Simplified TAVR Procedure: How Far Is It Possible to Go?
Abstract
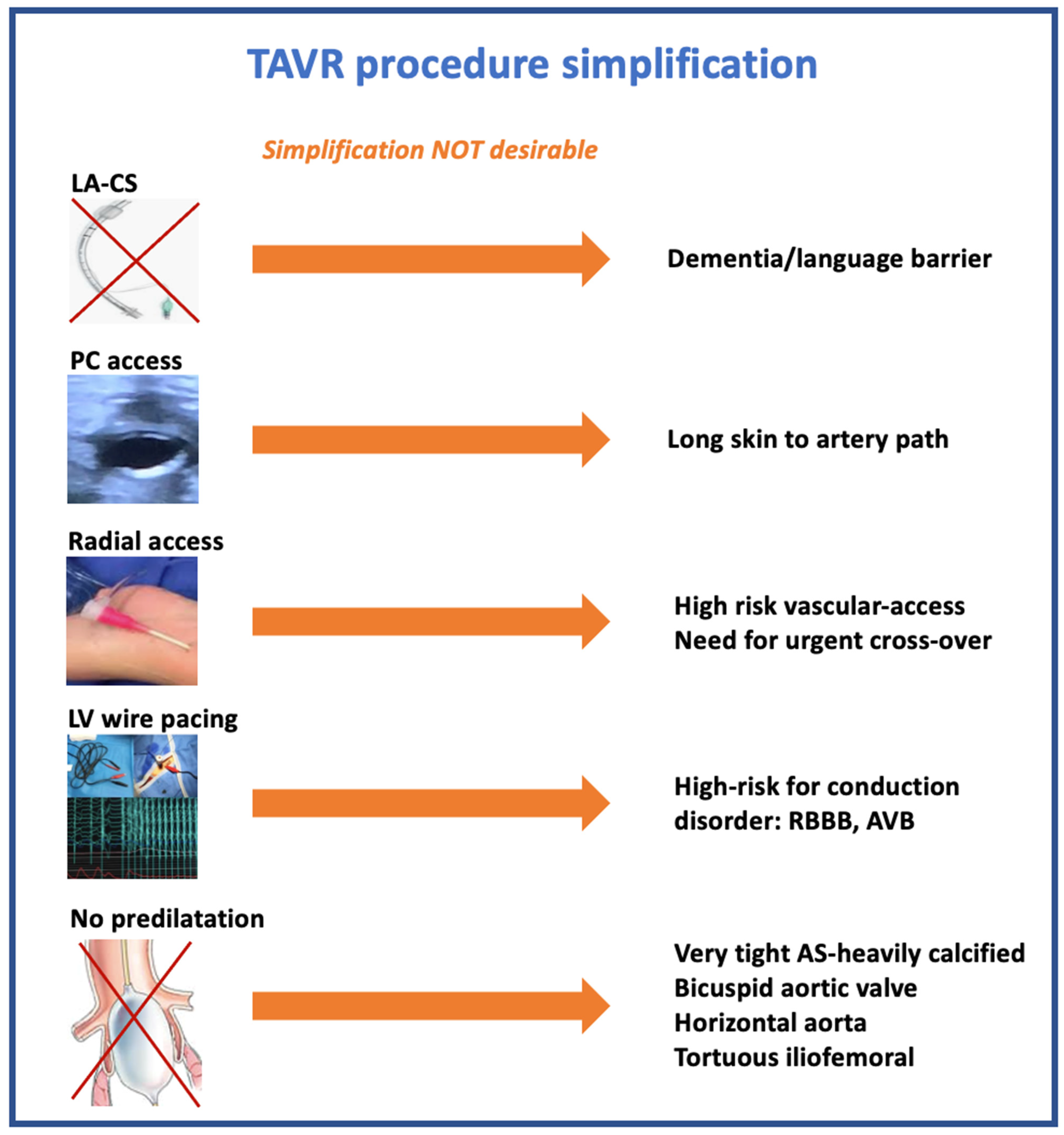
1. Introduction
2. Sedation or General Anesthesia
Hybrid Room or Catheterization Laboratory
3. Vascular Access
3.1. Percutaneous vs. Surgical Femoral Approach
3.2. Femoral vs. Alternative Access
3.3. Minimalist Secondary Access
Radial Access
4. Left Ventricle Wire Pacing
5. Direct Implantation of the THV
6. Per-Procedural Echography
7. Short or No Monitoring
8. Early Discharge
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, D.A.; Lauck, S.B.; Cairns, J.A.; Humphries, K.H.; Cook, R.; Welsh, R.; Leipsic, J.; Genereux, P.; Moss, R.; Jue, J.; et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. JACC Cardiovasc. Interv. 2019, 12, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Eng. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Eng. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Eng. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Eng. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Barbanti, M.; Van Mourik, M.S.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Santo Bortone, A.; Densem, C.G.; Van der Kley, F.; et al. Optimizing patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019, 15, 147–154. [Google Scholar] [CrossRef]
- Barker, M.; Sathananthan, J.; Perdoncin, E.; Devireddy, C.; Keegan, P.; Grubb, K.; Pop, A.M.; Depta, J.P.; Rai, D.; Abtahian, F.; et al. Same day discharge Post-Transcatheter Aortic Valve Replacement during the COVID-19 pandemic: The Multicenter PROTECT TAVR Study. JACC Cardiovasc. Interv. 2022, 15, 590–598. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Lansky, A.J.; Webb, J.; Roffi, M.; Toggweiler, S.; Reinthaler, M.; Wang, D.; Hutchinson, N.; Wendler, O.; Hildick-Smith, D.; et al. Local versus general anesthesia for transcatheter aortic valve implantation (TAVR)—Systematic review and meta-analysis. BMC Med. 2014, 12, 41. [Google Scholar] [CrossRef]
- Oguri, A.; Yamamoto, M.; Mouillet, G.; Gilard, M.; Laskar, M.; Eltchaninoff, H.; Fajadet, J.; Lung, B.; Donzeau-Gouge, P.; Leprince, P.; et al. Clinical outcomes and safety of transfemoral aortic valve implantation under general versus local anesthesia: Subanalysis of the French Aortic National CoreValve and Edwards 2 registry. Circ. Cardiovasc. Interv. 2014, 7, 602–610. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Lurz, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; et al. General versus Local Anesthesia with Conscious Sedation in Transcatheter Aortic Valve Implantation: The Randomized SOLVE-TAVI Trial. Circulation 2020, 142, 1437–1447. [Google Scholar] [CrossRef]
