture | 8 (14.8) | 10 (17.9) {56} | 3 (7.9) {38} | 8 (30.8) {26} | 0.121 |
| Number of pleural drains | 0.884 | ||||
| One | 12 (22.2) | 15 (25.4) | 8 (19.5) | 7 (25.9) | |
| Two | 42 (77.8) | 44 (74.6) | 33 (80.5) | 20 (74.1) | |
| Serratus preservation | 47 (88.7) {53} | 51 (87.9) {58} | 36 (94.7) {38} | 22 (84.6) {26} | 0.595 |
| Latissimus dorsi preservation | 19 (36.5) {52} | 28 (48.3) {58} | 17 (45.9) {37} | 7 (26.9) {26} | 0.244 |
| Transcostal suture | 13 (25.5) {51} | 21 (36.2) {58} | 14 (35.9) {39} | 3 (11.5) {26} | 0.082 |
| Extrapleural detachment | 7 (13.5) {52} | 7 (12.3) {57} | 6 (16.2) {37} | 3 (11.5) {26} | 0.947 |
| Trajectory 1 n = 54 | Trajectory 2 n = 59 | Trajectory 3 n = 41 | Trajectory 4 n = 27 | Global p Value | Intergroup p Value | |
|---|---|---|---|---|---|---|
| Type of postoperative analgesia | 0.788 | |||||
| Thoracic epidural analgesia | 48 (88.9) | 52 (88.1) | 34 (82.9) | 25 (92.6) | 0.707 | |
| Paravertebral block | 4 (7.4) | 2 (3.4) | 3 (7.3) | 1 (3.7) | 0.736 | |
| No locoregional procedure | 2 (3.7) | 5 (8.5) | 4 (9.8) | 1 (3.7) | 0.615 | |
| Chest tube in place at day 6 (±1) | 13 (24.1) | 13 (22.0) | 7 (17.1) | 6 (22.2) | 0.880 | |
| Pain at day 6 (±1 day) * | ||||||
| Pain score at rest | 2 (0–3) | 0 (0,1) | 1 (0–2) | 1 (0–3) | <0.0001 | T1 vs. T2; p < 0.0001 T2 vs. T4; p = 0.024 |
| Pain score for cough | 5 (4–7) {51} | 2 (1–3) | 4 (2–5) | 5 (3–6) {26} | <0.0001 | T1 vs. T2; p < 0.0001 T1 vs. T3; p = 0.005 T2 vs. T3; p = 0.034 T2 vs. T4; p < 0.0001 |
| Pain score for mobilization of the ipsilateral shoulder | 4 (2–6) | 2 (1–3) | 3 (2–4) | 4 (2–4) | <0.0001 | T1 vs. T2; p < 0.0001 T2 vs. T4; p = 0.018 |
| Neuropathic pain ** | 5 (9.3) | 1 (1.7) | 2 (4.9) | 2 (7.4) | 0.296 | |
| Height of hypoesthesia area around the scar, cm | ||||||
| On the breast line | 1.5 (0–7.7) {54} | 0 (0–4) {58} | 0 (0–7) {41} | 0 (0–8.5) {26} | 0.353 | |
| On the axillary line | 0 (0–3) {54} | 0 (0–5) {58} | 0 (0–5) {41} | 3 (0-5.5) {27} | 0.413 | |
| On the line tip of the scapula | 0 (0–0) {54} | 0 (0–0) {57} | 0 (0–0) {41} | 0 (0–0) {27} | 0.674 | |
| Scar length, cm | 15 (9–19) {54} | 12 (7–16) {54} | 12 (8–17) {41} | 14 (13–18) {26} | 0.047 | ns |
| Satisfaction score *** | 9 (8,9) {53} | 9 (8–10) {58} | 8 (7–10) {40} | 9 (6–10) {27} | 0.183 | |
| Postoperative complications ≥ IIIa **** | 2 (3.7) | 1 (1.7) | 4 (9.8) | 2 (7.4) | 0.247 | |
| Postoperative hospital stay, days (n) | 8 (7–13) | 9 (7–11) | 9 (6–13) | 8 (7–11) | 0.840 |
| Trajectory Group 1 OR (CI95%) | Trajectory Group 3 OR (CI95%) | Trajectory Group 4 OR (CI95%) | ||||
|---|---|---|---|---|---|---|
| Age | 0.96 | (0.91–1) | 0.99 | (0.94–1.03) | 0.98 | (0.93–1.04) |
| ASA class | ||||||
| II | 0.15 | (0.01–2.36) | 0.57 | (0.03–13.01) | 0.21 | (0.01–6.07) |
| III | 0.02 | (0.001–0.52) | 0.51 | (0.02–13.78) | 0.06 | (0.001–2.48) |
| Anxiety | 4.75 | (0.90–25.14) | 0.75 | (0.21–2.59) | 1.91 | (0.38–9.64) |
| Preoperative pain | 6.94 | (1.54–31.27) | 1.46 | (0.40–5.34) | 0.78 | (0.13–4.56) |
| At least one analgesic | 1.58 | (0.39–6.44) | 1.85 | (0.46–7.48) | 2.58 | (0.40–16.72) |
| Rib fracture | 0.86 | (0.15–4.78) | 0.13 | (0.01–1.33) | 2.51 | (0.49–12.85) |
| Transcostal suture | 0.59 | (0.14–2.54) | 1.49 | (0.39–5.73) | 0.32 | (0.05–2.08) |
| Scar length | 1.20 | (1.05–1.38) | 1.11 | (0.98–1.27) | 1.12 | (0.96–1.31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorges, P.; Michel-Cherqui, M.; Fessler, J.; Székély, B.; Sage, E.; Glorion, M.; Kennel, T.; Fischler, M.; Martinez, V.; Vallée, A.; et al. Early Postoperative Pain Trajectories after Posterolateral and Axillary Approaches to Thoracic Surgery: A Prospective Monocentric Observational Study. J. Clin. Med. 2022, 11, 5152. https://doi.org/10.3390/jcm11175152
Dorges P, Michel-Cherqui M, Fessler J, Székély B, Sage E, Glorion M, Kennel T, Fischler M, Martinez V, Vallée A, et al. Early Postoperative Pain Trajectories after Posterolateral and Axillary Approaches to Thoracic Surgery: A Prospective Monocentric Observational Study. Journal of Clinical Medicine. 2022; 11(17):5152. https://doi.org/10.3390/jcm11175152
Chicago/Turabian StyleDorges, Pascaline, Mireille Michel-Cherqui, Julien Fessler, Barbara Székély, Edouard Sage, Matthieu Glorion, Titouan Kennel, Marc Fischler, Valeria Martinez, Alexandre Vallée, and et al. 2022. "Early Postoperative Pain Trajectories after Posterolateral and Axillary Approaches to Thoracic Surgery: A Prospective Monocentric Observational Study" Journal of Clinical Medicine 11, no. 17: 5152. https://doi.org/10.3390/jcm11175152
APA StyleDorges, P., Michel-Cherqui, M., Fessler, J., Székély, B., Sage, E., Glorion, M., Kennel, T., Fischler, M., Martinez, V., Vallée, A., & Le Guen, M. (2022). Early Postoperative Pain Trajectories after Posterolateral and Axillary Approaches to Thoracic Surgery: A Prospective Monocentric Observational Study. Journal of Clinical Medicine, 11(17), 5152. https://doi.org/10.3390/jcm11175152






