Abstract
(1) Background: High-flow nasal cannula (HFNC) therapy or conventional oxygen therapy (COT) are typically applied during gastrointestinal (GI) endoscopic sedation. (2) Methods: We conducted a rigorous systematic review enrolling randomized controlled trials (RCTs) from five databases. Risk of bias was assessed using Cochrane’s RoB 2.0 tool; certainty of evidence (CoE) was assessed using GRADE framework. Meta-analysis was conducted using inverse-variance heterogeneity model and presented as relative risk (RR) with 95% confidence interval (CI). Trial sequential analysis was performed, and sensitivity analysis was conducted with Bayesian approach. (3) Results: Eight RCTs were included. Compared to COT, HFNC did not reduce the overall incidence of hypoxemia (RR 0.51; 95% CI 0.24–1.09; CoE: very low) but might reduce the incidence of hypoxemia in patients at moderate to high risk for hypoxemia (RR 0.54; 95% CI 0.31–0.96; and CoE: very low). HFNC might reduce the incidence of severe hypoxemia (RR 0.38; 95% CI 0.20–0.74; and CoE: low). HFNC might not affect the need of minor airway interventions (RR 0.31; 95% CI 0.08–1.22; and CoE: very low) and had no effect on procedure duration (CoE: very low); (4) Conclusions: During GI endoscopic sedation, HFNC might reduce the incidence of hypoxemia in patients at moderate to high risk for hypoxemia and prevent severe hypoxemia.
1. Introduction
Endoscopic procedures are common procedures performed worldwide for the diagnosis and treatment of gastrointestinal (GI) disorders. In 2019, there were a total of 22.2 million GI endoscopies performed in the United States, of which 64% were colonoscopies and 34% were esophagogastroduodenoscopies (EGD) [1]. While GI endoscopies are generally considered to be safe, patients’ willingness to receive procedures could be limited due to anxiety and peri-procedural discomfort. For that reason, administering intravenous (IV) sedatives during GI endoscopies has become a common approach for gastroenterologists in U.S. [2]. A nationwide survey in the U.S. showed that more than 98% of endoscopies were performed under sedation [3]. Although sedatives could improve patients’ satisfaction, they may have deleterious effects on hemodynamic profiles and respiratory functions. Incidence of hypoxemia during endoscopic sedation largely varies, based on the types of procedures and population. In the elder population receiving endoscopic retrograde cholangiopancreatography (ERCP), the incidence of hypoxemia could be as high as 21.4% [4]. Prolonged hypoxemia during sedation is a major risk factor of cardiac arrhythmia or myocardial infarction [5].
Conventional oxygen therapies (COT), such as low-flow nasal cannula, modified face mask, and nasopharyngeal airway, have been used to prevent hypoxemia during endoscopic sedation [6]. Nevertheless, the capability to provide a high fraction of inspired oxygen concentrations (FiO2) through those devices is limited because their low-flow rates may not meet patients’ peak inspiration flow, resulting in oxygen mixing with carbon dioxide (CO2) in the dead space. High-flow nasal cannula (HFNC) is an innovative high-flow oxygen-delivery system that can deliver humified oxygen up to 60 L per minute. HFNC not only makes higher FiO2 possible by providing a gas-flow rate which meets a patient’s peak inspiratory flow but also decreases the work of breathing by washing out CO2 in the dead space [7]. High-flow gas rates may generate small positive end-expiratory pressure (PEEP) by creating tracheal gas insufflation which prevents atelectasis [7]. HFNC has been studied in patients receiving bronchoscopy [8], awake craniotomy [9], and dental procedures [10] under IV sedation.
Several randomized controlled trials (RCTs) [11,12,13,14,15,16,17,18] had studied the use of HFNC in patients who received GI endoscopies during sedation. However, the study designs were heterogeneous, and study results were inconsistent. Four recent systematic reviews (SRs) were published to demonstrate the benefit in use of HFNC for prevention of hypoxemia in patients who received GI endoscopies [19,20,21,22]. Based on the tool of “A Measurement Tool to Assess Systematic Reviews II” (AMSTAR2) [23], these SRs were appraised as critically low or of low quality, mainly due to lack of discussing the influence of RoB on the pooled estimates (Table A1). Besides, certainty of evidence (CoE) in each outcome was not rated in these SRs. An additional RCT [18] was recently published on the same topic. Therefore, the present study aims to synthesize the updated evidence of using HFNC in patients who received GI endoscopies under IV sedation through comprehensive SR, multiple advanced methodologies in statistics, and rating the CoE.
2. Materials and Methods
2.1. Protocol and Registration
Two independent reviewers (CCL and HTL) performed a comprehensive search for relevant articles in multiple databases, including MEDLINE, Embase, Cochrane library, and Airiti library. We also searched ClinicalTrials.gov Database for unpublished or ongoing trials. No language limitation was applied. Relevant citations originating from references were eligible for additional review and selection. We conducted searches of electronic databases, both with controlled vocabulary (MeSH/EMTREE) terms and free text terms using the following keywords: (‘high flow nasal cannula’ OR ‘HFNC’ OR ‘high flow oxygen therapy’ OR ‘oxygen therapy’ OR ‘HFNO’ OR ‘high flow nasal prong’ OR ‘high flow nasal oxygenation’) AND (‘EGD’ OR ‘endoscope’ OR ‘ERCP’ OR ‘esophagogastroduodenoscopy’ OR ‘colonoscope’ OR ‘endoscopy’ OR ‘colonoscopy’ OR ‘endoscopic retrograde cholangiopancreatography’). The last search was conducted on 11 June 2022. The protocol for this SR was registered on PROSPERO (CRD42021272313) and designed according to Cochrane Handbook for Systematic Reviews of Interventions [24] and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline.
2.2. Study Selection
To avoid confounders and selection bias in non-RCTs, we only included studies that were designed as prospective RCTs. The inclusion criteria were: (1) participants who received GI endoscopies under procedural sedation, (2) participants who were randomly assigned to receive HFNC for oxygen therapy in the intervention group and receive COT (low-flow nasal cannula, nasal prong, mouthguard, or nasopharyngeal catheter) for oxygen therapy in the control group, and (3) studies which reported any of the following outcomes: incidence of hypoxemia, severe hypoxemia, hypercapnia, minor airway interventions (chin lift, jaw thrust, or insertion of nasopharyngeal airway), or duration of procedures. Studies were excluded if they were abstracts, conference articles, trial protocols, or performed in the pediatric population (age < 16 years old).
2.3. Data Extraction
Two independent reviewers (CCL and HTL) extracted the following data from selected articles: characteristics of the study (first author, year of publication, country, and sample size); characteristics of the participants (age, body mass index, and underlying medical conditions); characteristics of the procedures (types of procedures, sedation protocols, HFNC settings, COT settings); and study outcomes mentioned in Section 2.2. Incidence of hypoxemia, severe hypoxemia, or hypercapnia indicated the numbers of events when patients’ oxygen saturation by pulse oximetry (SpO2) or CO2 levels were below, or above, certain levels defined by each study. Minor airway interventions included chin lift, jaw thrust, or nasal airway insertion. If the incidences of minor airway interventions were reported separately, we extracted the incidence of the most common intervention for meta-analysis. Discrepancies of data collection were resolved in consultation with a third reviewer (TJ). We contacted primary investigators through email if additional information was required for analysis. Missing data were mentioned in the results section and excluded from data analysis.