- Butala, N.M.; Chung, M.; Secemsky, E.A.; Manandhar, P.; Marquis-Gravel, G.; Kosinski, A.S.; Vemulapalli, S.; Yeh, R.W.; Cohen, D.J. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Variation in Practice and Outcomes. JACC Cardiovasc. Interv. 2020, 13, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Fujita, B.; Hengstenberg, C.; Frerker, C.; Beckmann, A.; Möllmann, H.; Walther, T.; Bekeredjian, R.; Böhm, M.; Pellegrini, C.; et al. Conscious Sedation Versus General Anesthesia in Transcatheter Aortic Valve Replacement: The German Aortic Valve Registry. JACC Cardiovasc. Interv. 2018, 11, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Vemulapalli, S.; Kohsaka, S.; Shimamura, K.; Stebbins, A.; Kumamaru, H.; Nelson, A.J.; Kosinski, A.; Maeda, K.; Bavaria, J.E.; et al. Practice Patterns and Outcomes of Transcatheter Aortic Valve Replacement in the United States and Japan: A report from joint data harmonization initiative of STS/ACC TVT and J-TVT. J. Am. Heart Assoc. 2022, 11, e023848. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Akodad, M.; Aldhaheri, E.; Marin, G.; Roubille, F.; Macia, J.-C.; Gandet, T.; Delseny, D.; Schmutz, L.; Lattuca, B.; Robert, P.; et al. Transcatheter aortic valve replacement performed with selective telemetry monitoring: A prospective study. Int. J. Cardiol. 2021, 330, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, P.A.; Mohananey, D.; Nikolic, K.; Bangalore, S.; Slovut, D.P.; Mathew, V.; Thourani, V.H.; Rode’s-Cabaud, J.; Nuñez-Gil, I.; Shah, T.; et al. Comparison of local versus general anesthesia in patients undergoing transcatheter aortic valve replacement: A meta-analysis. Catheter. Cardiovasc. Interv. 2018, 91, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Spaziano, M.; Lefèvre, T.; Romano, M.; Eltchaninoff, H.; Leprince, P.; Motreff, P.; Iung, B.; Van Belle, E.; Koning, R.; Verhoye, J.P.; et al. Transcatheter Aortic Valve Replacement in the Catheterization Laboratory Versus Hybrid Operating Room: Insights From the FRANCE TAVI Registry. Focus on Trends in Surgical and Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N. Eng. J. Med. 2012, 366, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, M.; Yamawaki, M.; Tokuda, T.; Tsutumi, M.; Mori, S.; Sakamoto, Y.; Kobayashi, N.; Hirano, K.; Ito, Y. The novel echo-guided ProGlide technique during percutaneous transfemoral transcatheter aortic valve implantation. J. Interv. Cardiol. 2018, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.N.; Rao, S.V. Percutaneous or surgical access for transfemoral transcatheter aortic valve implantation. J. Thorac. Dis. 2018, 10, S3595–S3598. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Gurvitch, R.; Leipsic, J.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Cheung, A.; Ye, J.; Webb, J.G. Percutaneous aortic valve replacement: Vascular outcomes with a fully percutaneous procedure. J. Am. Coll. Cardiol. 2012, 59, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Spillemaeker, H.; Kyheng, M.; Belin-Vincent, C.; Delhaye, C.; Piérache, A.; Denimal, T.; Verdier, B.; Debry, N.; Moussa, M.; et al. Ultrasound Guidance to Reduce Vascular and Bleeding Complications of Percutaneous Transfemoral Transcatheter Aortic Valve Replacement: A Propensity Score-Matched Comparison. J. Am. Heart Assoc. 2020, 9, e014916. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, F.; Akodad, M.; Macia, J.C.; Gandet, T.; Lattuca, B.; Schmutz, L.; Gervasoni, R.; Nogue, E.; Nagot, N.; Levy, G.; et al. Vascular Complications and Bleeding After Transfemoral Transcatheter Aortic Valve Implantation Performed through Open Surgical Access. Am. J. Cardiol. 