Abstract
Multiple sclerosis is a chronic disease that may lead to different types of symptoms and disabilities. with the better quality of life and decreased disability due to early diagnosis and the availability of disease-modifying therapies (DMTs), the treating physician is increasingly asked to counsel patients on its effects on fertility and reproduction. In particular, reproductive issues are still scarcely studied and discussed in men. Among the still open questions are the following: (a) Does multiple sclerosis cause infertility per sè? (b) Is multiple sclerosis correlated with conditions that increase the risk of infertility? (c) Do DMTs or other therapies for multiple sclerosis impact gonadal function in men? The aim of this review is to provide an overview on the available literature data about the reproductive issues unique to men with multiple sclerosis, underlining the numerous areas where evidence is lacking and, therefore, the priorities for future research.
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disease of the central nervous system (CNS) [1,2], affecting 2.8 million people in 2020 [3]. Recent data [2] estimate the 2020 global prevalence to be 35.9 (95% CI: 35.87, 35.95) per 100,000 people. It is the most common cause of non-traumatic disability in young adults [4,5].
Its etiology is still unknown, as with most autoimmune diseases. Nevertheless, in recent decades, it has been proposed that several environmental factors (e.g., ultraviolet B light exposure, vitamin D levels, Epstein–Barr virus infection, obesity, and smoking) [6,7] may trigger MS within predisposed subjects. In particular, some genetic polymorphisms, mostly involving immune pathway genes [2], have been described as being associated with a higher risk of developing MS. The most significant genetic risk factor is the HLA DRB1*1501 haplotype [2,6,7]. Moreover, sex hormones possibly interfere with disease onset [8,9].
The typical pathological hallmark of MS is represented by distinct CNS lesions, easily recognized in white matter through magnetic resonance imaging (MRI), leading to plaques whose genesis involves the mechanism of demyelination, inflammation, and glial reaction [2,6,10]. The inflammatory infiltrates contain T-lymphocytes, dominated by MHC class I restricted CD8+ T-cells; B-cells and plasma cells are also present, although in much lower numbers [10]. Lesions of MS are typically located in periventricular, cortical, juxtacortical, and infratentorial brain regions and in the spinal cord [11]. A new lesion, in particular in an eloquent area, leads to corresponding symptoms that vary depending on location and size of the lesions. Moreover, macroscopic (or MRI-visible) lesions are just the tip of the iceberg; many more lesions can be seen at the microscopic level, and even more in deep and cortical grey matter, underlining the so-called “clinical-radiological paradox”, where clinical disability evidence does not always present a clear association with structural imaging studies.
MS is clinically characterized in most cases by the subacute onset of focal or multifocal neurological symptoms due to the involvement of different CNS systems (optic nerves, both hemispheres, brainstem, cerebellum, and spinal cord), which are either fully or partially reversible [6]. These episodes develop over hours to days and then reach a plateau lasting several weeks, followed by a gradual recovery that occurs spontaneously or with a high dose of steroidal therapy. These focal episodes are defined as “relapses”. When MS presents with multiple new focal symptoms occurring sequentially and ceasing afterwards, the onset of relapsing–remitting MS is signaled. Each relapse corresponds to at least one symptomatic lesion, but generally within a clinical attack, approximately 10 “asymptomatic” lesions are noted in MRI [6]. Later on in the course of the disease (usually 10–20 years from onset), after a period of tumultuous, inflammatory disease, most patients develop a slow worsening of their neurological condition with no distinct transition in between, shaping the so-called “progressive course”. In some patients (5–15%), MS starts as progressive from onset [2,6].
MS, as with most autoimmune diseases, is more common in women than in men, with the female-to-male ratio varying from 2:1 to 3:1 [12]. The age of onset increases after adolescence, reaching a peak at the third decade [13] in relapsing–remitting forms, between 25 and 35 years [7], and at the fifth decade [13] in primary–progressive forms. Nevertheless, some gender issues do exist. In particular, men receive diagnosis of MS at an older age than women [8,9,13], and the course of the disease is prognostically worse in men, with a higher tendency towards motor and disabling relapses and towards a progressive course [8,9,13].
However, MS incidence remains higher in both men and women of reproductive age. Therefore, especially as a consequence of earlier diagnoses and more efficacious therapies, it is common to treat patients with reproductive desires. In women, we know that the disease improves during pregnancy and worsens after birth [14,15], leading scientists to hypothesize a connection with sex hormones levels [9]. However, the possible effects of the disease and of disease-modifying therapies (DMTs) on fertility and pregnancy are not yet comprehensively determined in the literature [16], and in particular, they are severely under-investigated in men. Indeed, there are still unanswered questions on male fertility and MS; among them are the following: (a) Does MS cause infertility per se? (b) Is MS correlated with conditions that increase the risk of infertility? (c) Do DMTs or other therapies for MS impact gonadal function in men?
2. Methods
The main objective of this narrative review is to provide an overview of the possible reproductive issues unique to men with multiple sclerosis, with the aim of providing clinicians with a useful tool for counseling and highlighting possible research priorities. A review of literature published in English until July 2021 was conducted on PubMed, Scopus, and Google Scholar, using the search terms “multiple sclerosis” and/or “disease modifying agents” and “gonadotoxicity” and/or “fertility” and/or “male fertility”. In addition to that, reference lists of primary and review articles were reviewed for additional publications. Selected articles were verified by two separate authors and discrepancies were discussed within the team.
3. Results
3.1. Reproductive History of Men with Multiple Sclerosis
The issue of fertility in men with MS has not been exhaustively studied. There are currently no studies in the literature evaluating the prospective time to conceive of men with MS; only indirect information from population registries is available. Epidemiology studies have reported a decreased number of pregnancies in partners of males with MS. In particular, a Swedish population study described how men with a new diagnosis of MS were less likely to have had children in the 5 years before diagnosis [17]. A similar study in the Danish population reached similar conclusions some years before: out of more than 4 million Danish men, those who had become parents in the 5 years before had a significantly lower risk of being diagnosed with MS [18]. Of course, these results do not prove a correlation between the disease and reduced fertility, since these data are not calculated on the total number of men who searched for a pregnancy: those who had not had children may have not tried to conceive to begin with. Additional indirect information may be inferred from examining infertile couples. In 2008, a study reported worse seminal parameters (total count, motility, and morphology) in 68 MS patients, compared to 48 age-matched controls [19]. Data from the Danish population registry showed how the prevalence of multiple sclerosis was higher (odds ratio (OR) = 1.61, 95% confidence interval (CI): 1.04–2.51) in male partners of couples treated for male infertility than in male partners of couples whose infertility was not linked to a male factor [20].
