Abstract
This systematic review aimed to determine the comorbid dry eye (DE) and allergic conjunctivitis (AC) prevalence. We searched PubMed and EMBASE for articles published until 22 March 2022, combining the terms “(dry eye OR keratoconjunctivitis sicca) AND allergic conjunctivitis.” Study-specific estimates (DE and AC incidence rates among patients with AC and DE, respectively) were combined using the one-group meta-analysis in a random-effects model. The initial search yielded 700 studies. Five articles reporting AC incidence among individuals with DE and six articles reporting DE incidence among individuals with AC were included in the qualitative synthesis. In these nine articles, the total sample size was 7254 patients. The DE incidence among individuals with AC was 0.9–97.5%; the AC incidence among individuals with DE was 6.2–38.0%. One-group meta-analysis using a random-effects model showed that 47.2% (95% confidence interval: 0.165–0.779; 320/1932 cases) of patients with AC had comorbid DE and 17.8% (95% confidence interval: 0.120–0.236; 793/4855 cases) of patients with DE had comorbid AC, as defined by each article. Complimentary screening and treatment for patients with DE and AC may improve long-term outcomes and prevent chronic ocular damage in highly susceptible populations.
1. Introduction
Dry eye (DE) and allergic conjunctivitis (AC) are the two most common ocular surface diseases [1,2,3] that negatively affect one’s quality of life and work productivity [4,5]. DE and AC, particularly seasonal AC (SAC) and perennial AC (PAC), present with a wide range of symptoms that significantly overlap, such as itching, redness, and dryness [6]. Moreover, both diseases are highly multifactorial in terms of onset and aggravation and share a considerable number of risk factors [1,5,7]. Currently, the known risk factors for the two diseases include female sex, history of contact lens use, presence of allergic diseases, and hay fever [2,5,8,9]. As DE and AC share common ocular symptoms and risk factors, proper diagnosis and treatment of these diseases and their comorbidities are important, with tailored regimens for each of the three states.
The fundamental pathophysiology of DE and AC is rooted in the immunological alterations that result in inflammation of the ocular surface, and their shared pathogenesis paves the way to negative synergies that aggravate the other diseases [10,11,12]. Previous reports have suggested that the reduced tear volume caused by DE hinders the removal of allergenic antigens on the ocular surface in patients with hay fever, which exacerbates AC associated with hay fever [13,14]. Similarly, AC has been shown to disrupt the tear film stability, contributing to worse outcomes in patients with DE [13,14]. These negative interactions between the two diseases necessitate bidirectional diagnosis and management to prevent chronic damage to the ocular surface. However, to our knowledge, no study to date has described the differentiating characteristics and prevalence of these two diseases as comorbidities.
Therefore, we conducted this systematic review to identify the prevalence of comorbid DE and AC, including SAC and PAC.
2. Materials and Methods
2.1. Outcomes
The primary aim of this study was to systematically evaluate and characterize the current reports of DE and AC, including SAC and PAC. In particular, the primary analysis focused on the comorbidity rates of DE and AC.
2.2. Search Strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines [15]. An extensive search strategy was designed to retrieve all articles published until 22 March 2022, in PubMed and EMBASE, which are key electronic bibliographic databases, by combining the terms “(dry eye OR keratoconjunctivitis sicca) AND allergic conjunctivitis.” The inclusion and exclusion criteria for the study are listed in Table 1. Search results were compiled using EndNote X9.3.3 software (Clarivate Analytics, Philadelphia, PA, USA). In accordance with the quality standards for reporting systematic reviews and meta-analyses of observational studies [16], two independent researchers (Y.A. and T.I.) screened the retrieved articles. The same investigators independently assessed the full text of records deemed eligible by consensus.

Table 1.
Study inclusion and exclusion criteria.