2015, 116, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Du Cailar, C.; Gandet, T.; Du Cailar, M.; Albat, B. A simple sheath removal after open trans-femoral catheterization procedure: The ZIP technique. Eur. J. Cardiothorac. Surg. 2014, 45, 746–748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olasinska-Wisniewska, A.; Grygier, M.; Lesiak, M.; Araszkiewicz, A.; Trojnarska, O.; Komosa, A.; Misterski, M.; Jemielity, M.; Proch, M.; Grajek, S. Femoral artery anatomy-tailored approach in transcatheter aortic valve implantation. Postepy Kardiol. Interwencyjnej 2017, 13, 150–156. [Google Scholar] [CrossRef]
- Sinning, J.M.; Horack, M.; Grube, E.; Gerckens, U.; Erbel, R.; Eggebrecht, H.; Zahn, R.; Linke, A.; Sievert, H.; Figulla, H.R.; et al. The impact of peripheral arterial disease on early outcome after transcatheter aortic valve implantation: Results from the German Transcatheter aortic valve interventions registry. Am. Heart J. 2012, 164, 102–110. [Google Scholar] [CrossRef]
- Modine, T.; Lemesle, G.; Azzaoui, R.; Sudre, A. Aortic valve implantation with the CoreValve ReValving system via left carotid artery access: First case report. J. Thorac. Cardiovasc. Surg. 2010, 140, 928–929. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Schreiber, C.; Bleiziffer, S.; Ruge, H.; Mazzitelli, D.; Hutter, A.; Tassani, P.; Lange, R. Transcatheter aortic valve implantation through the ascending aorta: An alternative option for no-access patients. Heart Surg. Forum 2009, 12, E63–E64. [Google Scholar] [CrossRef]
- Ye, J.; Cheung, A.; Lichtenstein, S.V.; Carere, R.G.; Thompson, C.R.; Pasupati, S.; Webb, J.G. Transapical aortic valve implantation in humans. J. Thorac. Cardiovasc. Surg. 2006, 131, 1194–1196. [Google Scholar] [CrossRef]
- Petronio, A.S.; De Carlo, M.; Giannini, C.; De Caro, F.; Bortolotti, U. Subclavian TAVI: More than an alternative access route. EuroIntervention 2013, 9, S33–S37. [Google Scholar] [CrossRef]
- Watanabe, M.; Takahashi, S.; Yamaoka, H.; Sueda, T.; Piperata, A.; Zirphile, X.; Leroux, L.; Peltan, J.; Labrousse, L. Comparison of Transcarotid vs. Transfemoral Transcatheter aortic valve implantation. Circ. J. 2018, 82, 2518–2522. [Google Scholar] [CrossRef]
- Wee, I.J.Y.; Stonier, T.; Harrison, M.; Choong, A.M.T.L. Transcarotid transcatheter aortic valve implantation: A systematic review. J. Cardiol. 2018, 71, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, F.; Choteau, R.; Cayla, G.; Chamard, C.; Lounes, Y.; Lattuca, B.; Macia, J.C.; Delseny, D.; Akodad, M.; Gandet, T. Transcarotid versus transfemoral access in patients undergoing transcatheter aortic valve replacement with complex aortofemoral anatomy. Catheter. Cardiovasc. Interv. 2020, 97, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Chevalier, B.; Lefèvre, T.; Spaziano, M.; Unterseeh, T.; Champagne, S.; Benamer, H.; Sanguineti, F.; Garot, P.; Hovasse, T. Implementation of the transradial approach as an alternative vascular access for transcatheter aortic valve replacement guidance: Experience from a high-volume center. Catheter. Cardiovasc. Interv. 2019, 93, 1367–1373. [Google Scholar] [CrossRef]