3.2. Possible Rationale for Reduced Fertility in Men with Multiple Sclerosis
It is known that MS can directly affect sexual function in men, depending on the location of the plaques. Erectile function is controlled by parasympathetic fibers originating from S2 to S4; ejaculation depends on the coordinated action of thoracolumbar sympathetic fibers from segments T10–L2 and somatic fibers from S2 to S4. Central regulation also plays a role in ejaculation [21]. Impotence in MS can be ascribed to a suprasacral, parasympathetic, or peripheral autonomic lesion. Up to 70% of men with MS present with erection dysfunction, and up to 50% of men present with alterations in ejaculation [22,23]. Moreover, some medications like benzodiazepine or tricyclic antidepressants, used as symptomatic therapies, could affect sexual function as well. Possible therapeutic solutions include phosphodiesterase inhibitors (i.e., Sildenafil) for erectile dysfunction, assisted ejaculation, or surgical sperm retrieval coupled with assisted reproduction techniques [24,25,26]. Multiple sclerosis could also disrupt hypothalamus–pituitary function, with reduced levels of sex hormones as a consequence of central neurological damage [19,27,28,29,30]. Indeed, hypogonadotropic hypogonadism has been described, especially among male with more progressive disease [19].
Pathogenetic causes of MS may also have a cross sectional or longitudinal link with infertility. MS is defined as an autoimmune response to unidentified antigen(s) in genetically susceptible individuals [31]. Genetic predisposition, autoimmunity, and chronic inflammation could all be linked to male infertility pathogenesis. For example, genetic susceptibility to infertility has been hypothesized due to increasing evidence of a link between it and risk of degenerative diseases, but current data do not support a correlation with MS specifically [32]. A correlation between male infertility and autoimmunity has also been reported: various autoimmune conditions, such as type I diabetes mellitus or Crohn’s disease, were found to be more prevalent in infertile men [33,34]. Chronic inflammation has been linked to both MS and male infertility [20]. In particular, chronic inflammation was reported in men with low levels of testosterone [35], a connection worth examining when discussing MS. Bove et al. described a strong positive association between testicular hypofunction and subsequent MS (rate ratio = 4.62, 95% CI = 2.3–8.24, p < 0.0001), and low testosterone levels have been associated with higher disability in patients already diagnosed with MS [36]. These findings are in agreement with the fact that age at diagnosis in men is higher than in women, and that this age corresponds with the age when testosterone levels start to decline [37]. However, it is not possible to discriminate whether there is a causal relationship, a longitudinal association, or if the results are only a casualty [38]. An increased risk of MS has been reported in transgender women after hormonal transition that suppresses testosterone levels [39], suggesting a protective role of the hormone.
Oxidative stress, linked to chronic inflammation, plays a role in MS [40] and may be another cause of worse sperm quality, since it is well known that human semen is susceptible to an imbalance between reactive oxygen species (ROS) and antioxidants. In particular, spermatozoa’s plasma membranes are rich in polyunsaturated fatty acids and have low concentrations of scavenging enzymes, and therefore, their ROS-induced peroxidation causes a decrease in mobility. Moreover, ROS may cause direct damage to mitochondria, damaging sperm DNA and suppressing sperm capacitation and acrosome reaction [41,42].
In summary, men with MS could be subfertile due to a direct negative impact of the disease on erection and/or ejaculation or due to concurrent hypogonadism, both of central (direct action on the hypothalamus–pituitary–testis axis) and peripheral (chronic inflammation and autoimmunity) origin. To these possible causes, we need to add the gonadotoxic risk of the received medications.

Table 1.
Possible rationale for reduced fertility in men with multiple sclerosis.
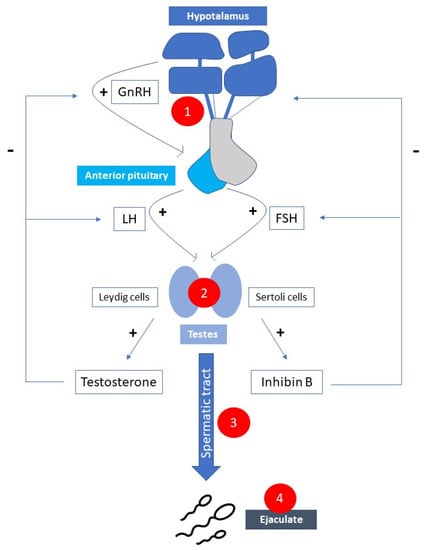
Figure 1.
Possible causes of male subfertility correlated with multiple sclerosis. 1. Hypogonadotropic hypogonadism; 2. Hypergonadotropic hypogonadism; 3. Chronic inflammation and/or oxidative stress; 4. Erection/ejaculation dysfunction. (GnRH—gonatropin-releasing hormone; FSH—follicle stimulation hormone; LH—luteinizing hormone).
3.3. Gonadotoxicity of Disease-Modifying Therapies in Male Patients
Data about the effect of DMTs on male reproduction, both as impact on spermatogenesis and as risk connected with fathering a child during therapies, are sparse and inconclusive [16]. In particular, no other data except those from preclinical animal studies reporting on prescribing information are available for most of the newest drugs approved for treatment [43]. The fact that patients are frequently subjected to the newest second-line therapies after the failure of first-line ones further complicates our ability to quantify the absolute damage of specific treatments.
Table 2 reports what is known about the effect of DMTs on male fertility.

Table 2.
Summary of potential gonadotoxicity of disease-modifying therapies (DMT) for multiple sclerosis in males.
Glatiramer Acetate and Interferon are both immunomodulatory drugs that have been used in the treatment of MS for more than 20 years. Two multicenter prospective studies reported the outcome of newborns fathered by men exposed to these drugs at the time of conception; no increased risk of spontaneous abortion, adverse fetal outcomes, or congenital malformations were reported [44,45]. As for the effects on spermatogenesis, no effect was reported in in vitro and animal registration studies [46], and while the aforementioned studies do not investigate sperm parameters, the reporting of healthy pregnancies and no increase in adverse fetal outcomes are reassuring for fertility as well.