2.3. Data Extraction
Two independent reviewers (Y.A. and T.I.) extracted data from each eligible study using a standardized data extraction sheet and subsequently cross-checked the results. Disagreements regarding the extracted data were resolved through discussion with a third reviewer (K.K.) [17]. Extracted data included the first author’s name; date of publication; type of study (retrospective or prospective); country of study; cohort size; characteristics of patients with DE and AC, including their age and sex; and the incidence rates of DE and AC among individuals with AC and DE, respectively.
2.4. Statistical Analysis
Study-specific estimates (incidence rate of DE among individuals with AC and incidence rate of AC among individuals with DE) were combined using the one-group meta-analysis in a random-effects model using OpenMetaAnalyst version 12.11.14 (Available from http://www.cebm.brown.edu/openmeta/, accessed on 22 June 2022) [18].
3. Results
The database search identified 700 articles. After removing 118 duplicates, 582 articles were reviewed based on the title and abstract. Of these articles, 488 studies were excluded because of the article type (clinical guidelines, consensus documents, reviews, and conference proceedings) and because they focused on other subtypes of AC (atopic keratoconjunctivitis, vernal keratoconjunctivitis, giant papillary conjunctivitis, and atopic blepharitis), had an animal-based design, focused on other unrelated topics and drug allergy, and were not written in English (Table 1). Thus, 94 articles were assessed for eligibility (Figure 1), and eventually, studies on nine studies met the inclusion criteria and were included in the systematic review (Table 2).
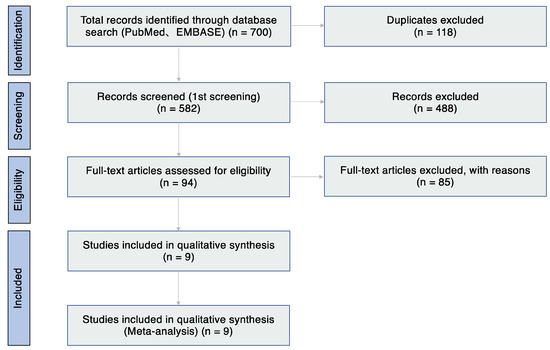
Figure 1.
Flow diagram of study selection.

Table 2.
Results of systematic reviews on the association between dry eye and allergic conjunctivitis.
3.1. Study Characteristics and Demographic Features
The articles included in this systematic review were published between 2012 and 2021 (Table 2). Four studies were from Japan [20,21,22,26], while one each was from Thailand [19], China [23], Turkey [24], Nigeria [25], and Ghana [27]. Seven were prospective studies [19,20,21,22,23,24,26], and two were retrospective studies [25,27]. The total sample size was 7254 patients. Three studies primarily included children [19,23,24]. The diagnostic examination for DED used in those included articles were shown in Table 3. Seven articles reported the mean age [19,21,22,23,24,26,27], and the mean age range of the included adults was 30.6 [27] to 69.1 [21] years, whereas the mean age range of children was 4.75 [23] to 11.79 [24] years. All articles mentioned the sex of the patients; there were 1892 men and 5362 women [19,20,21,22,23,24,25,26,27].

Table 3.
Diagnostic examinations for DE.
3.2. Incidence DE among Individuals with AC
Six articles reported the incidence of DE among individuals with AC [19,21,23,24,25,27], which ranged from 0.9% [25] to 97.5% [23]. Among these articles, 16.6% (320/1932 cases) of individuals with AC had DE. Three articles reported the incidence of DE among individuals with AC (110/158 patients (69.9%) [21], 9/972 eyes (0.9%) [25], and 126/696 patients (18.1%) [27]). Three articles reported the incidence of DE among children with AC (30/41 patients (73.2%) [19], 78/80 eyes (97.5%) [23], and 6/25 patients (24.0%) [24]). Two studies reported DE examinations findings and compared the findings from the AC group with those from the control group [23,24]. Chen L et al. [23] reported that the tear film break-up time (TFBUT) was lower in the AC children group (6.54 ± 1.48 s) than in the control group (10.04 ± 1.79 s; p < 0.001), and there was a negative correlation between the papillary reaction and the Schirmer test, TFBUT, and tear meniscus height reflex (TMH-R) values (r = −0.454, −0.412, and −0.419; p = 0.001, 0.003, and 0.002, respectively). However, Akil et al. [24] reported that the values of the Schirmer test, TFBUT, and TMH-R were lower in the AC than in the control group (p < 0.001 for all comparisons), and there was a negative correlation between the duration of AC and TFBUT (r = −0.45; p < 0.005).
