Abstract
Obese women are at high risk of developing pre-eclampsia (PE). As an altered angiogenic profile is characteristic for PE, measurement of soluble fms-like tyrosine kinase-1 (sFlt-1)/placental growth factor (PIGF) ratio in the maternal serum can be helpful for PE diagnosis, as well as for adverse perinatal outcome (APO) prediction. There is growing evidence that obesity might influence the level of sFlt-1/PIGF and, therefore, the aim of the study was the evaluation of sFlt-1/PIGF as an APO predictor in obese women with PE. Pre-eclamptic women who had an sFlt-1/PIGF measurement at the time of diagnosis were retrospectively included. Women were classified according to their pre-pregnancy body mass index (BMI) as normal weight (BMI < 25 kg/m2), overweight (BMI > 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2). APO was defined as the occurrence of one of the following outcomes: Small for gestational age, defined as a birthweight < 3rd centile, neonatal mortality, neonatal seizures, admission to neonatal unit required (NICU) or respiratory support. A total of 141 women were included. Of them, 28 (20%) patients were obese. ROC (receiver operating characteristic) analysis revealed a high predictive value for sFlt-1/PIGF and APO across the whole study cohort (AUC = 0.880, 95% CI: 0.826–0.936; p < 0.001). However, the subgroup of obese women showed a significantly lower level of sFlt-1 and, therefore, the performance of sFlt-1/PIGF as APO predictor was poorer compared to normal or overweight PE women (AUC = 0.754, 95% CI: 0.552–0.956, p = 0.025). In contrast to normal or overweight women, a ratio of sFlt-1/PIGF < 38 could not rule out APO in women with obesity.
1. Introduction
Pre-eclampsia (PE) is a disease affecting 2–8% of all pregnancies and a major cause for fetal and maternal morbidity [1,2,3]. An altered inflammatory and angiogenic profile is a main finding in the pathophysiology of PE and serum markers, such as soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PIGF), are now established for the diagnosis of PE [4,5].
Around a third of pregnant women in Western countries are affected by overweight and the percentage is steadily increasing [6,7]. In 2017, 15% of pregnant women in Germany were obese compared to 12% in 2003 [8]. Obesity is a risk factor for the development of PE [9] and is, as well as PE, characterized by an endothelial dysfunction and a proinflammatory microenvironment [10]. Therefore, the evaluation of the established predictive marker of PE, sFlt-1/PIGF ratio, is of the utmost importance in this high-risk cohort. Recent guidelines recommend the use of sFlt-1/PIGF for diagnosis or exclusion of PE [11]. The PROGNOSIS study, which evaluated and established sFlt-1/PIGF as a diagnostic tool, did not differentiate between obese or normal-weight women and did not, therefore, examine if different cutoffs are necessary or not [12].
The ratio of sFlt-1/PIGF is not only used for diagnosing or excluding PE, but also for the prediction of adverse perinatal outcome (APO) and adverse maternal outcome (AMO) [13,14,15,16]. Specifically, highly elevated levels of sFlt-1/PIGF correlate with APO and might be helpful for estimating the mean time until delivery [12,17,18,19,20]. Likewise, published data do not discriminate between different BMI groups.
It is important to notice that not only the severity of PE itself, but also other factors influence the serum level of sFlt-1/PIGF, such as twin pregnancies or, as mentioned above, BMI [21,22]. Levels of sFlt-1 seem to be inversely correlated with BMI and, therefore, APO prediction by sFlt-1/PIGF might be distorted [23].
The aim of this study was the evaluation of sFlt-1/PIGF as an APO and AMO predictor in obese women with PE and/or HELLP syndrome.
2. Materials and Methods
This is a retrospective single-center study from January 2018 to December 2020 at the University Hospital rechts der Isar, Department of Obstetrics and Gynecology (Technical University of Munich). All patients with diagnosed PE and/or HELLP syndrome were included if sFlt-1/PIGF was determined at the time of diagnosis. Cases with missing data on perinatal outcome as well as multiple pregnancies were excluded. Additionally, we included the study cohort of women with late-onset PE which was described before [24].
Elevated blood pressure was defined as a new onset hypertension with a systolic blood pressure of 140 mmHg and/or a diastolic blood pressure of 90 mmHg, on two occasions at least 4 h apart [25]. Large cuffs were used for obese women.
PE was defined as elevated blood pressure and one of the following symptoms: proteinuria (≥300 mg/24 h), thrombocytopenia (platelet count less than 100.000/microliter), poor liver function (elevated blood levels of liver transaminases to twice the normal concentration), new-onset renal insufficiency (elevated serum creatinine greater than 1.1 mg/dL or a doubling of serum creatinine in the absence of other renal disease), pulmonary edema or new-onset cerebral or visual disturbances [26]. HELLP syndrome was defined as the occurrence of hemolysis (haptoglobin < 10 mg/L), elevated liver enzymes (twice the normal concentration) and low platelets (<100.000/microliter) [27].