Mitoxantrone (no longer used in clinical practice for the treatment of young men with MS), is a type II topoisomerase inhibitor that disrupts DNA synthesis and DNA repair; it is known to cause oligo or azoospermia in men (usually reversible). Its use therefore carries a strong recommendation of semen cryopreservation before starting the therapy [47,48]. Of note, the evidence is mostly from data of onco-hematological patients using chemotherapy containing Mitoxantrone among other drugs. Three other drugs, used less frequently and off-label, are reported to have an effect on male reproduction: Cyclophosphamide, Azathioprine, and Methotrexate. All three have an FDA warning for fertility and pregnancy on their prescribing information documents. Cyclophosphamide, an alkylating agent that disrupts cell division in rapidly dividing cells, produces permanent damage to the seminiferous epithelium, causing dose-dependent azoospermia (not always reversible) as well as an increased risk of abortion in pregnancies fathered by men treated with this therapy [49,50,51]. Semen cryopreservation before therapy and a washout of 6 months (if fresh sperm is used to conceive) are recommended [52]. Azathioprine, an immunosuppressive agent that acts through its effects as an antagonist of purine metabolism, causes oligospermia in animal studies [53,54], but no alteration of sperm parameters during chronic treatment was reported in small cohorts of men with inflammatory bowel disease [55,56] and rheumatological diseases [57,58]. A washout of 6 months before trying to conceive is advised on prescribing documents. However, the concerns are mainly based on a prescription database study reporting 4 malformations out of 52 children (7.4%) fathered by treated men [59]. The study has several limitations (numerosity, consumption deduced based on filled prescriptions and not on directly collected data), and the same authors found no detrimental effect in a more recent bigger cohort [60]. Methotrexate, a dihydrofolate reductase inhibitor, was reported to cause dose-dependent reversible oligospermia, while other studies described minimal or no effect on spermatogenesis [61]. As for pregnancies, while it is abortifacient in women, no increased risk of complications was reported in pregnancies of babies fathered by men during therapy [62,63,64,65]. However, a 3-month washout period before trying to conceive is recommended.
All the other DMTs were approved for use in MS relatively recently, and very few data about their effect on male reproduction are available.
For example, we do not have enough data to exclude an effect on spermatogenesis and/or pregnancy of the more recently approved monoclonal antibodies, such as Natalizumab, Alemtuzumab, Ocrelizumab, Daclizumab, and Rituximab (off-label). Recently, a case-control study did not find any difference in terms of sperm parameters and gonadal steroid levels between 16 men treated with Natalizumab or Ocrelizumab for 12 months and healthy controls [66]. Since Alemtuzumab targets CD52s, which are also present in the male reproductive tract [67], a negative effect on sperm parameters was hypothesized [68], but a small human study (13 patients) did not find any significant variations after 1, 3, and 6 months of therapy [69].
Teriflunomide is a dihydro-orotate dehydrogenase inhibitor, a key mitochondrial enzyme in the de novo pyrimidine synthesis pathway, which leads to a reduction in proliferation of activated T- and B-lymphocytes without causing cell death. It has an FDA warning for pregnancy in women, since it has been associated with an increased risk of fetal malformation in some reports [70], but others concluded that outcomes were consistent with those of the general population [71,72]. We only have limited data for children fathered by men treated with this drug. Teriflunomide can be detected in semen, but two small studies (22 and 18 pregnancies) did not report an increased risk of malformation or other adverse events in pregnancies from fathers using Teriflunomide [71,73].
Teratogenic risk was described for mothers taking Fingolimod at conception [74], but no data are available for men.
We also have no evidence for the effect of Cladribine (approved for MS in 2019) on male reproduction, but, since it interferes with DNA synthesis, an effect on spermatogenesis has been hypothesized [75]. A washout period of 6 months before conception is suggested in the prescribing information documents.
3.4. Gonadotoxicity of Symptomatic and Supporting Therapies
Men with MS often find symptomatic (defined as therapies that aim to improve symptoms without modifying the course of the disease) and supporting therapies (treatment aimed to prevent, control, or relieve complications and/or side effects of the main treatment, to improve the patient’s comfort and quality of life) to be beneficial. However, some of them may have a detrimental effect on fertility (usually reversible). Of note, a relevant limitation to consider is that none of the human studies on the effects of such therapies on male fertility is focused specifically on men affected by MS. However, data extrapolated from published cohorts of men who underwent the same treatments in similar dosages are a good starting point to choose the treatment with the minimum possible effect and to help disentangle the complex interactions among the possible causes of subfertility in men with MS.
Antidepressant medications, which are widely used to treat both mood disorders and neuropathic pain, have been associated with sexual dysfunction (impaired libido, erectile dysfunction) and alteration of semen parameters [76]. Benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs) have been reported to cause hyperprolactinemia, which may cause hypogonadotropic hypogonadism and lower testosterone levels [77]. Animal and human studies have shown a worsening in sperm parameters using SSRIs [78]. In particular, some human studies have underlined an association between sperm DNA fragmentation and SSRI use: in a randomized, single-blind trial examining 60 men treated with Sertraline or behavioral therapy for 3 months, DNA fragmentation significantly increased in the treatment group, while sperm concentration and normal morphology decreased [79]. Increased DNA fragmentation was also reported in the absence of sperm parameter alterations after short periods of treatment with Paroxetine [80]. The described effects were correlated with the duration of antidepressant use [81].
On the other hand, Bupropion, a norepinephrine–dopamine reuptake inhibitor (NDRI) used to treat depression, showed no effect on sperm parameters in rats [82] and lower sexual side effects in humans compared to SSRIs [83]; thus, it is probably a better fit in depressed or anxious young male patients planning to have children [79]. A recent RCT aimed to investigate differences in DNA fragmentation among 68 healthy males after 6 weeks of treatment with 60 mg Duloxetine or placebo; no significant difference was found between the two groups. Furthermore, semen parameters and serum hormones were not altered by the treatment, showing how Duloxetine, and potentially other SNRIs, may be a good choice for young men desiring to conceive [84].