Using the one-group meta-analysis in a random-effects model, six studies that included 1932 individuals were further analyzed for the incidence rate of DE among individuals with AC (Figure 2); the results revealed that 47.2% (95% confidence interval: 0.165–0.779; 320/1932 cases) of patients with AC had DE.
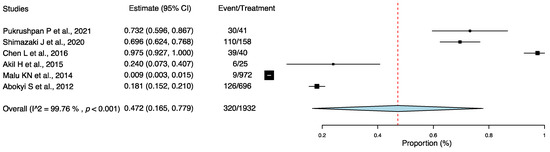
Figure 2.
Incidence rate of dry eye among individuals with allergic conjunctivitis. Abbreviation: 95% CI: 95% confidence interval [19,21,23,24,25,27].
3.3. Incidence of AC among Individuals with DE
Five studies reported the incidence of AC among individuals with DE [19,20,21,22,26], which ranged from 6.2% [22] to 38.0% [19]. Among these articles, 16.3% (793/4855 cases) of individuals with DE had AC. Four articles reported the incidence of AC among individuals with DE (97/580 (16.7%) [20], 110/551 (20.0%) [21], 28/449 (6.2%) [22], and 528/3196 patients (16.5%) [26]). These four studies were multicenter studies in Japan, two of which evaluated the safety and efficacy of 3% diquafosol [20,26]. One article reported the incidence of AC among children with DE (30/79 patients (38.0%) [19]).
Using the one-group meta-analysis in a random-effects model, five studies that included 4855 subjects were further analyzed for the incidence rate of AC among individuals with DE (Figure 3); the results revealed that 17.8% (95% confidence interval: 0.120–0.236; 793/4855 cases) of patients with DE had AC.
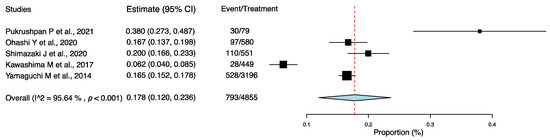
Figure 3.
Incidence rate of allergic conjunctivitis among individuals with dry eye. Abbreviation: 95% CI: 95% confidence interval [19,20,21,22,26].
4. Discussion
DE and AC are inflammatory diseases of the ocular surface that exhibit a synergistic effect in terms of pathology. In this study, we performed a systematic review and meta-analysis of the prevalence of comorbid DE and AC, including SAC and PAC. Our results indicated that comorbid DE and AC existed in almost half of the patients with AC and DE, respectively. Cautious diagnosis and bidirectional management of the two diseases, with due consideration of their existence as comorbidities, can improve the long-term outcomes.