- Rathore, S.; Stables, R.H.; Pauriah, M.; Hakeem, A.; Mills, J.D.; Palmer, N.D.; Perry, R.A.; Morris, J.L. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: A randomized study. JACC Cardiovasc. Interv. 2010, 3, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Szűcsborus, T.; Sasi, V.; Nagy, F.; Jambrik, Z.; Nemes, A.; Varga, A.; Bertrand, O.F.; Ruzsa, Z. Distal radial secondary access for transcatheter aortic valve implantation: The minimalistic approach. Cardiovasc. Revasc. Med. 2021. [Google Scholar] [CrossRef]
- Nazif, T.; Sanchez, C.; Whisenant, B.; Forrest, J.; Yakubov, S.J.; Grossman, P.; Arshi, A.; Menees, D.; Terre, J.; Orford, J.; et al. Analysis of the initial United States experience with the Biotrace Tempo temporary pacing lead in transcatheter aortic valve replacement and other cardiac procedures. J. Am. Coll. Cardiol. 2018, 71, 1285. [Google Scholar] [CrossRef]
- Faurie, B.; Souteyrand, G.; Staat, P.; Godin, M.; Caussin, C.; Van Belle, E.; Mangin, L.; Meyer, P.; Dumonteil, N.; Abdellaoui, M.; et al. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2449–2459. [Google Scholar] [CrossRef]
- Suarez, K.; Banchs, J.E. A Review of Temporary Permanent Pacemakers and a Comparison with Conventional Temporary Pacemakers. J. Innov. Card. Rhythm Manag. 2019, 10, 3652–3661. [Google Scholar] [CrossRef]
- Leon, M.B.; Piazza, N.; Nikolsky, E.; Blackstone, E.H.; Cutlip, D.E.; Kappetein, A.P.; Krucoff, M.W.; Mack, M.; Mehran, R.; Miller, C.; et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. Eur. Heart J. 2011, 32, 205–217. [Google Scholar] [CrossRef]
- Toutouzas, K.; Benetos, G.; Voudris, V.; Drakopoulou, M.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Antonopoulos, A.; Kosmas, E.; Iakovou, I.; et al. Pre-Dilatation Versus No Pre-Dilatation for Implantation of a Self-Expanding Valve in All Comers Undergoing TAVR: The DIRECT Trial. JACC Cardiovasc. Interv. 2019, 12, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, F.; Robert, P.; Akodad, M.; Macia, J.-C.; Gandet, T.; Delseny, D.; Chettouh, M.; Schmutz, L.; Robert, G.; Levy, G.; et al. Prior Balloon Valvuloplasty Versus Direct Transcatheter Aortic Valve Replacement: Results From the DIRECTAVI Trial. JACC Cardiovasc Interv. 2020, 13, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Okamoto, M.; Schymik, G.; Jacobshagen, C.; Rothe, J.; Treede, H.; Kerber, S.; Frank, D.; Bramlage, P.; Sykorova, L.; et al. Degree of valve calcification in patients undergoing transfemoral transcatheter aortic valve implantation with and without balloon aortic valvuloplasty: Findings from the multicenter EASE-IT TF registry. Catheter. Cardiovasc. Interv. 2019, 94, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Luk, N.H.V.; De Backer, O.; Søndergaard, L. Simplification and optimization of transcatheter aortic valve implantation—Fast-track course without compromising safety and efficacy. BMC Cardiovasc. Disord. 2018, 18, 231. [Google Scholar] [CrossRef]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Arsenault, M.; Beaudoin, J.; Bernier, M.; Dahou, A.; Khalique, O.K.; Asch, F.M.; Toubal, O.; et al. Association of Paravalvular Regurgitation With 1-Year Outcomes After Transcatheter Aortic Valve Replacement with the SAPIEN 3 Valve. JAMA Cardiol. 2017, 2, 1208–1216. [Google Scholar] [CrossRef]