Diagnosis of PE < 34 weeks was classified as early onset (eo) PE and diagnosis of PE ≥ 34 weeks as late-onset (lo) PE [28].
APO was defined as the presence of at least one of the following outcomes according to the Delphi consensus defining the core outcome of PE [29]: Small for gestational age defined as a birthweight < 3rd centile according to local standards [30], neonatal mortality, neonatal seizures, admission to neonatal unit required (NICU) or respiratory support. There was no case of stillbirth.
Adverse maternal outcome (AMO) was defined as the occurrence of at least one of the following core outcomes of PE: eclampsia, pulmonary edema, acute kidney injury (defined as elevated serum creatinine greater than 1.1 mg/dL or a doubling of serum creatinine in the absence of other renal disease), placental abruption, HELLP syndrome, admission to intensive care unit or the need of intubation and mechanical ventilation. There was no case of maternal mortality, cortical blindness, retinal detachment, liver capsule hematoma or rupture or stroke.
The sFlt-1/PIGF ratio was determined at the time of diagnosis according to our local chemistry guidelines as previously described [18].
We used the cutoff values for sFlt-1/PlGF, which are recommended by the consensus statement [31]: <38 exclusion of PE (gestational-age independent) for at least 1 week, >85 diagnosis of early onset (eo) PE (<34 week of gestation) and >110 diagnosis of late-onset (lo) PE (≥34 week of gestation).
Overweight and obesity were defined according to the WHO guidelines as a BMI from 25 to 29.9 kg/m2 or >30 kg/m2, respectively [32]. A BMI <25 kg/m2 was considered as normal weight. The pre-pregnancy BMI was used for classification.
2.1. Statistical Analysis
We used IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, NY, USA) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for our statistical analysis. Quantitative data are shown as means and standard deviations or median and interquartile range; categorical data are presented as absolute and relative frequencies. Differences in distributions of quantitative data between the three weight groups were tested using the Kruskal–Wallis test. If a significant difference was found pairwise group comparisons were performed with Mann–Whitney U tests. Categorical data were compared between groups using Fisher’s exact test. The predictive value of sFlt-1/PIGF for APO or AMO was analyzed with receiver operating characteristic (ROC) curves. Areas under the ROC curves were compared between groups using the method proposed by Delong et al. [33]. All statistical tests were conducted two sided and a p-value < 0.05 was considered statistically significant.
2.2. Ethical Approval
The study was approved by our local Institutional Ethics Board (Ethikkommission der Fakultät für Medizin der Technischen Universität München, protocol number 232/17). The study was not registered in a public trial registry.
3. Results
3.1. Baseline Characteristics and Perinatal Outcome
In total, 141 pregnancies with PE and/or HELLP were enrolled. Of these cases, 45 (32%) were diagnosed with early onset and 96 (68%) with late-onset PE and/or HELLP syndrome. Further, 84 women in our cohort had a normal pre-conceptional BMI (60%), 29 were overweight (20%) and 28 were obese (20%). Of all newborns, APO occurred in 69 cases (49%). Many newborns were affected by more than one event. The most common APO was admission to NICU in 55 cases (40%), followed by respiratory support in 42 cases (30%). There were 13 cases of newborns with a birthweight <3rd centile (9%), three cases of seizures (2%) and one case of neonatal death (0.7%).
AMO was observed in 36 cases (26%). The most common event was acute kidney injury in 15 cases (11%), followed by early onset HELLP syndrome affecting 10 pregnancies and late-onset HELLP syndrome affecting nine pregnancies (7% and 6%, respectively). Other AMO events were rarely observed.
Baseline characteristics of the study cohort and data on perinatal outcome are described in Table 1 and Table 2.

Table 1.
Baseline characteristics.

Table 2.
Perinatal outcome.
3.2. Levels of Angiogenic Factors in Preeclamptic Women Depending on BMI
Figure 1, Figure 2 and Figure 3 demonstrate the median level of sFlt-1/PIGF, sFlt-1 and PIGF in the different BMI subgroups. Obese women show a significantly lower sFlt-1 compared to normal-weight or overweight women (p = 0.001), whereas sFlt-1/PIGF and PIGF are not statistically different in the different subgroups.