This study found that the incidence rates of DE and AC among individuals with AC and DE were 47.2% and 17.8%, respectively. Previous studies on the epidemiology of DE have reported a global prevalence of 5–50% [1,28], and the relatively higher comorbidity rate in the AC cohort suggests that patients with AC may be predisposed to DE. Currently, the proposed pathological mechanisms include AC-induced lipid layer thickening of the tear film [14], instability of the tear film caused by the tear protein changes in AC [29], increased inflammatory cytokines secondary to a chronic disease [12,30], and prolonged use of antihistamines for symptomatic management [27,31], all of which may contribute to tear film disruption and the pathogenesis of DE [23,32]. In addition, studies have reported that AC may induce squamous metaplasia of the conjunctival epithelium and decrease conjunctival goblet cell density [33]. In an animal study, repeated exposure to allergens in a mouse model of AC showed a similar decrease in the number of conjunctival goblet cells, with a decrease in Muc5AC and Muc4 mRNA expression [34]. Damage to the goblet cells and altered mucin production can exacerbate the existing DE pathology [35,36], implying a direct link between AC and short-TFBUT-type DE [14,36]. The decreased tear production volume also hinders the removal of antigens from the ocular surface, creating a vicious cycle [13]. However, there are conflicting reports on the effects of AC on the clinical findings associated with DE. A study on patients with DE and mild conjunctivitis, including AC, revealed no significant effect of conjunctivitis on TFBUT [13]. Conversely, a study noted a significant decrease in TFBUT in patients with SAC [14]. In a study investigating the effects of AC and non-AC in a pediatric cohort, the AC group exhibited a decreased tear production volume, TFBUT, and central reflex tear meniscal height [24]. This finding indicates that various factors, such as the patient’s age and AC severity, may affect the comorbidity rates of AC and DE.
Contrarily, the incidence of DE among individuals with AC was presumed to be higher than the prevalence among the general population as the decreased tear production volume because DE hinders the removal of antigens from the ocular surface [13]. However, our study found that the incidence of DE among individuals with AC was 17.8%, and there was no significant difference compared with a previous report, which reported a prevalence of AC ranging from 15% to 20% [37,38]. This observation may be attributed to variations in the sample size, sampling bias, and differences in the diagnostic criteria for DE and AC.
Our results indicate that insufficient management of either disease in a comorbid patient could accelerate ocular damage and worsen the prognosis. This calls for a careful diagnosis and management with due consideration of their existence as comorbidities in patients exhibiting these conditions. Unfortunately, AC and DE have a wide range of overlapping manifestations, from eye dryness to itching, and clinicians must conduct detailed and comprehensive examinations to aid in their differentiation [6]. Determining any shared risk factors, including hay fever and contact lens use, is crucial during the initial evaluation [2,5,8,10,11]. In all patients with DE, the history of allergic diseases should be additionally investigated, and the findings of palpebral erythema and edema should be noted. Similarly, Schirmer test results, TFBUT, and tear film break patterns [39] should be examined in patients with AC to rule out the possibility of comorbid DE. To prevent DE onset in patients with AC, extensive and personalized strategies to avoid allergens should be implemented [40,41], starting with an allergen test to determine the causal allergenic factors.
An effort to promote societal education recommending appropriate clinical examinations for DE and AC may have a strong influence on the outcome of their management [5,7]. A study on DE and its association with video display terminal use revealed a higher DE rate among the working-age population, with existing concerns for a high prevalence of AC among the same population [40,41,42]. Recent findings also raised concerns regarding a high percentage of missed diagnoses of chronic DE in the working-age population [43]. By reaching such critical and wide populations in a timely manner, medicine driven by smartphone-based mHealth applications appears to hold potential in identifying symptoms and individual risk factors and in recommending appropriate management and hospital visits [40,41,43,44,45,46,47,48,49].
Missed diagnosis of DE in patients with AC may exacerbate DE and affect the long-term prognosis, as symptomatic management of AC through antihistamines can induce decreased tear production, with supporting evidence from a previous animal study [50]. Management of AC should be appropriately adjusted following a comprehensive examination to determine the presence of DE [51]. Such examinations include the Schirmer test and fluorescein dye examination, which focus on tear production and quality, and are relatively specific for DE diagnosis. Then, the results can be used to determine the need for tear replenishment or invasive techniques, such as punctal plug insertion and cautery. There have been a few reports describing the risk factors for comorbid DE and AC [27] and optimal treatment for comorbid DE and AC. Hence, further research is needed to establish appropriate management for patients with AC and DE.