2.4. Quality Assessment
The quality of included studies was assessed by two reviewers (CCL and HTL) independently and discrepancies were resolved in consultation with a third reviewer (TJ). The risk of bias (RoB) of studies was assessed according to Cochrane’s risk-of-bias tool for randomized trials, version 2.0 (RoB 2) [25]. Cochrane’s RoB 2 tool is structured to assess study bias in five domains. RoB judgments on each domain can be made as “low risk”, “some concerns” or “high risk”. An overall RoB judgment will be made based on the judgment of five domains.
2.5. Study Analysis
2.5.1. Meta-Analysis with Inverse Variance Heterogeneity Model
In contrast to the Mantel–Haenszel (M–H) model utilized by the previous meta-analysis [19], we conducted a meta-analysis using Microsoft Excel (Microsoft, Redmont, WA, USA) add-in MetaXL 5.3 (EpiGear International, Sunrise Beach, Australia) with the inverse variance heterogeneity (IVhet) model [26]. IVhet model, compared to random-effects (RE) model, might retain a correct coverage probability and lower observed variance than the RE model estimator, regardless of heterogeneity [26,27]. Risk ratio (RR) with 95% confidence level (CI) was reported for dichotomous outcome, and weighted mean difference (WMD) with 95% CI was reported for continuous outcome. For the study conducted by Teng et al. [12], we combined the nasal cannula group and mandibular advancement bite block group into one single control group, according to the Cochrane handbook, version 5.1 Chapter 16.5 [28]. A two-sided p value < 0.05 was considered statistically significant. Heterogeneity among studies was assessed using the I2 tests. An I2 higher than 50% represented substantial heterogeneity. Predefined subgroup analysis was conducted to compare studies which enrolled patients at low risk for hypoxemia (e.g., American Society of Anesthesiologists (ASA) class I–II) versus those at moderate to high risk for hypoxemia due to patients’ health conditions (e.g., obesity, higher ASA score, and history of sleep apnea) or requirements of complex procedures (e.g., ERCP). In addition, we performed subgroup analysis based on RoB among studies (i.e., studies with high overall RoB versus those with low or some concerns of overall RoB).
2.5.2. Sensitive Analysis
To better handle the zero events, we conducted a sensitive analysis with a Bayesian approach by utilizing the interactive web-based tool MetaInsight (https://crsu.shinyapps.io/metainsightc accessed on 16 March 2022). MetaInsight [29], a free and recently developed software created based on the existing netmeta and shiny packages for R, allows users to conduct Bayesian meta-analysis without the requirement of advanced statistical programming expertise. RR with 95% credible interval (CrI) was reported for dichotomous outcome, and WMD with 95% CrI was reported for continuous outcome. Bayesian RE meta-analysis naturally allows the presence of imprecision in the estimated between-study variance and carries better compatibility with studies including zero events [30]. Compared to other Frequentist approaches, a Bayesian approach likely performs better in case of a small number of studies (N < 5) or the presence of zero events [31].
2.5.3. Trial Sequential Analysis
To avoid the risk of type I and type II errors resulting from sequential testing on a constant significance level, we conducted a trial sequential analysis (TSA) using TSA version 0.9.5.10 beta (Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark). Types I and II errors were set at 5 and 20%, respectively. A RE model with Biggerstaff–Tweedie (BT) method was used. For zero events, a constant addition of 0.001 was chosen in the software. O’Brien–Fleming α-spending monitoring boundaries were applied for hypothesis testing.
Results were considered true positive if the Z curve crossed the O’Brien–Fleming monitoring boundaries and considered true negative if the Z curve entered the futility area. An underpower was detected if total sample size of included studies did not achieve the required information size (RIS). The RIS was calculated considering the proportion of investigational and control events and the anticipated heterogeneity variance of the meta-analysis. The incidence of intervention and control arms was filled in the “overall events/total cases” of the enrolled studies.
2.6. CoE
The CoE of outcomes was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [32]. The overall CoE was judged by five downgrading domains for the SR of RCTs. We downgraded CoE in the domain of RoB based on the proportion of RCTs with some-concern and/or high overall RoB. In the domain of inconsistency, we downgraded one or two levels if I2 ratio was more than 50% or 90%, respectively. If inappropriate combination in population/intervention/comparator/outcome was noted, we downgraded level(s) in the domain of indirectness. Apart from wide ranges of interval, probability of false positive or negative and insufficient sample size were evaluated in the domain of imprecision. We considered the major asymmetry of Doi plot as probability of publication bias. Disagreements of GRADE assessments were discussed and resolved in the groups.
3. Results
3.1. Summary of the Characteristics of Studies
Figure 1 showed the study selection process. Overall, we identified 2593 articles from Medline, Embase, Cochrane library, Airiti library and ClinicalTrials.gov. After 234 duplicate articles were removed, we screened 2359 articles. A total of 13 full-text articles which met the inclusion criteria were assessed for eligibility. Five articles were not enrolled since they were study protocols, conference abstracts, or study performed in pediatric population. Finally, we included eight RCTs [11,12,13,14,15,16,17,18] involving a total of 3236 patients in the analysis.
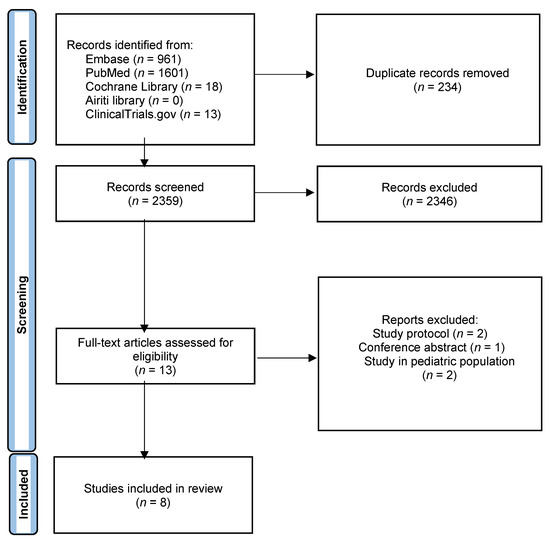
Figure 1.
Study flow diagram.
Table 1 showed study characteristics. Two studies included patients who received EGD and were at low risk for hypoxemia. Six studies included patients who received EGD, EGD and interventions, ERCP, or colonoscopy and were at moderate to high risk for hypoxemia. Two studies received academic funding and four studies received industrial as well as academic funding. Participants’ demographic profiles were heterogenous across studies. Two studies did not specify sedation protocols and allowed physicians to administer sedatives to reach targeted level of sedation at their discretion. In terms of the initial choice of sedatives: one study used midazolam plus alfentanil IV push; one study used propofol with targeted controlled infusion (TCI); and other studies used propofol with or without fentanyl IV push. In terms of choice of sedatives to maintain targeted levels of sedation: two studies used propofol IV push as needed; two studies used propofol IV continuous infusion; one study used either propofol IV push or continuous infusion; and one study used propofol TCI. Depth of sedation ranged from moderate sedation to general anesthesia. All but one study [12] reported the significant difference of total dose of sedatives or analgesics between HFNC group and COT group. Most studies used Optiflow as their HFNC devices. Regarding HFNC settings, three studies [13,16,18] matched FiO2 in HFNC groups to those in control groups, while other studies used 100% of FiO2. Flow of oxygen in HFNC groups ranged from 20 to 70 L per minute. In contrast, flow of oxygen in COT groups ranged from 2–6 L per minute.