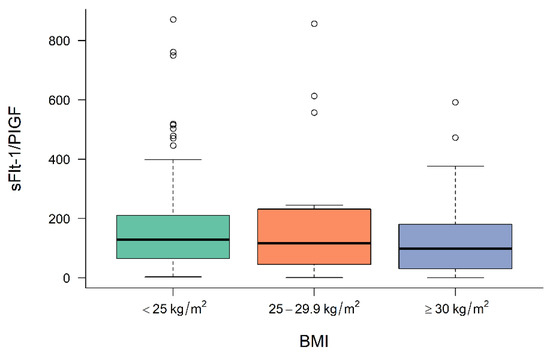
Figure 1.
Level of sFlt-1/PIGF at diagnosis in normal-weight, overweight and obese women. BMI body mass index, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.
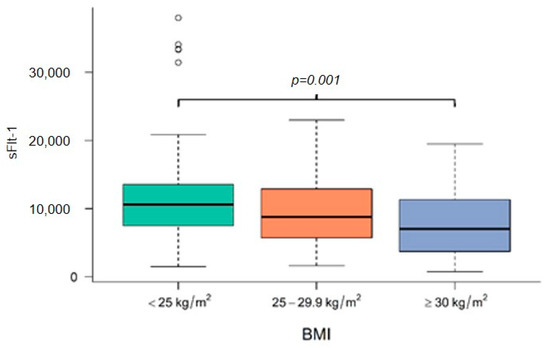
Figure 2.
Level of sFlt-1 at diagnosis in normal-weight, overweight and obese women. BMI body mass index, sFlt-1 soluble fms-like tyrosine kinase-1.
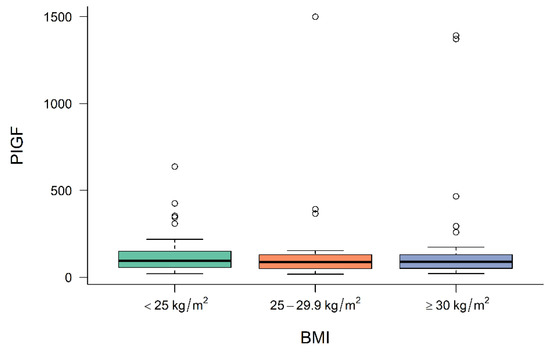
Figure 3.
Level of PIGF at diagnosis in normal-weight, overweight and obese women. BMI body mass index, PIGF placental growth factor.
3.3. APO Prediction by sFlt-1/PlGF
For the whole study cohort, ROC analysis revealed a significant predictive value for sFlt-1/PIGF and APO (AUC = 0.880, 95% CI: 0.824–0.936; p < 0.001), as well as for PIGF or sFlt-1 alone (AUC = 0.866, 95% CI: 0.807–0.925, p < 0.001 and AUC = 0.721, 95% CI: 0.637–0.804, p < 0.001, respectively). The area under the ROC curve (AUC) was significantly larger for sFlt-1/PIGF and for PIGF than for sFlt-1 alone (p < 0.001 and p = 0.005).
In all the subgroups of normal weight, overweight and obese women, sFlt-1/PIGF was significantly associated with APO, although the prognostic value was reduced in obese women compared to the other two subgroups (AUC = 0.914, 95% CI: 0.857–0.972, p < 0.001; AUC = 0.931, 95% CI: 0.845–0.999, p < 0.001; AUC = 0.754, 95% CI: 0.552–0.956, p = 0.025). In contrast, sFlt-1 failed to be statistically significant as an outcome predictor in obese women (AUC = 0.642, 95% CI: 0.428–0.855, p = 0.213).
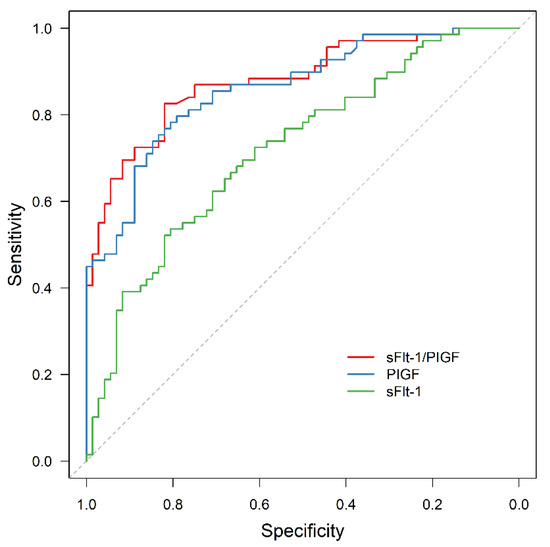
Figure 4.
ROC curve for APO prediction by sFlt-1 (AUC = 0.721, 95% CI: 0.637–0.804, p < 0.001), PIGF (AUC = 0.866, 95% CI: 0.807–0.925, p < 0.001) and sFlt-1/PIGF (AUC = 0.880, 95% CI: 0.824–0.936, p < 0.001). ROC receiver operating characteristic, APO adverse perinatal outcome, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.