This study had a few limitations. First, there was a relatively high variation in the sample size per study among the included publications. Second, compared with the sample size in the prevalence studies independently conducted for either DE or AC, the total sample size in the present study might be considered small [1,37,52]. Third, sampling bias concerning limited geographic coverage may be present, as a significant portion of the included studies were conducted in Japan and other Asian countries [19,20,21,22,23,26]. Fourth, there were discrepancies in the diagnostic criteria for DE and AC, as well as varied individual characteristics, such as age, in each included study. In addition, symptoms of DE and AC can be difficult to distinguish because of the symptoms overlap. Future studies concerning the epidemiology of comorbid DE and AC should establish and apply standardized criteria for these two diseases in a larger sample size.
In conclusion, our study results suggest that almost half of the patients with AC may exhibit comorbid DE, and almost 20% of patients with DE may exhibit comorbid AC. This warrants careful examinations for the counterpart disease in patients presenting with either disease, followed by appropriate modification of treatment regimens to minimize the exacerbation of AC and DE.
Author Contributions
Conceptualization, T.I.; methodology, Y.A. and T.I.; investigation, Y.A. and T.I.; statistical analysis, Y.A., T.I., M.N., K.N. and A.M.-I.; result interpretation, M.N., K.K., K.C.S. and T.A.; writing—original draft preparation, Y.A., T.I., J.S., K.K., K.C.S., T.A., Y.O., K.F., K.N., A.M.-I., M.K., K.H., T.H., Y.M., H.S., A.E. and A.M.; funding acquisition, T.I., A.M.-I., A.E. and Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Agency for Medical Research and Development Grant Number JP21ek0410063 (T.I.); JSPS KAKENHI Grand Numbers 20KK0207 (T.I.), 20K23168 (A.M.-I.), 21K17311 (A.M.-I.), 21K20998 (A.E.), and 22K16983 (A.E.); Medical Research Encouragement Prize 2018 and 2021, the Institute for Environmental & Gender-specific Medicine, Juntendo University (T.I.), Medical Research Encouragement Prize 2020, Kondou Kinen Medical Foundation (T.I.), Medical Research Encouragement Prize 2022, the Cell Science Research Foundation (T.I.), and Medical Research Encouragement Prize 2021, and the OTC Self-Medication Promotion Foundation (Y.O.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank all members of the Department of Ophthalmology, Juntendo University Graduate School of Medicine for providing critical comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish.
Abbreviations
DE, dry eye; AC, allergic conjunctivitis; SAC, seasonal allergic conjunctivitis; PAC, perennial allergic conjunctivitis; TFBUT, tear film break-up time; TMH-R, tear meniscus height reflex.
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Castegnaro, A.; Valerio, A.L.G.; Lazzarini, D. Epidemiology of allergic conjunctivitis: Clinical appearance and treatment patterns in a population-based study. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, P.; Prokopich, C.L.; Hynes, A.; Kim, H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Sung, J.; Nakamura, M.; Fujisawa, K.; Muto, K.; Ebihara, N.; Iwagami, M.; Nakamura, M.; Fujio, K.; Okumura, Y.; et al. New medical big data for P4 medicine on allergic conjunctivitis. Allergol. Int. 2020, 69, 510–518. [Google Scholar] [CrossRef]