Scant data exist for the effect of TCAs on spermatogenesis. An animal study examining semen parameters of 40 mice after treatment with venlafaxine, venlafaxine plus ascorbic acid, amitriptyline, amitriptyline plus ascorbic acid, or placebo found decreased parameters and increased oxidative stress in the amitriptyline group [85]. Also, two animal studies suggested a mutagenic effect of amitriptyline on germ cells [86,87].
Antiepileptic drugs, used for the treatment of neuropathic pain in men with MS, may affect male reproduction [88]. In particular, Carbamazepine was associated with altered sexual hormone levels in men with epilepsy, especially decreasing bioactive testosterone [89]. Sperm parameters were reported to be altered as well: Azadi-Pooya et al. found a significant reduction in concentration, motility, and morphology in 8 men after 3 months of Carbamazepine therapy for epilepsy [90]. Reis et al. compared 63 men with epilepsy taking Carbamazepine with 55 healthy controls and found a 10-times higher rate of erectile dysfunction in the first group, even after having controlled for confounders [91].
The effects of delta-9-tetrahydrocannabinol (THC) on human semen are yet to be better quantified. We know that deltha-9-THC can cross the blood–testis barrier in certain individuals [92], but there is no definitive evidence of a deleterious effect on seminal parameters. Some studies have reported lower sperm concentrations and lower total sperm counts in marijuana users: a Danish cohort study on marijuana use (1215 participants, where 45% had smoked marijuana within the last 3 months) found 28% lower sperm concentration and 29% lower total sperm count in regular users after adjustment for confounders [93]. However, another recent cohort study (662 subfertile men) showed significantly higher sperm concentrations and total sperm counts in users [94]. Whan et al. incubated sperm with THC at therapeutic dosages (0.032 μM) and reported a 28% reduction in motility in samples with worse general parameters and no reduction in those with better parameters. Higher concentrations, similar to those for recreational use, instead caused a significant reduction in the motility of all samples (56% reduced motility in the fraction with best fertilizing potential and 28% in the poorer sperm population) [95]. In addition, THC may play a role in male libido, with augmented short-term libido and diminished ability to achieve erection [96,97]. Paternal THC use was also linked to neurobehavioral effects in offspring (decreased attentional accuracy), using an animal model [98].
Of note, an important limitation to consider is that most of these cohorts are almost entirely comprised of subfertile men smoking the psychoactive drug and not medical cannabis (CBD—cannabidiol) users. Animal studies suggest an effect on male fertility (reduced fertilization rate and number of litters) for CBD, probably due to a negative impact on sperm acrosome reaction [99].
Therapies specifically used to address MS-related fatigue, such as Modafinil, Naltrexone, 4-aminopyrine, and Amantadine (off-label), could potentially have a positive effect on male reproduction by improving sexual function. Modafinil has also been associated with a therapeutic effect on premature ejaculation in animal studies [100], some case reports [101], and a small proof-of-concept study of 55 patients [102]. There are no studies evaluating effects on fertility, but the registration studies using animal models reported no adverse effect in the prescribing information.
Concerns were raised with respect to the effect of phosphodiesterase inhibitors, used to treat erectile dysfunction, on male fertility after the report of premature acrosome reaction in vitro [103]. However, a systematic review and meta-analysis including 11 studies (1317 participants) concluded that there are no adverse effects and reported a small increase in sperm motility and morphology in infertile men [104].
In conclusion, there are several limitations to the application of the current literature to evidence-based practice. Some of them (lack of power, heterogeneity of the population, lack of standardization among different studies) are generic [105], while others (interactions with MS, disability, and DMTs) are specific to MS patients.
Table 3 summarizes the available evidence on the effect of MS symptomatic/supporting therapies on male fertility.

Table 3.
Summary of potential gonadotoxicity of symptomatic/supporting therapies for multiple sclerosis in males.
4. Concluding Remarks
An evaluation of the effect of MS on male reproductive function is complex, as it is the result of several potential factors: the effects of the disease itself on sexual function and/or fertility, the use of multiple DMTs, and symptomatic/supporting therapies.
In this review, we highlighted how limited the currently available data on each of these issues and their interactions are. On the basis of the pathogenesis of the disease and the data reported in the few observational studies available, we know that sexual function is frequently impaired and that an effect on fertility cannot be excluded. As for the medications used, few of them have a demonstrated gonadotoxic/teratogenic effect, but, for the majority of the new DMTs, data are severely lacking. As for supporting/symptomatic therapies, the available evidence, mainly extrapolated from other populations, may aid the clinician in choosing the drug with the minimum potential effect on fertility and pregnancy [111].
The still open questions indicate the research priorities in this field.
Epidemiological data on the reproductive history of affected men should be gathered on a national and supra-national level, to better understand if the potential mechanisms described in the manuscript are related to causing subfertility.
Moreover, the long-term effects of the DMTs, especially the newest immunomodulatory drugs, on sperm quality and their safety for fathering a pregnancy must be collected rigorously and reported to the scientific community. To overcome the limitation due to the relatively low number of patients affected and the great number of therapies available, multicenter studies are desirable.
Gynaecologists, andrologists, and neurologists must be up to date on the issue and able to conduct an in-depth reproductive anamnesis of males with MS. Indeed, an effective collaboration between the different specialists, similar to the networks between oncologists and fertility units [112], would greatly help us to better understand the issue and improve patient management.