Figure 5.
ROC curve for APO prediction in the subgroup of normal-weight women by sFlt-1 (AUC = 0.723, 95% CI: 0.615–0.832, p < 0.001), PIGF (AUC = 0.892, 95% CI: 0.826–0.958, p < 0.001) and sFlt-1/PIGF (AUC = 0.914, 95% CI: 0.857–0.972, p < 0.001). ROC receiver operating characteristic, APO adverse perinatal outcome, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.

Figure 6.
ROC curve for APO prediction in the subgroup of overweight women by sFlt-1 (AUC = 0.721, 95% CI: 0.531–0.910, p = 0.046), PIGF (AUC = 0.912, 95% CI: 0.811–1.000, p < 0.001) and sFlt-1/PIGF (AUC = 0.931, 95% CI: 0.845–0.999, p < 0.001). ROC receiver operating characteristic, APO adverse perinatal outcome, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.
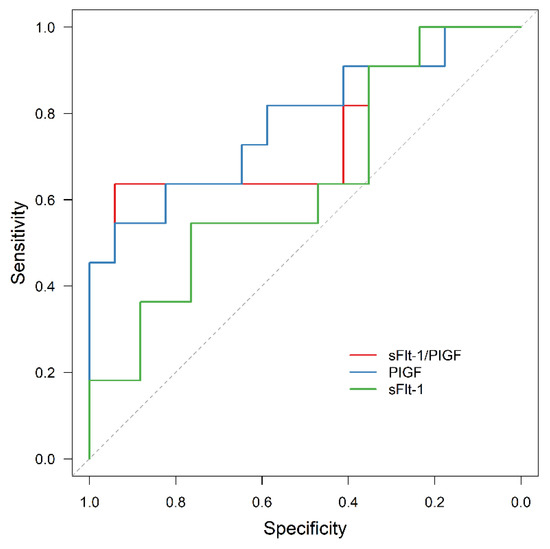
Figure 7.
ROC curve for APO prediction in the subgroup of obese women by sFlt-1 (AUC = 0.642, 95% CI: 0.428–0.855, p = 0.213), PIGF (AUC = 0.781, 95% CI: 0.596–0.966, p = 0.014) and sFlt-1/PIGF (AUC = 0.754, 95% CI: 0.552–0.956, p = 0.025). ROC receiver operating characteristic, APO adverse perinatal outcome, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.
3.4. AMO Prediction by sFlt-1/PIGF
For the whole study cohort, ROC analysis revealed a predictive value for sFlt-1/PIGF and AMO (AUC = 0.667, 95% CI: 0.566–0.768; p = 0.003), as well as for sFlt-1 (AUC = 0.694, 95% CI: 0.598–0.790; p = 0.001), but not for PIGF (AUC = 0.605, 95% CI: 0.495–0.715, p = 0.061). Due to the small number of events, we did not perform a subgroup analysis.
ROC analysis is shown in Figure 8.
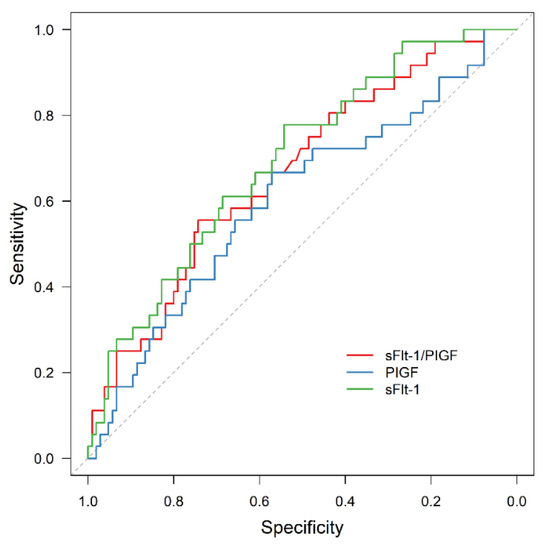
Figure 8.
ROC curve for AMO prediction by sFlt-1 (AUC = 0.694, 95% CI: 0.598–0.790, p = 0.001), PIGF (AUC = 0.605, 95% CI: 0.495–0.715, p = 0.061) and sFlt-1/PIGF (AUC = 0.667, 95% CI: 0.566–0.768, p = 0.003). ROC receiver operating characteristic, APO adverse perinatal outcome, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor.
3.5. Exclusion of APO by sFlt-1/PIGF
In the group of normal-weight women, 8 of 84 (10%) women showed an sFlt-1/PIGF level < 38 and in the overweight cohort, 5 of 29 (17%) women. None of these women were affected by APO. In contrast, 7 of 28 (25%) obese women showed an sFlt-1/PIGF level < 38, but two of them were affected by APO. Both women developed superimposed PE and high blood pressure despite their antihypertensive medications, which led to iatrogenic preterm delivery with admission of the newborn to NICU.