- Hom, M.M.; Nguyen, A.L.; Bielory, L. Allergic conjunctivitis and dry eye syndrome. Ann. Allergy Asthma Immunol. 2012, 108, 163–166. [Google Scholar] [CrossRef]
- Inomata, T.; Sung, J.; Nakamura, M.; Iwagami, M.; Okumura, Y.; Iwata, N.; Midorikawa-Inomata, A.; Fujimoto, K.; Eguchi, A.; Nagino, K.; et al. Using Medical Big Data to Develop Personalized Medicine for Dry Eye Disease. Cornea 2020, 39, S39–S46. [Google Scholar] [CrossRef]
- Inomata, T.; Nakamura, M.; Iwagami, M.; Shiang, T.; Yoshimura, Y.; Fujimoto, K.; Okumura, Y.; Eguchi, A.; Iwata, N.; Miura, M.; et al. Risk Factors for Severe Dry Eye Disease: Crowdsourced Research Using DryEyeRhythm. Ophthalmology 2019, 126, 766–768. [Google Scholar] [CrossRef]
- Kosrirukvongs, P.; Visitsunthorn, N.; Vichyanond, P.; Bunnag, C. Allergic conjunctivitis. Asian Pac. J. Allergy Immunol. 2001, 19, 237–244. [Google Scholar] [PubMed]
- Ayaki, M.; Kawashima, M.; Uchino, M.; Tsubota, K.; Negishi, K. Possible association between subtypes of dry eye disease and seasonal variation. Clin. Ophthalmol. 2017, 11, 1769–1775. [Google Scholar] [CrossRef]
- Leonardi, A.; Modugno, R.L.; Salami, E. Allergy and Dry Eye Disease. Ocul. Immunol. Inflamm. 2021, 29, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Opthalmol. Vis. Sci. 2018, 59, Des192–Des199. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Imanaga, Y. Effect of mild conjunctivitis complication on tear balance in dry eye. Contact Lens Anterior Eye 2012, 35, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Goto, E.; Dogru, M.; Asano-Kato, N.; Matsumoto, Y.; Hara, Y.; Fujishima, H.; Tsubota, K. Tear film lipid layer alterations in allergic conjunctivitis. Cornea 2006, 25, 277–280. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Kuno, T.; Takagi, H.; Ando, T.; Sugiyama, T.; Miyashita, S.; Valentin, N.; Shimada, Y.J.; Kodaira, M.; Numasawa, Y.; Briasoulis, A.; et al. Oral Anticoagulation for Patients with Atrial Fibrillation on Long-Term Hemodialysis. J. Am. Coll. Cardiol. 2020, 75, 273–285. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Kawai, N.; Ando, T. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ. 2020, 29, 729–741. [Google Scholar] [CrossRef]
- Pukrushpan, P.; Kummaraphat, V.; Praneeprachachon, P.; Reinprayoon, U.; Pityaratstian, N.; Honglertnapakul, W. Excessive blinking in children and its association with dry eyes and visual display terminal: A 200 case-control study. J. Med. Assoc. Thail. 2021, 104, 1671–1677. [Google Scholar] [CrossRef]
- Ohashi, Y.; Munesue, M.; Shimazaki, J.; Takamura, E.; Yokoi, N.; Watanabe, H.; Nomura, A.; Shimada, F. Long-Term Safety and Effectiveness of Diquafosol for the Treatment of Dry Eye in a Real-World Setting: A Prospective Observational Study. Adv. Ther. 2020, 37, 707–717. [Google Scholar] [CrossRef]
- Shimazaki, J.; Nomura, Y.; Numa, S.; Murase, Y.; Kakinoki, K.; Abe, F.; Kato, Y.; Okabe, H.; Kishimoto, H.; Yamada, Y. Prospective, Multicenter, Cross-Sectional Survey on Dry Eye Disease in Japan. Adv. Ther. 2020, 37, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Yamada, M.; Suwaki, K.; Shigeyasu, C.; Uchino, M.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K. A Clinic-based Survey of Clinical Characteristics and Practice Pattern of Dry Eye in Japan. Adv. Ther. 2017, 34, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pi, L.; Fang, J.; Chen, X.; Ke, N.; Liu, Q. High incidence of dry eye in young children with allergic conjunctivitis in Southwest China. Acta Ophthalmol. 