Author Contributions
Conceptualization, C.M. and P.A.; literature search, C.M., E.S., I.G. and S.S.; writing—original draft preparation, C.M. and I.G.; writing—review and editing, E.S., S.S., M.I. and P.A.; supervision, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Inglese, M.; Petracca, M. MRI in multiple sclerosis: Clinical and research update. Curr. Opin. Neurol. 2018, 31, 249–255. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. MSCOI Study Group; European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Bansal, A.; Culberson, J.; Peiris, A.N. The Role of Sex Hormones in Multiple Sclerosis. Eur. Neurol. 2018, 80, 93–99. [Google Scholar] [CrossRef]
- Lassmann, H. Pathology and disease mechanisms in different stages of multiple sclerosis. J. Neurol. Sci. 2013, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Ribbons, K.A.; McElduff, P.; Boz, C.; Trojano, M.; Izquierdo, G.; Duquette, P.; Girard, M.; Grand’Maison, F.; Hupperts, R.; Grammond, P.; et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS ONE 2015, 10, e0122686. [Google Scholar] [CrossRef]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 9, 285–291. [Google Scholar] [CrossRef]
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C.; Pregnancy in Multiple Sclerosis Group. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain 2004, 127, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Van Der Walt, A.; Nguyen, A.L.; Jokubaitis, V. Family planning, antenatal and post partum care in multiple sclerosis: A review and update. Med. J. Aust. 2019, 211, 230–236. [Google Scholar] [CrossRef]
- Hedström, A.K.; Hillert, J.; Olsson, T.; Alfredsson, L. Reverse causality behind the association between reproductive history and MS. Mult. Scler. 2014, 20, 406–411. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Jørgensen, K.T.; Stenager, E.; Jensen, A.; Pedersen, B.; Hjalgrim, H.; Kjær, S.; Frisch, M. Reproductive history and risk of multiple sclerosis. Epidemiology 2011, 22, 546–552. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Evaluation of endocrine profile, hypothalamic-pituitary-testis axis and semen quality in multiple sclerosis. J. Neuroendocrinol. 2008, 20, 1368–1375. [Google Scholar] [CrossRef]
- Glazer, C.H.; Tøttenborg, S.S.; Giwercman, A.; Bräuner, E.V.; Eisenberg, M.L.; Vassard, D.; Magyari, M.; Pinborg, A.; Schmidt, L.; Bonde, J.P. Male factor infertility and risk of multiple sclerosis: A register-based cohort study. Mult. Scler. 2018, 24, 1835–1842. [Google Scholar] [CrossRef]
- Fode, M.; Krogh-Jespersen, S.; Brackett, N.L.; Ohl, D.A.; Lynne, C.M.; Sønksen, J. Male sexual dysfunction and infertility associated with neurological disorders. Asian J. Androl. 2012, 14, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Haensch, C.A.; Jorg, J. Autonomic dysfunction in multiple sclerosis. J. Neurol. 2006, 253, I3–I9. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Russo, M.; Dattola, V.; de Luca, R.; Leo, A.; Grisolaghi, J.; Bramanti, P.; Quattrini, F. Sexual Function in Young Individuals with Multiple Sclerosis: Does Disability Matter? J. Neurosci Nurs. 2018, 50, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, J.; Luo, H. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis. Cochrane Database Syst. Rev. 2012, 4, CD009427. [Google Scholar] [CrossRef]
- Lombardi, G.; Macchiarella, A.; del Popolo, G. Efficacy and safety of tadalafil for erectile dysfunction in patients with multiple sclerosis. J. Sex. Med. 2010, 7, 2192–2200. [Google Scholar] [CrossRef]
- Pinter, A.; Cseh, D.; Sarkozi, A.; Illigens, B.M.; Siepmann, T. Autonomic dysregulation in multiple sclerosis. Int. J. Mol. Sci 2015, 16, 16920–16952. [Google Scholar] [CrossRef]
- Michelson, D.; Stone, L.; Galliven, E.; Magiakou, M.A.; Chrousos, G.P.; Sternberg, E.M.; Gold, P.W. Multiple sclerosis is associated with alterations in hypo-thalamic-pituitary-adrenal axis function. J. Clin. Endocrinol. Metab. 1994, 79, 848–853. [Google Scholar]
- Reder, A.T.; Makowiec, R.L.; Lowy, M.T. Adrenal size is increased in multiple sclerosis. Arch Neurol. 1994, 51, 151–154. [Google Scholar] [CrossRef]
- Heesen, C.; Gold, S.M.; Huitinga, I.; Reul, J.M. Stress and hypothalamic-pituitary-adrenal axis function in experimental autoimmune encephalomyelitis and multiple sclerosis—A review. Psychoneuroendocrinology 2007, 32, 604–618. [Google Scholar] [CrossRef]
- Huitinga, I.; Erkut, Z.A.; van Beurden, D.; Swaab, D.F. Impaired hypothalamus-pituitary-adrenal axis activity and more severe multiple sclerosis with hypothalamic lesions. Ann. Neurol. 2004, 55, 37–45. [Google Scholar] [CrossRef]
- Podbielska, M.; O’Keeffe, J.; Hogan, E.L. Autoimmunity in multiple sclerosis: Role of sphingolipids, invariant NKT cells and other immune elements in control of inflammation and neurodegeneration. J. Neurol. Sci. 2018, 385, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Tarin, J.J.; Garcia-Perez, M.A.; Hamatani, T.; Cano, A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod. Biol. Endocrinol. 2015, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Jangir, R.N.; Jain, G.C. Diabetes mellitus induced impairment of male reproductive functions: A review. Curr. Diabetes Rev. 2014, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, W.D.; Li, S.; Baker, L.C.; Eisenberg, M.L. Increased risk of autoimmune disorders in infertile men: Analysis of US claims data. Andrology 2018, 6, 94–98. [Google Scholar] [CrossRef]
- Bobjer, J.; Katrinaki, M.; Tsatsanis, C.; Giwercman, Y.; Giwercman, A. Negative association between testosterone concentration and inflammatory markers in young men: A nested cross sectional study. PLoS ONE 2013, 8, e61466. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Musallam, A.; Healy, B.C.; Raghavan, K.; Glanz, B.; Bakshi, R.; Weiner, H.; De Jager, P.; Miller, K.; Chitnis, T. Low testosterone is associated with disability in men with multiple sclerosis. Mult. Scler. 2014, 20, 1584–1592. [Google Scholar] [CrossRef]
- Bove, R.; Chitnis, T. Sexual disparities in the incidence and course of MS. Clin. Immunol. 2013, 149, 201–210. [Google Scholar] [CrossRef]
- Voci, C. Testicular hypofunction and multiple sclerosis: Cause or consequence? Ann. Neurol. 2014, 76, 765. [Google Scholar] [CrossRef]
- Pakpoor, J.; Wotton, C.J.; Schmierer, K.; Giovannoni, G.; Goldacre, M.J. Gender identity disorders and multiple sclerosis risk: A national record-linkage study. Mult. Scler. 2016, 22, 1759–1762. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Opuwari, C.S.; Henkel, R.R. An update on oxidative damage to spermatozoa and oocytes. Biomed. Res. Int. 2016, 2016, 9540142. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Adoni, T.; Brooks, J.B.B.; Finkelsztejn, A.; da Gama, P.; Grzesiuk, A.; Marques, V.; Parolin, M.; Sato, H.; Varela, D.; et al. Practical Evidence-Based Recommendations for Patients with Multiple Sclerosis Who Want to Have Children. Neurol. Ther. 2018, 7, 207–232. [Google Scholar] [CrossRef]
- Pecori, C.; Giannini, M.; Portaccio, E.; Ghezzi, A.; Hakiki, B.; Pastò, L.; Razzolini, L.; Sturchio, A.; de Giglio, L.; Pozzilli, C.; et al. Paternal therapy with disease modifying drugs in multiple sclerosis and pregnancy outcomes: A prospective observational multicentric study. BMC Neurol. 2014, 14, 114. [Google Scholar] [CrossRef]
- Lu, E.; Zhu, F.; Zhao, Y.; van der Kop, M.; Synnes, A.; Dahlgren, L.; Sadovnick, A.D.; Traboulsee, A.; Tremlett, H. Birth outcomes in newborns fathered by men with multiple sclerosis exposed to disease-modifying drugs. CNS Drugs 2014, 28, 475–482. [Google Scholar] [CrossRef] [PubMed]
- TEVA: Copaxone® Full Prescribing Information. Revised 2016. Available online: http://copaxone.com/pdfs/PrescribingInformation.aspx (accessed on 25 September 2021).