4. Discussion
This study confirmed that obese women with PE show significantly lower levels of sFlt 1 compared to normal or overweight women. However, APO prediction by sFlt-1/PIGF is still possible, although with a poorer prognostic value compared to normal or overweight women.
As obesity is an epidemic disease affecting more and more pregnancies, outcome prediction in this subgroup is of urgent need. Outcome prediction in pre-eclamptic women using (anti-)angiogenic factors, especially sFlt-1/PIGF, was evaluated over the last decade [16,19,34]. In our study cohort, sFlt-1/PIGF had a high prognostic value for APO, a finding in line with previous publications of our working group [20,24]. Saleh et al. recently suggested a model using sFlt-1/PIGF, proteinuria and gestational age for outcome prediction in pregnancies diagnosed with PE [35]. Patients with normal sFlt-1/PIGF were affected by APO in less than 5%. However, in several studies APO prediction by sFlt-1/PIGF remains inconclusive or with a reduced prognostic value compared to our data [36,37]. One explanation for this observation might be the inconsistency in the reported outcome data; therefore, we decided to define APO according to the recently published Delphi consensus by Duffy et al. [29]. Another reason might be the focus on special cohorts, such as twin pregnancies [38]. Our results suggest that it might be necessary to investigate obese women as an independent subgroup to avoid a distorted result.
The main finding of our study is that APO can be predicted by sFlt-1/PIGF, not only in the group of normal and overweight women, but also in women with obesity, considering the decreasing prognostic value. This should be taken into account, especially if sFlt-1/PIGF levels are low. In contrast to normal and overweight women [39], obese women are at a higher risk of developing APO, even with sFlt-1/PIGF-levels < 38 and, therefore, these women might be in need of intensified clinical surveillance.
It is unclear why sFlt-1 levels are lower in obese women. In a Finnish case-control study, including 1450 women, sFlt-1 levels in pre-eclamptic obese women were significantly lower compared to women with normal BMI, whereas sFlt-1 levels in the first trimester did not differ [22]. In line with our own results, Suwaki et al. reported an inverse correlation of sFlt-1 and BMI in pre-eclamptic women [40], but no difference in the angiogenic profile of normotensive women. Lobmaier et al. could not find a significant influence of BMI on sFlt-1/PlGF levels, considering that the study group showed a relatively low BMI (22.5 (20.5–25.6) kg/m2) [20]. The profile of sFlt-1/PIGF in obese women without PE remains controversial, as some studies report a higher level of sFlt-1 and PIGF, as well as the opposite [41,42]. A possible explanation might be the above-mentioned “low BMI” study cohorts. Nevertheless, obese women affected by AMO, APO or PE show elevated levels of sFlt-1/PIGF compared to obese women without further complications [43].
Increased plasma volume, as a confounder, might, at least, contribute to our finding of a lower sFlt-1 level, as animal studies rather implicate a higher sFlt-1 release in obese pregnancies [44,45]. On the other hand, TNF-α, a proinflammatory factor elevated in obese women, decreases the sFlt-1 expression in fat tissue [46]. Another reason might be the higher mass of extracellular matrix in fat tissue containing heparin sulfate proteoglycans, which leads to sequestration of sFlt-1 from blood circulation [47]. However, PIGF concentrations were not different in obese and normal-weight women, which is in line with other studies [40]. Therefore, further research needs to be conducted to analyze the role of adipose tissue in the processing of angiogenic factors in PE.
The ratio of sFlt-1 and PIGF was not only a predictor of APO, but also of AMO, although its prognostic value is limited due to the low incidence of AMO in our study cohort. HELLP syndrome, as well as acute kidney failure, were more common in normal-weight women and other events were rare. This finding was unexpected, as obesity itself is a risk factor for HELLP syndrome or other pregnancy complications [48]. One explanation for this finding might be that women with the risk factor of obesity are carefully observed during pregnancy and, therefore, AMO might be prevented in some cases. Rana et al. demonstrated that pre-eclamptic women with a normal angiogenic profile were more likely to be obese and AMO was rare, except for iatrogenic preterm delivery [49]. Therefore, there might be a subgroup of obese women diagnosed with only mild symptoms of PE in the late-onset group, as obesity especially increases the risk of late-onset PE [50].