Table 1.
Study characteristics.
3.2. RoB Accessment
Figure 2 showed the study bias of included studies. Two studies did not detail the methods of allocation concealment. Although blinding of participants or physicians cannot be avoided in all studies due to different appearances of oxygen devices, no deviations from the intended interventions were observed. Missing outcome data was not observed during the short duration of study period. Four studies did not detail the methods of blinding to outcome assessors. Only one study did not detail the pre-specified analysis plan. After appraisal, two studies were deemed high overall RoB due to some concerns of bias in two different domains. Only three RCTs fit the criteria of low overall RoB.
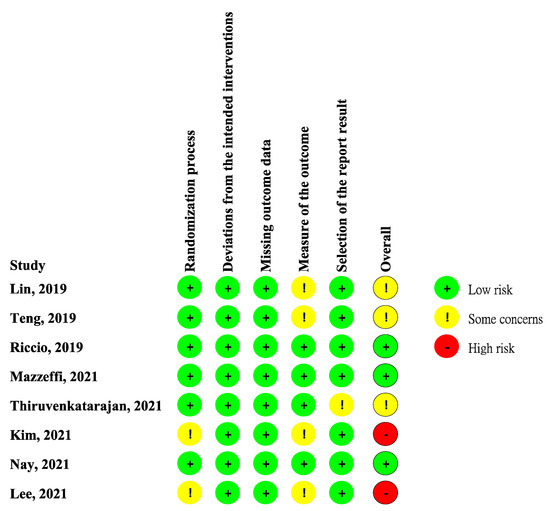
Figure 2.
Risk of bias. Studies included Lin et al. [11], Teng et al. [12], Riccio et al. [13], Mazzeffi et al. [14], Thiruvenkataragan et al. [15], Kim et al. [17], Nay et al. [16], Lee et al. [18].
3.3. Study Outcome
3.3.1. Incidence of Hypoxemia
All eight studies reported incidence of hypoxemia. Overall, meta-analysis conducted with IVhet model showed HFNC did not reduce the incidence of hypoxemia in comparison with COT (RR 0.51; 95% CI 0.24–1.09; Figure 3a). Significant heterogeneities among studies were observed (I2 = 71%). TSA depicted a fluctuated Z-curve, and the end of Z-curve lies on the O’Brien–Fleming monitoring boundaries, indicating the possibility of an uncertain result (Figure 3b). Besides, TSA also showed that studies were underpowered since the total sample size of included studies did not achieve RIS (Figure 3b). Doi plot showed major asymmetry (Figure A1a), and CoE was rated as very low which was downgraded in the domains of RoB, inconsistency, imprecision, and publication bias (Table 2). Similarly, when considering the three RCTs with low overall RoB, non-significant results were observed (RR 0.56; 95% CI 0.28–1.11; I2 = 77%; Figure A2). Although the end of the Z-curve crossed the O’Brien–Fleming α-spending monitoring boundaries, it did not reach the line of RIS which indicated the existence of uncertainty (Figure A3).
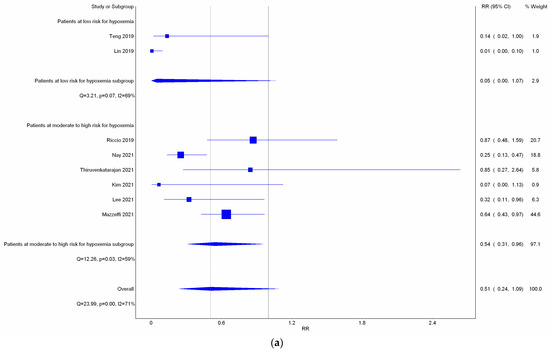
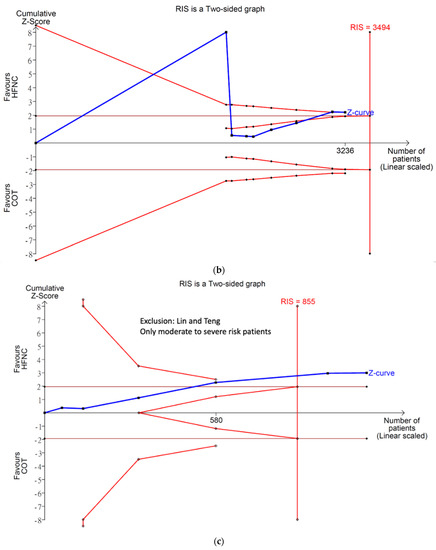
Figure 3.
Incidence of hypoxemia. (a) Meta-analysis with IVhet model; (b) trial sequential analysis; and (c) trial sequential analysis: Patients with moderate to high risk for hypoxemia. Studies in Figure 3a include Riccio et al. [3], Nay et al. [16], Thiruyenkatarajan et al. [15], Kim et al. [17], Lee et al. [18], Mazzeffi et al. [14].

Table 2.
Certainty of evidence: HFNC compared to COT for patients receiving gastrointestinal endoscopies.
Subgroup analysis (Figure 3a) showed HFNC might not reduce the incidence of hypoxemia in patients at low risk for hypoxemia with a very wide range of interval (RR 0.05; 95% CI 0.00–1.07; I2 = 69%). However, HFNC may reduce the incidence of hypoxemia in patients at moderate to high risk for hypoxemia (RR 0.54; 95% CI 0.31–0.96; and I2 = 59%). TSA of studies which only included patients at moderate to high risk for hypoxemia yielded a true positive result since the Z-line crossed the O’Brien–Fleming monitoring boundaries and exceeded the line of RIS (Figure 3c). CoE for incidence of hypoxemia in patients at moderate to high risk for hypoxemia was rated as very low based on downgrading in the domains of RoB, inconsistency, and publication bias (Table 2).
Bayesian meta-analysis (Figure A4), in contrast to the analysis conducted with IVhet model, showed that HFNC might reduce the incidence of hypoxemia in comparison with COT (RR 0.155; 95% CrI 0.014–0.862). Subgroup analysis showed HFNC may not reduce the incidence of hypoxemia in patients at low risk for hypoxemia (RR 0.00569; 95% CrI 0.00–1.04) but may reduce the incidence of hypoxemia for patients at moderate to high risk for hypoxemia (RR 0.404; 95% CrI 0.12–0.971).
3.3.2. Incidence of Severe Hypoxemia
Only four studies reported the incidence of severe hypoxemia. Severe hypoxemia was defined as SpO2 < 80% in two studies, <85% in one study, and <75% in one study. Meta-analysis conducted with IVhet model revealed HFNC might reduce the incidence of severe hypoxemia (RR 0.38; 95% CI 0.20–0.74; Figure 4a) in comparison with COT. TSA of all four studies showed a true positive effect with sufficient sample sizes but fluctuated curve (Figure 4b). CoE was rated as low because the domains of RoB and publication bias (Figure A1b) were downgraded (Table 2). Subgroup analysis showed HFNC may reduce the incidence of hypoxemia for patients at moderate to high risk for hypoxemia (RR 0.42; 95% CI 0.21–0.83). However, TSA of studies including patients at moderate to high risk for hypoxemia may be false positive and underpowered (Figure 4c).