2016, 94, e727–e730. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Celik, F.; Ulas, F.; Kara, I.S. Dry Eye Syndrome and Allergic Conjunctivitis in the Pediatric Population. Middle East Afr. J. Ophthalmol. 2015, 22, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Malu, K.N. Allergic conjunctivitis in Jos-Nigeria. Niger. Med. J. 2014, 55, 166–170. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nishijima, T.; Shimazaki, J.; Takamura, E.; Yokoi, N.; Watanabe, H.; Ohashi, Y. Clinical usefulness of diquafosol for real-world dry eye patients: A prospective, open-label, non-interventional, observational study. Adv. Ther. 2014, 31, 1169–1181. [Google Scholar] [CrossRef]
- Abokyi, S.; Koffuor, G.A.; Abu, E.K.; Kyei, S.; Abraham, C.H. Dry eyes: An adverse effect of systemic antihis-tamine use in allergic conjunctivitis management. Res. J. Pharmacol. 2012, 6, 71–77. [Google Scholar]
- Inomata, T.; Shiang, T.; Iwagami, M.; Sakemi, F.; Fujimoto, K.; Okumura, Y.; Ohno, M.; Murakami, A. Changes in Distribution of Dry Eye Disease by the New 2016 Diagnostic Criteria from the Asia Dry Eye Society. Sci. Rep. 2018, 8, 1918. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Chen, Z.; Huang, Q.; Wu, K. Quantification of tear proteins and sPLA2-IIa alteration in patients with allergic conjunctivitis. Mol. Vis. 2010, 16, 2084–2091. [Google Scholar]
- Leonardi, A.; Curnow, S.J.; Zhan, H.; Calder, V.L. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin. Exp. Allergy 2006, 36, 777–784. [Google Scholar] [CrossRef]
- Dogru, M.; Gunay, M.; Celik, G.; Aktas, A. Evaluation of the tear film instability in children with allergic diseases. Cutan. Ocul. Toxicol. 2016, 35, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lobefalo, L.; D’Antonio, E.; Colangelo, L.; Della Loggia, G.; Di Gioacchino, M.; Angelucci, D.; Di Iorio, A.; Gallenga, P.E. Dry eye in allergic conjunctivitis: Role of inflammatory infiltrate. Int. J. Immunopathol. Pharmacol. 1999, 12, 133–137. [Google Scholar] [CrossRef]
- Borazan, M.; Karalezli, A.; Akova, Y.A.; Akman, A.; Kiyici, H.; Erbek, S.S. Efficacy of olopatadine HCI 0.1%, ketotifen fumarate 0.025%, epinastine HCI 0.05%, emedastine 0.05% and fluorometholone acetate 0.1% ophthalmic solutions for seasonal allergic conjunctivitis: A placebo-controlled environmental trial. Acta Ophthalmol. 2009, 87, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Keane-Myers, A.M.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2483–2489. [Google Scholar]
- Shirai, K.; Saika, S. Ocular surface mucins and local inflammation—Studies in genetically modified mouse lines. BMC Ophthalmol. 2015, 15 (Suppl. 1), 154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toda, I.; Shimazaki, J.; Tsubota, K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology 1995, 102, 302–309. [Google Scholar] [CrossRef]
- La Rosa, M.; Lionetti, E.; Reibaldi, M.; Russo, A.; Longo, A.; Leonardi, S.; Tomarchio, S.; Avitabile, T.; Reibaldi, A. Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 2013, 39, 18. [Google Scholar] [CrossRef]
- Wong, A.H.; Barg, S.S.; Leung, A.K. Seasonal and perennial allergic conjunctivitis. Recent Patents Inflamm. Allergy Drug Discov. 2014, 8, 139–153. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of Fluorescein Breakup Patterns: A Novel Method of Differential Diagnosis for Dry Eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef]