The limitations of our study were the retrospective design and a relatively small sample size. Furthermore, data on weight gain during pregnancy were not available for all women. We used the levels of sFlt-1/PIGF at the time of diagnosis of PE, but another measurement prior to delivery might give further and more detailed information about the angiogenic profile in PE women. Further, clinicians were aware of the test results. Therefore, sFlt-1/PIGF might have influenced the decision making, especially regarding the timing of delivery. On the one hand, this might have prolonged pregnancies if a low sFlt-1/PIGF was measured and helped to prevent APO. On the other hand, it might have led to an iatrogenic delivery if high serum levels were detected, especially if the mother was severely affected by PE and, therefore, might have prevented AMO. An impact of sFlt-1/PIGF in the decision-making process regarding further therapy of women with PE was described before [51]. Pre-pregnancy BMI was used for the statistical analysis; however, weight gain during pregnancy might influence our results. Interestingly, mean weight gain during pregnancy was lower in obese women. These results might be biased, as severe PE is often accompanied by excessive oedema. Another effect might be the diatetic counselling all obese women received during pregnancy.
5. Conclusions
In conclusion, we could demonstrate that sFlt-1/PIGF is a helpful tool for APO prediction in normal and overweight, but also in obese women. However, for decisions on the timing of delivery and perinatal management, it should be taken into account that the performance is not as precise in obese women as it is in normal-weight women. Clinicians should be aware that normal sFlt-1/PIGF might not rule out APO and, therefore, obese women with PE are a subgroup of patients who should be managed carefully.
Author Contributions
Conceptualization, A.K., B.K. and O.G.; methodology, A.K., B.K. and O.G.; software, A.K. and B.H.; validation, A.K., L.D., J.U.O., S.M.L., B.K. and O.G.; formal analysis, A.K., L.D. and B.H.; investigation, A.K. and L.D.; resources, B.K.; data curation, A.K. and L.D.; writing—original draft preparation, A.K. and O.G.; writing—review and editing, A.K., L.D., J.U.O., S.M.L., B.K. and O.G.; visualization, A.K.; supervision, B.K. and O.G.; project administration A.K., B.K. and O.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by our local Institutional Ethics Board (Ethikkommission der Fakultät für Medizin der Technischen Universität München, protocol number 232/17). The study was not registered in a public trial registry.
Informed Consent Statement
In accordance with article §27, Section 4 of the Bavarian hospital law (“Bayrisches Krankenhausgesetz—BayKrG”), patient data, collected in the context of the hospital medical treatment relationship, may be used for training, further education, research purposes in the hospital, and statistics for the hospital. However, the patient data must remain in the custody of the hospital. For this reason, a separate declaration of informed consent of the patients in this retrospective hospital data collection was waived. There were no minors included in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- van Esch, J.J.A.; van Heijst, A.F.; de Haan, A.F.J.; van der Heijden, O.W.H. Early-onset preeclampsia is associated with perinatal mortality and severe neonatal morbidity. J. Matern. Fetal Neonatal Med. 2017, 30, 2789–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Guidelines Approved by the Guidelines Review Committee. In WHO Recommendations: Policy of Interventionist versus Expectant Management of Severe Pre-Eclampsia before Term; World Health Organization: Geneva, Switzerland, 2018.
- Tomimatsu, T.; Mimura, K.; Endo, M.; Kumasawa, K.; Kimura, T. Pathophysiology of preeclampsia: An angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens. Res. 2017, 40, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Schaefer-Graf, U.; Ensenauer, R.; Gembruch, U.; Groten, T.; Flothkötter, M.; Hennicke, J.; Köhrle, J.; Möhler, J.; Kühnert, M.; Schmittendorf, A.; et al. Obesity and Pregnancy. Guideline of the German Society of Gynecology and Obstetrics (S3-Level, AWMF Registry No. 015-081, June 2019). Geburtshilfe Frauenheilkd 2021, 81, 279–303. [Google Scholar] [CrossRef]
- Strauss, A.; Rochow, N.; Kunze, M.; Hesse, V.; Dudenhausen, J.W.; Voigt, M. Obesity in pregnant women: A 20-year analysis of the German experience. Eur. J. Clin. Nutr. 2021, 75, 1757–1763. [Google Scholar] [CrossRef]
- Schummers, L.; Hutcheon, J.A.; Bodnar, L.M.; Lieberman, E.; Himes, K.P. Risk of Adverse Pregnancy Outcomes by Prepregnancy Body Mass Index. Obstet. Gynecol. 2015, 125, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Hypertensive Pregnancy Disorders: Diagnosis and Therapy. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AMWF Registry No. 015/018, March 2019). Available online: http://www.awmf.org/leitlinien/detail/II/015-018.html (accessed on 27 April 2022).