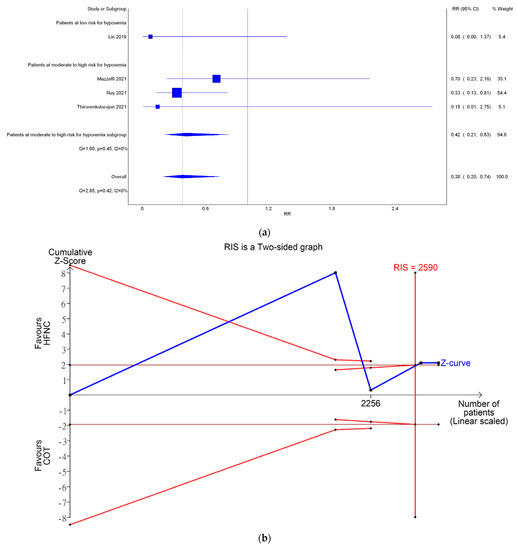
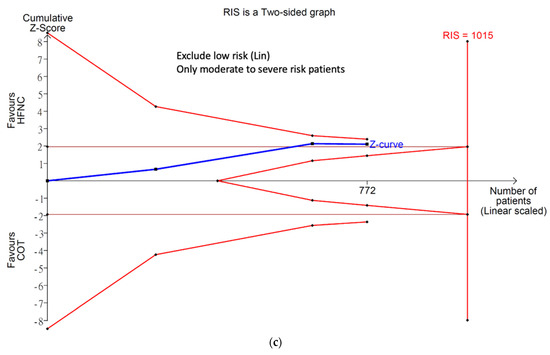
Figure 4.
Incidence of severe hypoxemia. (a) Meta-analysis with IVhet model; (b) trial sequential analysis; and (c) trial sequential analysis: patients with moderate to high risk for hypoxemia. Studies in Figure 4a include: Mazzeffi et al. [14], Nay et al. [16], and Thiruvenkatarajan et al. [15].
Bayesian meta-analysis (Figure A5) also showed HFNC might reduce the incidence of hypoxemia in comparison with COT (RR 0.177; 95% CrI 0.014–0.806). However, subgroup analysis showed HFNC might not reduce the incidence of severe hypoxemia for patients at moderate to high risk for hypoxemia (RR 0.3; 95% CrI 0.0493–1.12).
3.3.3. Incidence of Hypercapnia
Mazzeffi et al. and Thiruvenkatarajan et al. assessed incidence of hypercapnia with transcutaneous blood carbon dioxide (PtCO2) measuring device and used PtCO2 > 20 mmHg as the cutoff value to diagnose hypercapnia. Meta-analysis depicted no statistical difference (RR 1.24; 95% CI 0.97–1.58; I2 = 0%; Figure A6). The end of Z-curve in TSA did not reach futility area, indicating the possibility of false negative (Figure A7). Reasonably, the Z-curve did not cross line of RIS in a pooling of just two RCTs, so more studies are warranted to give a more solid conclusion in this outcome. CoE was rated as low because the domain of imprecision was downgraded by two levels (Table 2).
3.3.4. Need for Minor Airway Interventions
Six studies reported the need for minor airway interventions (e.g., jaw thrust, chin lift, or insertion of nasal airway). Although the need for minor airway interventions was lower in the HFNC group in comparison with COT (RR 0.31; Figure 5a), a very wide range crossing the non-significant line was noted (95% CI (0.08–1.22)). High heterogeneity was observed (I2 = 91%). Doi plot depicted minor asymmetry (Figure A1c). CoE was rated as very low (Table 2). In contrast, TSA depicted an absolute benefit of HFNC from the first study, so the O’Brien–Fleming monitoring boundaries and the line of RIS were not renderable (Figure 5b). Bayesian meta-analysis (Figure A8) showed that HFNC reduced the need for minor airway interventions in comparison with COT (RR 0.178; 95% CrI 0.0256–0.919).
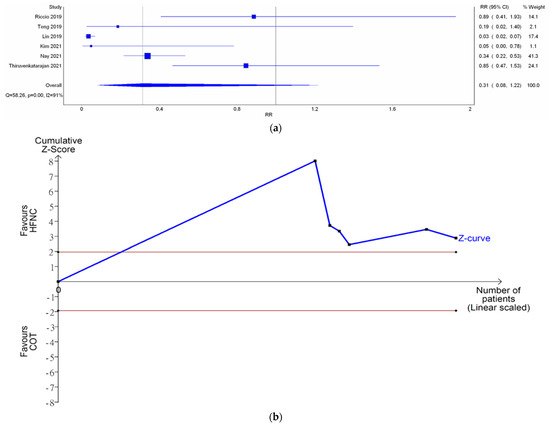
Figure 5.
Need for minor airway interventions. (a) Meta-analysis with IVhet model; and (b) trial sequential analysis. Riccio et al. [13], Teng et al. [12], Lin et al. [11], Kim et al. [17], Nay et al. [16], Thiruvenkatarajan et al. [15].
3.3.5. Duration of Procedure
There was no significant difference in the procedure time between HFNC group and COT group (WMD 0.12 min; 95% CI −0.04 to 0.28; I2 = 0%; Figure A9). TSA showed the pooled estimates may be underpowered and existed the probability of false negative (Figure A10). Besides, major asymmetry of Doi plot was observed (Figure A1d). CoE was rated as very low (Table 2). Bayesian meta-analysis also showed there was no significant difference in mean difference (MD) of procedure time between the two groups (MD 0.173 min; 95% CrI −0.617 to 1.63; Figure A11).
4. Discussion
The present analysis investigated the clinical efficacy of HFNC therapy in patients who received IV sedation during GI endoscopies. Overall, HFNC therapy did not reduce the incidence of hypoxemia. However, HFNC therapy might reduce the incidence of hypoxemia in patients at moderate to high risk for hypoxemia and overall incidence of severe hypoxemia. The evidence was insufficient to suggest the impact of HFNC therapy on the incidence of hypercapnia. HFNC therapy might reduce the incidence of minor airway interventions. The duration of endoscopic procedures might not be different between the two groups.
In contrast to previous SRs, our analysis showed HFNC did not reduce the overall incidence of hypoxemia [19,20,21]. This phenomenon could be explained by the different statistical models used by each SR, especially when the included studies had heterogenous study designs or zero events (i.e., zero incidence in one or both study group) [33]. In our study, we utilized three different advanced statistical methodologies to compare the analysis results and minimize study bias. IVhet model, compared to the traditional model of meta-analysis, has been reported to minimize the possibility of under- or over-estimating the heterogeneity compared to traditional model of meta-analysis with sparse studies [26]. TSA are warranted for containment of the pooled estimates given the small numbers of studies. Due to the presence of zero events in some studies of HFNC group, sensitivity analysis using Bayesian approach was also used for duplicate confirmation [33,34]. Overall, we believe the current evidence was insufficient to make strong recommendations for the use of HFNC therapy routinely in cases who received GI endoscopies on IV sedatives. TSA in our study further revealed the results in most of the endpoints were unpowered, which highlighted the importance of including more studies to confirm the therapeutic effects. The discrepant pooled estimates between IVhet model and Bayesian framework could be explained by the applications of different methodologies and statistical models in the analysis, which further explained the uncertain benefits of HFNC on this issue.