- Inomata, T.; Nakamura, M.; Iwagami, M.; Sung, J.; Nakamura, M.; Ebihara, N.; Fujisawa, K.; Muto, K.; Nojiri, S.; Ide, T.; et al. Individual characteristics and associated factors of hay fever: A large-scale mHealth study using AllerSearch. Allergol. Int. 2022. [Google Scholar] [CrossRef]
- Inomata, T.; Nakamura, M.; Iwagami, M.; Sung, J.; Nakamura, M.; Ebihara, N.; Fujisawa, K.; Muto, K.; Nojiri, S.; Ide, T.; et al. Symptom-based stratification for hay fever: A crowdsourced study using the smartphone application AllerSearch. Allergy 2021, 76, 3820–3824. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am. J. Ophthalmol. 2013, 156, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Iwagami, M.; Nakamura, M.; Shiang, T.; Yoshimura, Y.; Fujimoto, K.; Okumura, Y.; Eguchi, A.; Iwata, N.; Miura, M.; et al. Characteristics and Risk Factors Associated with Diagnosed and Undiagnosed Symptomatic Dry Eye Using a Smartphone Application. JAMA Ophthalmol. 2019, 138, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Nakamura, M.; Sung, J.; Midorikawa-Inomata, A.; Iwagami, M.; Fujio, K.; Akasaki, Y.; Okumura, Y.; Fujimoto, K.; Eguchi, A.; et al. Smartphone-based digital phenotyping for dry eye toward P4 medicine: A crowdsourced cross-sectional study. NPJ Digit. Med. 2021, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Nakamura, M.; Iwagami, M.; Midorikawa-Inomata, A.; Sung, J.; Fujimoto, K.; Okumura, Y.; Eguchi, A.; Iwata, N.; Miura, M.; et al. Stratification of Individual Symptoms of Contact Lens-Associated Dry Eye Using the iPhone App DryEyeRhythm: Crowdsourced Cross-Sectional Study. J. Med. Internet Res. 2020, 22, e18996. [Google Scholar] [CrossRef]
- Inomata, T.; Iwagami, M.; Nakamura, M.; Shiang, T.; Fujimoto, K.; Okumura, Y.; Iwata, N.; Fujio, K.; Hiratsuka, Y.; Hori, S.; et al. Association between dry eye and depressive symptoms: Large-scale crowdsourced research using the DryEyeRhythm iPhone application. Ocul. Surf. 2020, 18, 312–319. [Google Scholar] [CrossRef]
- Eguchi, A.; Inomata, T.; Nakamura, M.; Nagino, K.; Iwagami, M.; Sung, J.; Midorikawa-Inomata, A.; Okumura, Y.; Fujio, K.; Fujimoto, K.; et al. Heterogeneity of eye drop use among symptomatic dry eye individuals in Japan: Large-scale crowdsourced research using DryEyeRhythm application. Jpn. J. Ophthalmol. 2021, 65, 271–281. [Google Scholar] [CrossRef]
- Okumura, Y.; Inomata, T.; Midorikawa-Inomata, A.; Sung, J.; Fujio, K.; Akasaki, Y.; Nakamura, M.; Iwagami, M.; Fujimoto, K.; Eguchi, A.; et al. DryEyeRhythm: A reliable and valid smartphone application for the diagnosis assistance of dry eye. Ocul. Surf. 2022, 25, 19–25. [Google Scholar] [CrossRef]
- Inomata, T.; Sung, J. Changing Medical Paradigm on Inflammatory Eye Disease: Technology and Its Implications for P4 Medicine. J. Clin. Med. 2022, 11, 2964. [Google Scholar] [CrossRef]
- Villareal, A.L.; Farley, W.; Pflugfelder, S.C. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens 2006, 32, 272–276. [Google Scholar] [CrossRef]
- Nye, M.; Rudner, S.; Bielory, L. Emerging therapies in allergic conjunctivitis and dry eye syndrome. Expert Opin. Pharmacother. 2013, 14, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.; Barg, S.S.; Leung, A.K. Seasonal and perennial allergic conjunctivitis. Recent Patents Inflamm. Allergy Drug Discov. 2009, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).