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New Gestational Phase–Specific Cutoff Values for the Use of the Soluble fms-Like Tyrosine Kinase-1/Placental Growth Factor Ratio as a Diagnostic Test for Preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef]
- Rana, S.; Salahuddin, S.; Mueller, A.; Berg, A.H.; Thadhani, R.I.; Karumanchi, S.A. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2018, 13, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Chen, C.-N.; Jeng, S.-F.; Su, Y.-N.; Chen, C.-Y.; Chou, H.-C.; Tsao, P.-N.; Hsieh, W.-S. The sFlt-1/PlGF ratio as a predictor for poor pregnancy and neonatal outcomes. Pediatr. Neonatol. 2017, 58, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; on behalf of the Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karge, A.; Beckert, L.; Moog, P.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Abel, K.; Flechsenhar, S.; Kuschel, B.; Graupner, O. Role of sFlt-1/PIGF ratio and uterine Doppler in pregnancies with chronic kidney disease suspected with Pre-eclampsia or HELLP syndrome. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2020, 22, 160–166. [Google Scholar] [CrossRef]
- Gómez-Arriaga, P.I.; Herraiz, I.; López-Jiménez, E.A.; Escribano, D.; Denk, B.; Galindo, A. Uterine artery Doppler and sFlt-1/PlGF ratio: Prognostic value in early-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2014, 43, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Graupner, O.; Lobmaier, S.M.; Ortiz, J.U.; Karge, A.; Kuschel, B. sFlt-1/PlGF ratio for the prediction of the time of delivery. Arch. Gynecol. Obstet. 2018, 298, 567–577. [Google Scholar] [CrossRef]
- Karge, A.; Seiler, A.; Flechsenhar, S.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Axt-Fliedner, R.; Enzensberger, C.; Abel, K.; Kuschel, B.; et al. Prediction of adverse perinatal outcome and the mean time until delivery in twin pregnancies with suspected pre-eclampsia using sFlt-1/PIGF ratio. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2021, 24, 37–43. [Google Scholar] [CrossRef]
- Lobmaier, S.M.; Figueras, F.; Mercade, I.; Perello, M.; Peguero, A.; Crovetto, F.; Ortiz, J.U.; Crispi, F.; Gratacos, E. Angiogenic factorsvsDoppler surveillance in the prediction of adverse outcome among late-pregnancy small-for- gestational-age fetuses. Ultrasound Obstet. Gynecol. 2014, 43, 533–540. [Google Scholar] [CrossRef]
- Dröge, L.; Herraiz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, T.; Finnpec, F.T.; Heinonen, S.; Hämäläinen, E.; Pulkki, K.; Romppanen, J.; Laivuori, H. Impact of obesity on angiogenic and inflammatory markers in the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort. Int. J. Obes. 2019, 43, 1070–1081. [Google Scholar] [CrossRef] [Green Version]
- Zera, C.A.; Seely, E.W.; Wilkins-Haug, L.E.; Lim, K.-H.; Parry, S.I.; McElrath, T.F. The association of body mass index with serum angiogenic markers in normal and abnormal pregnancies. Am. J. Obstet. Gynecol. 2014, 211, 247.e1–247.e7. [Google Scholar] [CrossRef] [PubMed]
- Graupner, O.; Karge, A.; Flechsenhar, S.; Seiler, A.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Axt-Fliedner, R.; Enzensberger, C.; Abel, K.; et al. Role of sFlt-1/PlGF ratio and feto-maternal Doppler for the prediction of adverse perinatal outcome in late-onset pre-eclampsia. Arch. Gynecol. Obstet. 2020, 301, 375–385. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Weinstein, L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1982, 142, 159–167. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of Preeclampsia. Hypertens. Pregnancy 2003, 22, 143–148. [Google Scholar] [CrossRef]
- Duffy, J.M.; Cairns, A.E.; Richards-Doran, D.; Hooft, J.V.; Gale, C.; Brown, M.; Chappell, L.; Grobman, W.A.; Fitzpatrick, R.; Karumanchi, S.A.; et al. A core outcome set for pre-eclampsia research: An international consensus development study. BJOG 2020, 127, 1516–1526. [Google Scholar] [CrossRef]