It is worth mentioning that in patients who were at moderate to high risk for hypoxemia, the subgroup analysis conducted by three different methodologies universally suggested HFNC may be beneficial to prevent hypoxemia. This finding is more consistent with our real-world practice. Sedation for GI endoscopies, even for simple EGD, should be considered high-risk anesthetics for some cases [35]. Endoscopies, especially ERCP, frequently require deeper sedation to suppress gag, cough, and laryngospasm reflex. Sedatives that are administered to achieve targeted levels of sedation potentially lead to airway collapse, hypoventilation, or deleterious effect on hemodynamic profiles, especially in cases of obesity, higher ASA score, or history of sleep apnea. Upper endoscopies, placed in aerodigestive tracts, often causes partial obstruction of airway since their diameters frequently exceeds the cross-sectional area of oropharynx [36]. HFNC may be able to mitigate complications, such as hypoxemia, during GI endoscopic sedation in patients with higher anesthetic risks. The CoE based on the methodology of GRADE was only rated as very low, which indicated there was high likelihood that the actual effect would be substantially different from our study results. Future large prospective studies are needed to delineate the types of population that will benefit most from HFNC therapy in this setting.
The incidence of severe hypoxemia, typically defined as SpO2 less than 80%, may be more clinically relevant than the incidence of hypoxemia when comparing the efficacy of different oxygen therapies. Our analysis suggested that HFNC therapy might be beneficial in preventing severe hypoxemia in comparison with COT based on three statistical methodologies. The primary physiologic benefit of HFNC to prevent severe hypoxemia is the capability to maintain high FiO2. During anesthetic induction, a recent published meta-analysis of 14 studies showed HFNC was superior to COT in improving oxygenation and prolonging safe apnea time [37]. However, it is worth mentioning high gas flow may reduce the oxygen content by pulling nitrogen content from environmental air. The high gas flow passing through a constricted area could decrease the downstream pressure leading to a vacuum effect, a phenomenon also observed in venturi mask [38]. Wetsch et al. [39] performed a simulation study which used HFNC for apnea oxygenation. Interestingly, highest oxygen content was reached at a flow of 10 to 20 L per minutes. Higher flow resulted in a slight decrease in oxygen content. Further studies are needed to establish the gas flow of HFNC that can achieve optimal oxygen delivery in the setting of apnea oxygenation. HFNC could also provide PEEP at roughly 1 cm H2O for every 10 L per minute of gas flow by causing tracheal gas insufflation [40], but the effect may be reduced during GI endoscopies when patients open their mouths. Given current existing literature, the evidence was limited since only a few studies reported this outcome. In addition, the CoE was rated as low in GRADE methodology. More RCTs are warranted to confirm the benefit of HFNC on this endpoint.
The effect of HFNC therapy on ventilation is complex. Most studies suggested HFNC improved hypercapnia and work of breathing by washing out CO2 from dead space and improving thoracoabdominal synchrony [41,42]. On the other hand, unnecessary oxygen administration from HFNC, especially in patients with chronic obstructive pulmonary disease, could lead to hypercapnia due to the change of dead space and modulation of Haldane effect [43]. In our analysis, only two studies reported incidence of hypercapnia using PtCO2. While both studies showed a non-statistical increase in the incidence of hypercapnia in the HFNC group, it was too early to draw conclusions of HFNC’s effect on ventilation during endoscopic sedation. Our pooled estimates in three methodologies and the CoE in GRADE supported the above-mentioned conclusion. Since hypoventilation from sedative use is a major concern, further studies are needed to address the effect of HFNC therapy on CO2 change during sedation. End tidal CO2, while widely available, might not be the ideal tool to assess ventilation in this clinical setting since high-flow gas would severely dilute exhaled CO2, leading to a falsely low reading [44].
Minor airway interventions are typically used to correct partial airway closure resulting from sedation. Increased needs for minor airway interventions can lead to interruptions of endoscopies and prolong the duration of procedures. In our analysis, the meta-analysis conducted by IVhent model and Bayesian model showed discordant results. This was likely due to heterogeneity of study designs and different methodologies in handling zero events. The need for minor airway interventions, to a certain degree, is reflective of the severity of hypoventilation. Since most included studies did not apply CO2 monitoring devices, the need for minor interventions was mostly determined by low SpO2 level, a late feature of hypoventilation. We believe future evidence is needed to assess the impact of HFNC therapy on the incidence of minor airway interventions.
There was no overall difference in the pooled mean duration of endoscopic procedure between HFNC group and COT group. Frequent need for minor airway interventions could possibly prolong the duration of procedure. However, the average numbers of minor airway interventions needed for patients in each study were unclear and the incidence of minor airway interventions varied among included studies.
Strengths of this review includes a comprehensive literature search, pre-registered protocols at PROSPERO, compliance to the PRISMA guideline for systematic review, the use of both Frequentist and Bayesian approaches for meta-analyses, advanced application of TSA, and using GRADE methodology to assess the CoE. Limitation of this review includes the heterogeneity of study designs and small number of included studies which resulted in the uncertain conclusion. In addition, meta-regression cannot be performed to elucidate the relationship among heterogeneities due to the small number of enrolled RCTs.
5. Conclusions
During GI endoscopies, HFNC may not reduce the overall incidence of hypoxemia. However, HFNC may be beneficial in preventing hypoxemia in patients who were at moderate to high risk for hypoxemia and severe hypoxemia. HFNC might not affect the need of minor airway interventions and had no effect on procedure duration. Future large RCTs are needed to elucidate the population who will benefit most from HFNC therapy while balancing the costs of devices.
Author Contributions
Conceptualization, C.C.L. and T.R.J.; methodology, P.C.L.; software, Y.T.H.; validation, C.C.L., T.R.J. and H.-T.L.; formal analysis, C.C.L.; investigation, Y.T.H.; resources, H.-T.L.; data curation, P.C.L. and Y.T.H.; writing—original draft preparation, C.C.L.; writing—review and editing, Y.T.H.; visualization, P.C.L.; supervision, Y.T.H.; project administration, T.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Appraisal of currently available systematic review by AMSTAR2.
Table A1.
Appraisal of currently available systematic review by AMSTAR2.
| No. | Items | References | |||
|---|---|---|---|---|---|
| Hung et al. (2022) | Zhang et al. (2022) | Gu et al. (2022) | Gu et al. (2022) | ||
| 1. | Coincident PICO in this S/R. | Yes | Yes | Yes | Yes |
| 2. | Protocolized processing of S/R. | Partial Yes | Partial Yes | Yes | Yes |
| 3. | Explain the selection of study type. | No | No | No | No |
| 4. | Comprehensive search strategy. | Partial Yes | Partial Yes | Yes | Yes |
| 5. | Double check the selected studies. | Yes | Yes | Partial Yes | Partial Yes |
| 6. | Double check data extraction. | Yes | Yes | Yes | Yes |
| 7. | List and explanation of excluded studies. | Yes | Yes | Yes | Yes |
| 8. | Describe the details of included studies. | Partial Yes | Partial Yes | Yes | Yes |
| 9. | Satisfactory technique for assessing the RoB. | Yes | Yes | Yes | Yes |
| 10. | Report on the sources of funding in the enrolled studies. | No | No | No | No |
| 11. | Appropriate methods for statistical combination. | Yes | Yes | Yes | Yes |
| 12. | Assess the potential impact of RoB in studies on the M/A results | No | No | No | No |
| 13. | Discuss the influence of RoB on the M/A results. | No | No | No | No |
| 14. | Explain or discuss the heterogeneity. | Yes | Yes | Yes | Yes |
| 15. | Analyze and discuss publication bias. | N/N | N/N | N/N | N/N |
| 16. | Report COI to conduct this S/R M/A. | Yes | Yes | Yes | Yes |
| Overall score | Low | Low | Critically low | Low | |
Abbreviations: AMSTAR = A Measurement Tool to Assess Systematic Reviews; PICO = population, intervention, comparison, and outcome; S/R = systemic review; M/A = meta-analysis; N/N = not necessary; RoB = risk of bias; and COI = conflict of interest. Boldface of the words in the domains indicates the critical domain of AMSTAR2. Critically low is rated when a S/R has more than one flaw in critical domain, which may not provide an accurate and comprehensive summary in this topic. Hung et al. [19], Zhang et al. [21], Gu et al. [22].