- Frias, S.; Van Hummelen, P.; Meistrich, M.L.; Lowe, X.R.; Hagemeister, F.B.; Shelby, M.D.; Bishop, J.B.; Wyrobek, A.J. NOVP chemotherapy for Hodgkin’s disease transiently induces sperm aneuploidies associated with the major clinical aneuploidy syndromes involving chromosomes X, Y, 18, and 21. Cancer Res. 2003, 63, 44–51. [Google Scholar]
- Lemez, P.; Urbánek, V. Chemotherapy for acute myeloid leukemias with cytosine arabinoside, daunorubicin, etoposide, and mitoxantrone may cause permanent oligoasthenozoospermia or amenorrhea in middle-aged patients. Neoplasma 2005, 52, 398–401. [Google Scholar] [PubMed]
- Bermas, B.L. Paternal safety of anti-rheumatic medications. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 64, 77–84. [Google Scholar] [CrossRef]
- Ghobadi, E.; Moloudizargari, M.; Asghari, M.H.; Abdollahi, M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin. Drug Metab. Toxicol. 2017, 13, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.M.; Robaire, B.; Hales, B.F.; Kelly, S.M. Paternal cyclophosphamide exposure causes decreased cell proliferation in cleavage-stage embryos. Biol. Reprod. 1994, 50, 55–64, Correction in Biol. Reprod. 1994, 50, 711. [Google Scholar] [CrossRef][Green Version]
- Samplaski, M.K.; Nangia, A.K. Adverse effects of common medications on male fertility. Nat. Rev. Urol. 2015, 12, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Bendre, S.V.; Shaddock, J.G.; Patton, R.E.; Dobrovolsky, V.N.; Albertini, R.J.; Heflich, R.H. Lymphocyte Hprt mutant frequency and sperm toxicity in C57BL/6 mice treated chronically with Azathioprine. Mutat. Res. 2005, 578, 1–14. [Google Scholar] [CrossRef]
- Ligumsky, M.; Badaan, S.; Lewis, H.; Meirow, D. Effects of 6-mercaptopurine treatment on sperm production and reproductive performance: A study in male mice. Scand. J. Gastroenterol. 2005, 40, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Mittermaier, C.; Reinisch, W.; Gasche, C.; Waldhoer, T.; Strohmer, H.; Moser, G. Azathioprine treatment and male fertility in inflammatory bowel disease. Gastroenterology 2001, 121, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Valer, P.; Algaba, A.; Santos, D.; Fuentes, M.E.; Nieto, E.; Gisbert, J.P.; López, P.; Quintanilla, E.; García-Alonso, F.J.; Guerra, I.; et al. Evaluation of the quality of semen and sexual function in men with inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 1144–1153. [Google Scholar] [CrossRef]
- Soares, P.M.; Borba, E.F.; Bonfa, E.; Hallak, J.; Correa, A.L.; Silva, C.A. Gonad evaluation in male systemic lupus erythematosus. Arthr. Rheum. 2007, 56, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Uzunaslan, D.; Saygin, C.; Hatemi, G.; Tascilar, K.; Yazici, H. No appreciable decrease in fertility in Behcet’s syndrome. Rheumatology 2014, 53, 828–833. [Google Scholar] [CrossRef][Green Version]
- Norgard, B.; Pedersen, L.; Jacobsen, J.; Rasmussen, S.N.; Sorensen, H.T. The risk of congenital abnormalities in children fathered by men treated with azathioprine or mercaptopurine before conception. Aliment. Pharmacol. Ther. 2004, 19, 679–685. [Google Scholar] [CrossRef]
- Norgard, B.M.; Magnussen, B.; Larsen, M.D.; Friedman, S. Reassuring results on birth outcomes in children fathered by men treated with azathioprine/6-mercaptopurine within 3 months before conception: A nationwide cohort study. Gut 2017, 66, 1761–1766. [Google Scholar] [CrossRef]
- Grosen, A.; Kelsen, J.; Hvas, C.L.; Bellaguarda, E.; Hanauer, S.B. The Influence of Methotrexate Treatment on Male Fertility and Pregnancy Outcome After Paternal Exposure. Inflamm. Bowel Dis. 2017, 23, 561–569. [Google Scholar] [CrossRef]
- Viktil, K.K.; Engeland, A.; Furu, K. Outcomes after anti-rheumatic drug use before and during pregnancy: A cohort study among 150,000 pregnant women and expectant fathers. Scand. J. Rheumatol. 2012, 41, 196–201. [Google Scholar] [CrossRef]
- Wallenius, M.; Lie, E.; Daltveit, A.K.; Salvesen, K.Å.; Skomsvoll, J.F.; Kalstad, S.; Lexberg, Å.S.; Mikkelsen, K.; Kvien, T.K.; Østensen, M. No excess risks in offspring with paternal preconception exposure to disease-modifying antirheumatic drugs. Arthritis Rheumatol. 2015, 67, 296–301. [Google Scholar] [CrossRef]
- Weber-Schoendorfer, C.; Hoeltzenbein, M.; Wacker, E.; Meister, R.; Schaefer, C. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose Methotrexate: An observational cohort study. Rheumatology 2014, 53, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Eck, L.K.; Jensen, T.B.; Mastrogiannis, D.; Torp-Pedersen, A.; Askaa, B.; Nielsen, T.K.; Poulsen, H.E.; Jimenez-Solem, E.; Andersen, J.T. Risk of adverse pregnancy outcome after paternal exposure to methotrexate within 90 days before pregnancy. Obstet. Gynecol. 2017, 129, 707–714. [Google Scholar] [CrossRef]