- Toggweiler, S.; Stortecky, S.; Holy, E.; Zuk, K.; Cuculi, F.; Nietlispach, F.; Sabti, Z.; Suciu, R.; Maier, W.; Jamshidi, P.; et al. The Electrocardiogram After Transcatheter Aortic Valve Replacement Determines the Risk for Post-Procedural High-Degree AV Block and the Need for Telemetry Monitoring. JACC Cardiovasc. Interv. 2016, 9, 1269–1276. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Sammour, Y.; Mangieri, A.; Kadri, A.; Karrthik, A.; Banerjee, K.; Kaur, M.; Giannini, F.; Pagliaro, B.; Ancona, M.; et al. The Utility of Rapid Atrial Pacing Immediately Post-TAVR to Predict the Need for Pacemaker Implantation. JACC Cardiovasc. Interv. 2020, 13, 1046–1054. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement. JACC Sci. Expert. Panel J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Durand, E.; Avinée, G.; Gillibert, A.; Tron, C.; Bettinger, N.; Bouhzam, N.; Gilard, M.; Verhoye, J.-P.; Koning, R.; Lefevre, T.; et al. Analysis of length of stay after transfemoral transcatheter aortic valve replacement: Results from the FRANCE TAVI registry. Clin. Res. Cardiol. 2021, 110, 40–49. [Google Scholar] [CrossRef]
- Lauck, S.B.; Sathananthan, J.; Park, J.; Achtem, L.; Smith, A.; Keegan, P.; Hawkey, M.; Brandwein, R.; Webb, J.G.; Wood, D.A.; et al. Post-procedure protocol to facilitate next-day discharge: Results of the multidisciplinary, multimodality but minimalist TAVR study. Catheter. Cardiovasc. Interv. 2020, 96, 450–458. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leclercq, F.; Meunier, P.A.; Gandet, T.; Macia, J.-C.; Delseny, D.; Gaudard, P.; Mourad, M.; Schmutz, L.; Robert, P.; Roubille, F.; et al. Simplified TAVR Procedure: How Far Is It Possible to Go? J. Clin. Med. 2022, 11, 2793. https://doi.org/10.3390/jcm11102793
Leclercq F, Meunier PA, Gandet T, Macia J-C, Delseny D, Gaudard P, Mourad M, Schmutz L, Robert P, Roubille F, et al. Simplified TAVR Procedure: How Far Is It Possible to Go? Journal of Clinical Medicine. 2022; 11(10):2793. https://doi.org/10.3390/jcm11102793
Chicago/Turabian StyleLeclercq, Florence, Pierre Alain Meunier, Thomas Gandet, Jean-Christophe Macia, Delphine Delseny, Philippe Gaudard, Marc Mourad, Laurent Schmutz, Pierre Robert, François Roubille, and et al. 2022. "Simplified TAVR Procedure: How Far Is It Possible to Go?" Journal of Clinical Medicine 11, no. 10: 2793. https://doi.org/10.3390/jcm11102793
APA StyleLeclercq, F., Meunier, P. A., Gandet, T., Macia, J.-C., Delseny, D., Gaudard, P., Mourad, M., Schmutz, L., Robert, P., Roubille, F., Cayla, G., & Akodad, M. (2022). Simplified TAVR Procedure: How Far Is It Possible to Go? Journal of Clinical Medicine, 11(10), 2793. https://doi.org/10.3390/jcm11102793