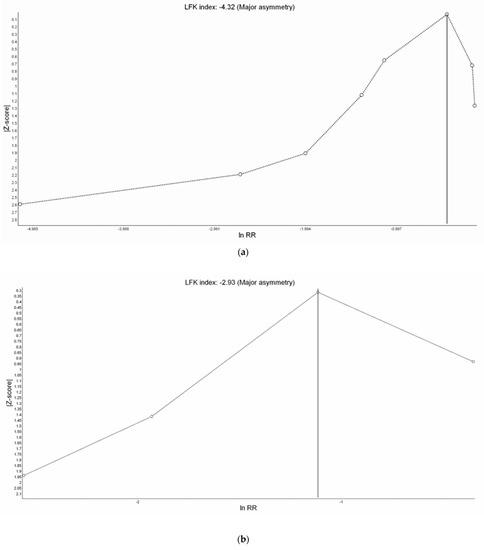
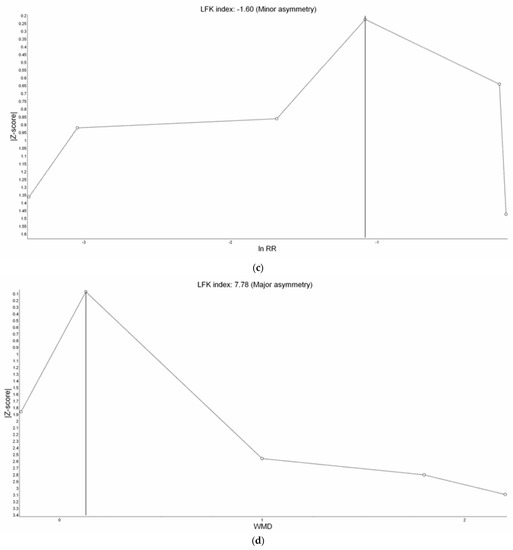
Figure A1.
Doi Plot of study outcomes. (a) Incidence of hypoxemia, (b) incidence of severe hypoxemia, (c) need for minor airway interventions, and (d) duration of procedure.
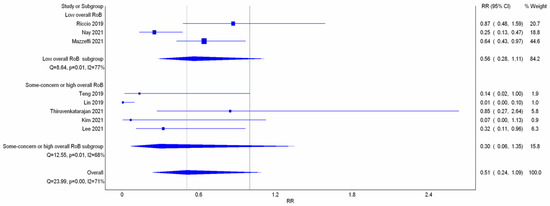
Figure A2.
Meta-analysis with IVhet model: incidence of hypoxemia (subgroup analysis with overall risk of bias). Studies included: Riccio et al. [13], Nay et al. [16], Mazzeffi et al. [14], Teng et al. [12], Lin et al. [11], Thiruvenkatarajan et al. [15], Kim et al. [17], Lee et al. [18].
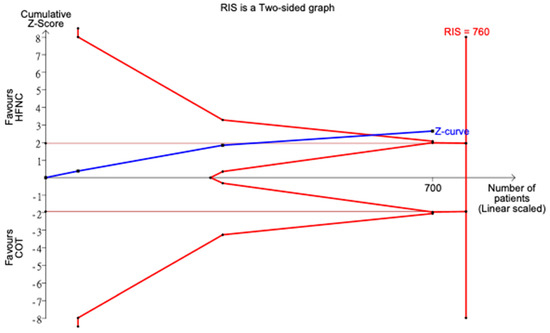
Figure A3.
Trial sequential analysis: incidence of hypoxemia (studies with overall low risk of bias).
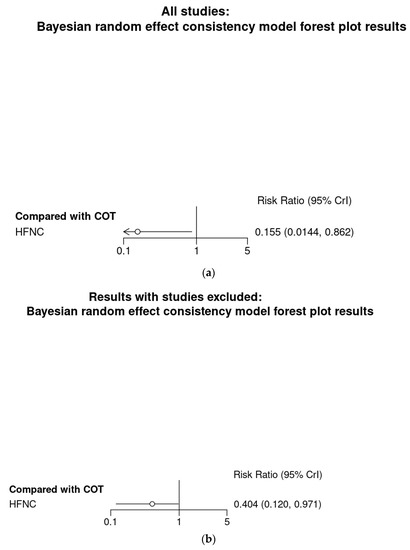
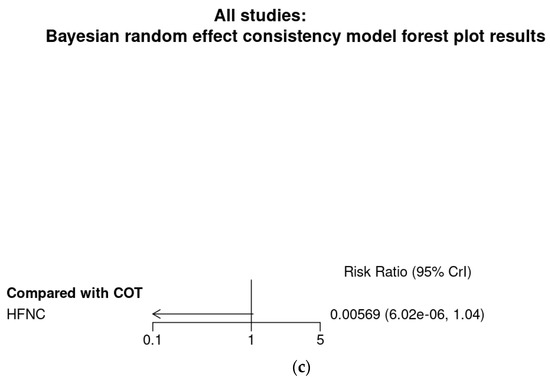
Figure A4.
Bayesian random effect consistency model Outcome: Hypoxemia. (a) All eight studies. (b) Six studies with patients at moderate to high risk for hypoxemia. (c) Two studies (Lin et al. [11]) and Teng et al. [12]) with patients at low risk for hypoxemia.
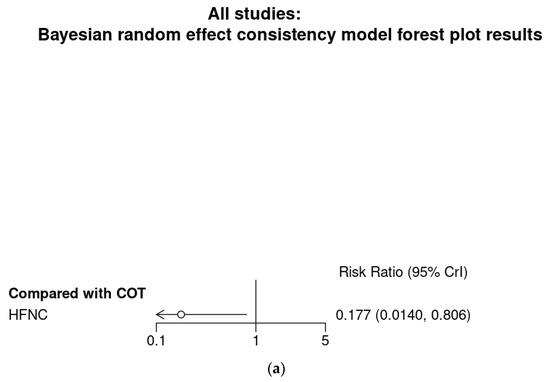
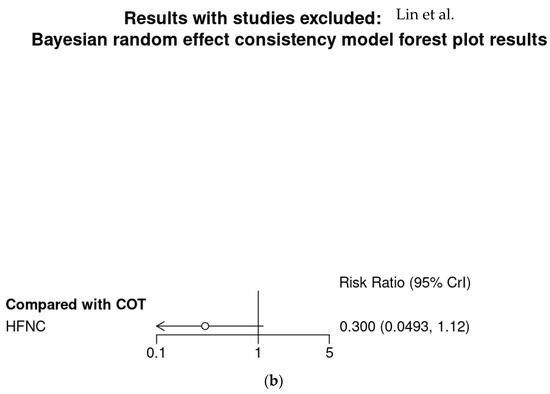
Figure A5.
Bayesian random effect consistency model Outcome: Severe hypoxemia. (a) All four studies. (b) Three studies with patients at moderate to high risk for hypoxemia.