- D’Amico, E.; Zanghì, A.; Calogero, A.E.; Patti, F. Male fertility in relapsing-remitting multiple sclerosis patients treated with natalizumab and ocrelizumab: A prospective case-control study. Mult. Scler. 2021, 19, 13524585211009208. [Google Scholar] [CrossRef]
- Kirchhoff, C. CD52 is the ’major maturation-associated’ sperm membrane antigen. Mol. Hum. Reprod. 1996, 2, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G.; Nieschlag, E. Human epididymal secreted protein CD52 on ejaculated spermatozoa: Correlations with semen characteristics and the effect of its antibody. Mol. Hum. Reprod. 1997, 3, 1045–1051. [Google Scholar] [CrossRef][Green Version]
- Margolin, D.H.; Rizzo, M.A.; Smith, G.; Arnold, D.L.; Cohen, J.A.; Coles, A.J.; Confavreux, C.; Fox, E.; Hartung, H.; Havrdova, E.; et al. Alemtuzumab treatment has no adverse impact on sperm quality, quantity, or motility: A CARE-MS substudy. J. Neurol. Sci. 2013, 333, e375–e376. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Moberg, J.Y.; Spelman, T.; Magyari, M. Pregnancy Outcomes in Men and Women Treated with Teriflunomide. A Population-Based Nationwide Danish Register Study. Front. Immunol. 2018, 9, 2706. [Google Scholar] [CrossRef]
- Vukusic, S.; Coyle, P.K.; Jurgensen, S.; Truffinet, P.; Benamor, M.; Afsar, S.; Purvis, A.; Poole, E.M.; Chambers, C. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: Clinical study data and 5 years of post-marketing experience. Mult. Scler. 2020, 26, 829–836. [Google Scholar] [CrossRef]
- Kieseier, B.C.; Benamor, M. Pregnancy Outcomes Following Maternal and Paternal Exposure to Teriflunomide During Treatment for Relapsing–Remitting Multiple Sclerosis. Neurol. Ther. 2014, 3, 133–138. [Google Scholar] [CrossRef]
- Karlsson, G.; Francis, G.; Koren, G.; Heining, P.; Zhang, X.; Cohen, J.A.; Kappos, L.; Collins, W. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology 2014, 82, 674–680. [Google Scholar] [CrossRef]
- Cree, B.A. Update on reproductive safety of current and emerging disease-modifying therapies for multiple sclerosis. Mult. Scler. J. 2013, 19, 835–843. [Google Scholar] [CrossRef]
- Beeder, L.A.; Samplaski, M.K. Effect of antidepressant medications on semen parameters and male fertility. Int. J. Urol. 2020, 27, 39–46. [Google Scholar] [CrossRef]
- Coker, F.; Taylor, D. Antidepressant-induced hyperprolactinaemia: Incidence, mechanisms and management. CNS Drugs 2010, 24, 563–574. [Google Scholar] [CrossRef]
- Milosavljević, J.Z.; Milosavljević, M.N.; Arsenijević, P.S.; Milentijević, M.N.; Stefanović, S.M. The effects of selective serotonin reuptake inhibitors on male and female fertility: A brief literature review. Int. J. Psychiatry Clin. Pract. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Akasheh, G.; Sirati, L.; Noshad Kamran, A.R.; Sepehrmanesh, Z. Comparison of the effect of sertraline with behavioral therapy on semen parameters in men with primary premature ejaculation. Urology 2014, 83, 800–804. [Google Scholar] [CrossRef]
- Tanrikut, C.; Feldman, A.S.; Altemus, M.; Paduch, D.A.; Schlegel, P.N. Adverse effect of paroxetine on sperm. Fertil. Steril. 2010, 94, 1021–1026. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Sperm DNA damage and semen quality impairment after treatment with selective serotonin reuptake inhibitors detected using semen analysis and sperm chromatin structure assay. J. Urol. 2008, 180, 2124–2128. [Google Scholar] [CrossRef]
- Cavariani, M.M.; de Almeida Kiguti, L.R.; de Lima Rosa, J.; de Araujo Leite, G.A.; Silva, P.V.E.; Pupo, A.S.; De Grava Kempinas, W. Bupropion treatment increases epididymal contractility and impairs sperm quality with no effects on the epididymal sperm transit time of male rats. J. Appl. Toxicol. 2015, 35, 1007–1016. [Google Scholar] [CrossRef]
- Modell, J.G.; Katholi, C.R.; Modell, J.D.; DePalma, R.L. Comparative sexual side effects of bupropion, fluoxetine, paroxetine, and sertraline. Clin. Pharmacol. Ther. 1997, 61, 476–487. [Google Scholar] [CrossRef]
- Punjani, N.; Kang, C.; Flannigan, R.; Bach, P.; Altemus, M.; Kocsis, J.H.; Wu, A.; Pierce, H.; Schlegel, P.N. Impact of duloxetine on male fertility: A randomised controlled clinical trial. Andrologia 2021, 53, e14207. [Google Scholar] [CrossRef]
- Bandegi, L.; Anvari, M.; Vakili, M.; Khoradmehr, A.; Mirjalili, A.; Talebi, A.R. Effects of antidepressants on parameters, melondiadehyde, and diphenyl-2-picryl-hydrazyl levels in mice spermatozoa. Int. J. Reprod Biomed. 2018, 16, 365–372. [Google Scholar] [CrossRef]
- Chowdary, P.S.; Rao, M.S. Cytogenetic effects of amitriptyline hydrochloride in somatic and germ cells of mice. Toxicol. Lett. 1987, 39, 199–204. [Google Scholar] [CrossRef]