- Voigt, M.; Rochow, N.; Schneider, K.T.M.; Hagenah, H.-P.; Scholz, R.; Hesse, V.; Wittwer-Backofen, U.; Straube, S.; Olbertz, D. New percentile values for the anthropometric dimensions of singleton neonates: Analysis of perinatal survey data of 2007–2011 from all 16 states of Germany. Z. Geburtshilfe Neonatol. 2014, 218, 210–217. [Google Scholar] [CrossRef]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating charac-teristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Graupner, O.; Enzensberger, C. Prediction of Adverse Pregnancy Outcome Related to Placental Dysfunction Using the sFlt-1/PlGF Ratio: A Narrative Review. Geburtshilfe Frauenheilkd. 2021, 81, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; Alblas, M.M.; Nieboer, D.; Neuman, R.I.; Vergouwe, Y.; Brussé, I.A.; Duvekot, J.J.; Steyerberg, E.W.; Versendaal, H.J.; Danser, A.H.J.; et al. Prediction of pre-eclampsia-related complications in women with suspected or confirmed pre-eclampsia: Development and internal validation of clinical prediction model. Ultrasound Obstet. Gynecol. 2021, 58, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Stolz, M.; Zeisler, H.; Heinzl, F.; Binder, J.; Farr, A. An sFlt-1:PlGF ratio of 655 is not a reliable cut-off value for predicting perinatal outcomes in women with preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2018, 11, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Simón, E.; Permuy, C.; Sacristán, L.; Zamoro-Lorenci, M.J.; Villalaín, C.; Galindo, A.; Herraiz, I. sFlt-1/PlGF ratio for the prediction of delivery within 48 hours and adverse outcomes in expectantly managed early-onset preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2020, 22, 17–23. [Google Scholar] [CrossRef]
- Saleh, L.; Tahitu, S.I.; Danser, A.J.; Meiracker, A.H.V.D.; Visser, W. The predictive value of the sFlt-1/PlGF ratio on short-term absence of preeclampsia and maternal and fetal or neonatal complications in twin pregnancies. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2018, 14, 222–227. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Suwaki, N.; Masuyama, H.; Nakatsukasa, H.; Masumoto, A.; Sumida, Y.; Takamoto, N.; Hiramatrsu, Y. Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am. J. Obstet. Gynecol. 2006, 195, 1687–1692. [Google Scholar] [CrossRef]
- Mijal, R.S.; Holzman, C.B.; Rana, S.; Karumanchi, S.A.; Wang, J.; Sikorskii, A. Midpregnancy levels of angiogenic markers in relation to maternal characteristics. Am. J. Obstet. Gynecol. 2011, 204, 244.e1–244.e12. [Google Scholar] [CrossRef] [Green Version]
- Faupel-Badger, J.M.; Staff, A.C.; Thadhani, R.; Powe, C.E.; Potischman, N.; Hoover, R.N.; Troisi, R. Maternal angiogenic profile in pregnancies that remain normotensive. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Heimberger, S.; Mueller, A.; Ratnaparkhi, R.; Perdigao, J.L.; Rana, S. Angiogenic factor abnormalities and risk of peripartum complications and prematurity among urban predominantly obese parturients with chronic hypertension. Pregnancy Hypertens. Int. J. Women Cardiovasc. Health 2020, 20, 124–130. [Google Scholar] [CrossRef]
- Spradley, F.T.; Palei, A.C.; Granger, J.P. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol. Rep. 2013, 1, e00081. [Google Scholar] [CrossRef] [PubMed]
- Spradley, F.T.; Palei, A.C.; Granger, J.P. Increased risk for the development of preeclampsia in obese pregnancies: Weighing in on the mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1326–R1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herse, F.; Fain, J.N.; Janke, J.; Engeli, S.; Kuhn, C.; Frey, N.; Weich, H.A.; Bergmann, A.; Kappert, K.; Karumanchi, S.A.; et al. Adipose Tissue-Derived Soluble Fms-Like Tyrosine Kinase 1 Is an Obesity-Relevant Endogenous Paracrine Adipokine. Hypertension 2011, 58, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef] [Green Version]
- Lisonkova, S.; Razaz, N.; Sabr, Y.; Muraca, G.M.; Boutin, A.; Mayer, C.; Joseph, K.; Kramer, M.S. Maternal risk factors and adverse birth outcomes associated with HELLP syndrome: A population-based study. BJOG 2020, 127, 1189–1198. [Google Scholar] [CrossRef]
- Rana, S.; Schnettler, W.T.; Powe, C.; Wenger, J.; Salahuddin, S.; Cerdeira, A.S.; Verlohren, S.; Perschel, F.H.; Arany, Z.; Lim, K.-H.; et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens. Pregnancy 2013, 32, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Mbah, A.K.; Kornosky, J.L.; Kristensen, S.; August, E.M.; Alio, A.P.; Marty, P.J.; Belogolovkin, V.; Bruder, K.; Salihu, H.M. Super-obesity and risk for early and late pre-eclampsia. BJOG 2010, 117, 997–1004. [Google Scholar] [CrossRef]
- Klein, E.; Schlembach, D.; Ramoni, A.; Langer, E.; Bahlmann, F.; Grill, S.; Schaffenrath, H.; van der Does, R.; Messinger, D.; Verhagen-Kamerbeek, W.D.; et al. Influence of the sFlt-1/PlGF Ratio on Clinical Decision-Making in Women with Sus-pected Preeclampsia. PLoS ONE 2016, 11, e0156013. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).