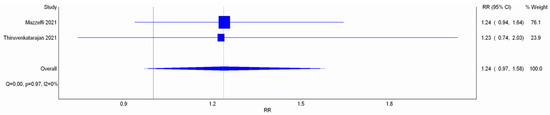
Figure A6.
Meta-analysis with IVhet model: incidence of hypercapnia. Studies included: Mazzeffi et al. [14] Thiruvenkatarajan et al. [15].
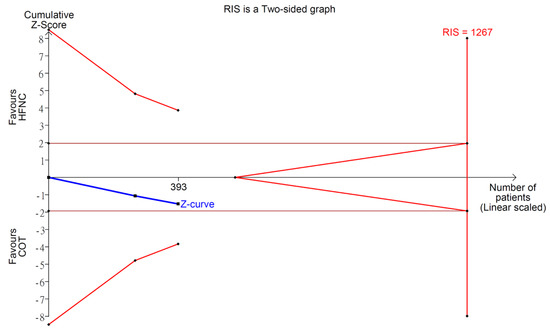
Figure A7.
Trial sequential analysis: incidence of hypercapnia.
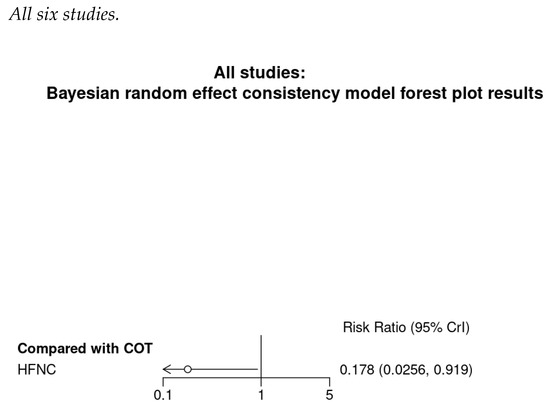
Figure A8.
Bayesian random effect consistency model Outcome: Need for minor airway interventions.
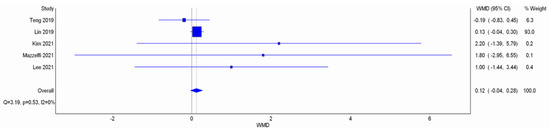
Figure A9.
Meta-analysis with IVhet model: duration of procedure. Studies included: Teng et al. [12], Lin et al. [11], Kim et al. [17], Lee et al. [18].
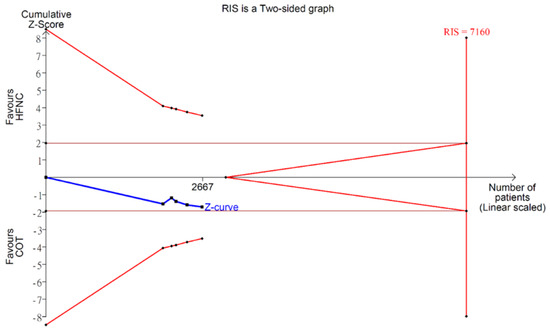
Figure A10.
Trial sequential analysis: duration of procedure.
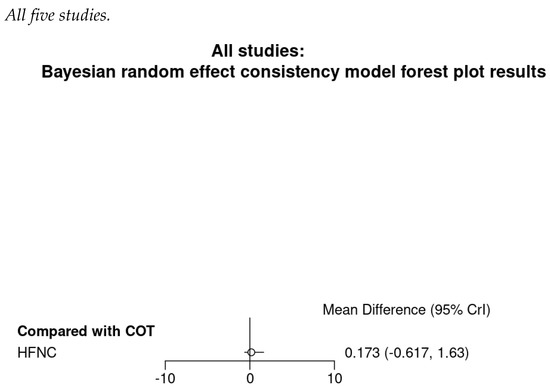
Figure A11.
Bayesian random effect consistency model Outcome: Duration of procedure.
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Early, D.S.; Lightdale, J.R.; Vargo, J.J.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Evans, J.A.; Fisher, D.A.; Fonkalsrud, L.; Hwang, J.H. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2018, 87, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.B.; Wecsler, J.S.; Gaetano, J.N.; Benson, A.A.; Miller, K.M.; Durkalski, V.; Aisenberg, J. Endoscopic sedation in the United States: Results from a nationwide survey. Am. J. Gastroenterol. 2006, 101, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Fisher, A.; Thomson, A. Cardiopulmonary complications of ERCP in older patients. Gastrointest. Endosc. 2006, 63, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, M.A.; Lopez, A.R.; Dumot, J.A.; Vargo, J.J. Risk factors for hypoxemia during ambulatory gastrointestinal endoscopy in ASA I–II patients. Dig. Dis. Sci. 2009, 54, 1035. [Google Scholar] [CrossRef]
- Goudra, B.; Gouda, G.; Singh, P.M. Recent Developments in Devices Used for Gastrointestinal Endoscopy Sedation. Clin. Endosc. 2021, 54, 182. [Google Scholar] [CrossRef]
- Drake, M.G. High-flow nasal cannula oxygen in adults: An evidence-based assessment. Ann. Am. Thorac. Soc. 2018, 15, 145–155. [Google Scholar] [CrossRef]
- Su, C.-L.; Chiang, L.-L.; Tam, K.-W.; Chen, T.-T.; Hu, M.-C. High-flow nasal cannula for reducing hypoxemic events in patients undergoing bronchoscopy: A systematic review and meta-analysis of randomized trials. PLoS ONE 2021, 16, e0260716. [Google Scholar] [CrossRef]
- Yi, P.; Li, Q.; Yang, Z.; Cao, L.; Hu, X.; Gu, H. High-flow nasal cannula improves clinical efficacy of airway management in patients undergoing awake craniotomy. BMC Anesthesiol. 2020, 20, 156. [Google Scholar] [CrossRef]
- Higuchi, H.; Takaya-Ishida, K.; Miyake, S.; Fujimoto, M.; Nishioka, Y.; Maeda, S.; Miyawaki, T. Comparison of Oxygen Saturation between Nasal High-Flow Oxygen and Conventional Nasal Cannula in Obese Patients Undergoing Dental Procedures with Deep Sedation: A Randomized Crossover Trial. J. Oral Maxillofac. Surg. 2021, 79, 1842–1850. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Li, L.; Wei, M.; Zhao, B.; Wang, X.; Pan, Z.; Tian, J.; Yu, W.; Su, D. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: A randomized multicenter clinical trial. Gastrointest. Endosc. 2019, 90, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.-N.; Ting, C.K.; Wang, Y.-T.; Hou, M.-C.; Chang, W.-K.; Tsou, M.-Y.; Chiang, H.; Lin, C.-L. High-flow nasal cannula and mandibular advancement bite block decrease hypoxic events during sedative esophagogastroduodenoscopy: A randomized clinical trial. BioMed Res. Int. 2019, 2019, 4206795. [Google Scholar] [CrossRef] [PubMed]
- Riccio, C.A.; Sarmiento, S.; Minhajuddin, A.; Nasir, D.; Fox, A.A. High-flow versus standard nasal cannula in morbidly obese patients during colonoscopy: A prospective, randomized clinical trial. J. Clin. Anesth. 2019, 54, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.A.; Petrick, K.M.; Magder, L.; Greenwald, B.D.; Darwin, P.; Goldberg, E.M.; Bigeleisen, P.; Chow, J.H.; Anders, M.; Boyd, C.M. High-flow nasal cannula oxygen in patients having anesthesia for advanced esophagogastroduodenoscopy: HIFLOW-ENDO, a randomized clinical trial. Anesth. Analg. 2021, 132, 743–751. [Google Scholar] [CrossRef]
- Thiruvenkatarajan, V.; Dharmalingam, A.; Arenas, G.; Wahba, M.; Steiner, R.; Kadam, V.R.; Tran, A.; Currie, J.; Van Wijk, R.; Quail, A. High-flow nasal cannula versus standard oxygen therapy assisting sedation during endoscopic retrograde cholangiopancreatography in high risk cases (OTHER): Study protocol of a randomised multicentric trial. Trials 2020, 21, 444. [Google Scholar] [CrossRef]
- Nay, M.-A.; Fromont, L.; Eugene, A.; Marcueyz, J.-L.; Mfam, W.-S.; Baert, O.; Remerand, F.; Ravry, C.; Auvet, A.; Boulain, T. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: A multicentre randomised controlled trial (ODEPHI trial). Br. J. Anaesth. 2021, 27, 133–142. [Google Scholar] [CrossRef]
- Kim, S.H.; Bang, S.; Lee, K.-Y.; Park, S.W.; Park, J.Y.; Lee, H.S.; Oh, H.; Oh, Y.J. Comparison of high flow nasal oxygen and conventional nasal cannula during gastrointestinal endoscopic sedation in the prone position: A randomized trial. Can. J. Anesth./J. Can. D’anesthésie 2021, 68, 460–466. [Google Scholar] [CrossRef]
- Lee, M.-J.; Cha, B.; Park, J.-S.; Kim, J.S.; Cho, S.Y.; Han, J.-H.; Park, M.H.; Yang, C.; Jeong, S. Impact of High-Flow Nasal Cannula Oxygenation on the Prevention of Hypoxia During Endoscopic Retrograde Cholangiopancreatography in Elderly Patients: A Randomized Clinical Trial. Dig. Dis. Sci. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Hung, K.-C.; Chang, Y.-J.; Chen, I.-W.; Soong, T.-C.; Ho, C.-N.; Hsing, C.-H.; Chu, C.-C.; Chen, J.-Y.; Sun, C.-K. Efficacy of high flow nasal oxygenation against hypoxemia in sedated patients receiving gastrointestinal endoscopic procedures: A systematic review and meta-analysis. J. Clin. Anesth. 2022, 77, 110651. [Google Scholar] [CrossRef]
- Doulberis, M.; Sampsonas, F.; Papaefthymiou, A.; Karamouzos, V.; Lagadinou, M.; Karampitsakos, T.; Stratakos, G.; Kuntzen, T.; Tzouvelekis, A. High-flow versus conventional nasal cannula oxygen supplementation therapy and risk of hypoxia in gastrointestinal endoscopies: A systematic review and meta-analysis. Expert Rev. Respir Med. 2022, 16, 323–332. [Google Scholar] [CrossRef]
- Zhang, Y.X.; He, X.X.; Chen, Y.P.; Yang, S. The effectiveness of high-flow nasal cannula during sedated digestive endoscopy: A systematic review and meta-analysis. Eur J. Med. Res. 2022, 27, 30. [Google Scholar] [CrossRef]
- Gu, W.J.; Wang, H.T.; Huang, J.; Pei, J.P.; Nishiyama, K.; Abe, M.; Zhao, Z.M.; Zhang, C.D. High flow nasal oxygen versus conventional oxygen therapy in gastrointestinal endoscopy with conscious sedation: A systematic review and meta-analysis with trial sequential analysis. Dig. Endosc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Doi, S.A.; Barendregt, J.J.; Khan, S.; Thalib, L.; Williams, G.M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp. Clin. Trials 2015, 45, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, S.A.; Furuya-Kanamori, L. Selecting the best meta-analytic estimator for evidence-based practice: A simulation study. JBI Evid. Implement. 2020, 18, 86–94. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. How to include multiple groups from one study. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: Amsterdam, The Netherlands, 2011; Volume 5. [Google Scholar]
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.; Higgins, J.; Altman, D. Cochrane Statistical Methods Group. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Seide, S.E.; Röver, C.; Friede, T. Likelihood-based random-effects meta-analysis with few studies: Empirical and simulation studies. BMC Med. Res. Methodol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Goldet, G.; Howick, J. Understanding GRADE: An introduction. J. Evid.-Based Med. 2013, 6, 50–54. [Google Scholar] [CrossRef]
- Xu, C.; Furuya-Kanamori, L.; Zorzela, L.; Lin, L.; Vohra, S. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J. Clin. Epidemiol. 2021, 135, 70–78. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Lian, Q.; Zou, H.; Chu, H. Real-world Performance of Meta-analysis Methods for Double-Zero-Event Studies with Dichotomous Outcomes Using the Cochrane Database of Systematic Reviews. J. Gen. Intern. Med. 2019, 34, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.M. Hypoxia during Upper Endoscopy: A Serious Problem and a new solution. Anesthesiology News, 1 November 2019. [Google Scholar]
- Avrahami, E.; Englender, M. Relation between CT axial cross-sectional area of the oropharynx and obstructive sleep apnea syndrome in adults. Am. J. Neuroradiol. 1995, 16, 135–140. [Google Scholar] [PubMed]
- Song, J.-L.; Sun, Y.; Shi, Y.-B.; Liu, X.-Y.; Su, Z.-B. Comparison of the effectiveness of high-flow nasal oxygen vs. standard facemask oxygenation for pre-and apneic oxygenation during anesthesia induction: A systematic review and meta-analysis. BMC Anesthesiol. 2022, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Jones, J. The ‘fixed performance’venturi: Effect of downstream pressure on outflow and FiO2. Anaesthesia 2004, 59, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Wetsch, W.; Herff, H.; Schroeder, D.; Sander, D.; Böttiger, B.; Finke, S. Efficiency of different flows for apneic oxygenation when using high flow nasal oxygen application—A technical simulation. BMC Anesthesiol. 2021, 21, 239. [Google Scholar] [CrossRef]
- Parke, R.L.; Eccleston, M.L.; McGuinness, S.P. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir. Care 2011, 56, 1151–1155. [Google Scholar] [CrossRef]
- Pisani, L.; Fasano, L.; Corcione, N.; Comellini, V.; Musti, M.A.; Brandao, M.; Bottone, D.; Calderini, E.; Navalesi, P.; Nava, S. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017, 72, 373–375. [Google Scholar] [CrossRef] [Green Version]
- Biselli, P.; Fricke, K.; Grote, L.; Braun, A.T.; Kirkness, J.; Smith, P.; Schwartz, A.; Schneider, H. Reductions in dead space ventilation with nasal high flow depend on physiological dead space volume: Metabolic hood measurements during sleep in patients with COPD and controls. Eur. Respir. J. 2018, 51, 1702251. [Google Scholar] [CrossRef] [Green Version]
- Hanson, C.W., III; Marshall, B.E.; Frasch, H.F.; Marshall, C. Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease. Crit. Care Med. 1996, 24, 23–28. [Google Scholar] [CrossRef]
- Phillips, J.S.; Pangilinan, L.P.; Mangalindan, E.R.; Booze, J.L.; Kallet, R.H. A comparison of different techniques for interfacing capnography with adult and pediatric supplemental oxygen masks. Respir. Care 2017, 62, 78–85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).