- Hassanane, M.S.; Hafiz, N.; Radwan, W.; El-Ghor, A.A. Genotoxic evaluation for the tricyclic antidepressant drug, amitriptyline. Drug Chem. Toxicol. 2012, 35, 450–455. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Nangia, A.K. Psychotropics and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 63–101. [Google Scholar] [CrossRef]
- Herzog, A.G.; Drislane, F.W.; Schomer, D.L.; Pennell, P.B.; Bromfield, E.B.; Dworetzky, B.A.; Farina, E.L.; Frye, C.A. Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 2005, 65, 1016–1020. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.; Farazdaghi, M.; Ashjazadeh, N. Effects of carbamazepine on semen parameters in men with newly diagnosed epilepsy. Iran. J. Neurol. 2015, 14, 168–170. [Google Scholar]
- Reis, R.M.; de Angelo, A.G.; Sakamoto, A.C.; Ferriani, R.A.; Lara, L.A. Altered sexual and reproductive functions in epileptic men taking carbamazepine. J. Sex. Med. 2013, 10, 493–499. [Google Scholar] [CrossRef]
- Lee, M.S.; Lanes, A.; Ginsburg, E.S.; Fox, J.H. Delta-9 THC can be detected and quantified in the semen of men who are chronic users of inhaled cannabis. J. Assist. Reprod. Genet. 2020, 37, 1497–1504. [Google Scholar] [CrossRef]
- Gundersen, T.D.; Jørgensen, N.; Andersson, A.M.; Bang, A.K.; Nordkap, L.; Skakkebæk, N.E.; Priskorn, L.; Juul, A.; Jensen, T.K. Association between use of marijuana and male reproductive hormones and semen quality: A study among 1215 healthy young men. Am. J. Epidemiol. 2015, 182, 473–481. [Google Scholar] [CrossRef]
- Nassan, F.L.; Arvizu, M.; Mínguez-Alarcón, L.; Williams, P.L.; Attaman, J.; Petrozza, J.; Hauser, R.; Chavarro, J.; EARTH Study Team. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. 2019, 34, 715–723. [Google Scholar] [CrossRef]
- Whan, L.B.; West, M.C.; McClure, N.; Lewis, S. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil. Steril. 2006, 85, 653. [Google Scholar] [CrossRef]
- Aversa, A.; Rossi, F.; Francomano, D.; Bruzziches, R.; Bertone, C.; Santiemma, V.; Spera, G. Early endothelial dysfunction as a marker of vasculogenic erectile dysfunction in young habitual cannabis users. Int. J. Impot. Res. 2008, 20, 566. [Google Scholar] [CrossRef]
- Payne, K.S.; Mazur, D.J.; Hotaling, J.M.; Pastuszak, A.W. Cannabis and Male Fertility: A Systematic Review. J. Urol. 2019, 202, 674–681. [Google Scholar] [CrossRef]
- Levin, E.D.; Hawkey, A.B.; Hall, B.J.; Cauley, M.; Slade, S.; Yazdani, E.; Kenou, B.; White, H.; Wells, C.; Rezvani, A.H.; et al. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 2019, 74, 106806. [Google Scholar] [CrossRef]
- Carvalho, R.K.; Souza, M.R.; Santos, M.L.; Guimarães, F.S.; Pobbe, R.L.H.; Andersen, M.L.; Mazaro-Costa, R. Chronic cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reprod. Toxicol. 2018, 81, 34–40. [Google Scholar] [CrossRef]
- Marson, L.; Yu, G.; Farber, N.M. The effects of oral administration of d-modafinil on male rat ejaculatory behavior. J. Sex. Med. 2010, 7 Pt 1, 70–78. [Google Scholar] [CrossRef]
- Serefoglu, E.C. On-demand d-modafinil may be an effective treatment option for lifelong premature ejaculation: A case report. Andrologia 2016, 48, 121–122. [Google Scholar] [CrossRef]
- Tuken, M.; Kiremit, M.C.; Serefoglu, E.C. On-demand Modafinil Improves Ejaculation Time and Patient-reported Outcomes in Men with Lifelong Premature Ejaculation. Urology 2016, 94, 139–142. [Google Scholar] [CrossRef]
- Glenn, D.R.; McVicar, C.M.; McClure, N.; Lewis, S.E. Sildenafil citrate improves sperm motility but causes a premature acrosome reaction in vitro. Fertil. Steril. 2007, 87, 1064–1070. [Google Scholar] [CrossRef]
- Tan, P.; Liu, L.; Wei, S.; Tang, Z.; Yang, L.; Wei, Q. The Effect of Oral Phosphodiesterase-5 Inhibitors on Sperm Parameters: A Meta-analysis and Systematic Review. Urology 2017, 105, 54–61. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Nangia, A.K. Challenges of Obtaining Evidence-Based Information Regarding Medications and Male Fertility. Adv. Exp. Med. Biol. 2017, 1034, 5–11. [Google Scholar] [CrossRef]
- Calabrò, R.S.; D’Aleo, G.; Sessa, E.; Leo, A.; De Cola, M.C.; Bramanti, P. Sexual dysfunction induced by intrathecal baclofen administration: Is this the price to pay for severe spasticity management? J. Sex. Med. 2014, 11, 1807–1815. [Google Scholar] [CrossRef]
- Taneja, N.; Kucheria, K.; Jain, S.; Maheshwari, M.C. Effect of phenytoin on semen. Epilepsia 1994, 35, 136–140. [Google Scholar] [CrossRef]
- Otoom, S.; Batieneh, H.; Hassan, Z.; Daoud, A. Effects of long-term use Topiramate on fertility and growth parameter in adult male rats. Neuro Endocrinol. Lett. 2004, 25, 351–355. [Google Scholar]
- Osuntokun, O.S.; Olayiwola, G.; Oladele, A.; Ola, I.; AyokaAbiodun, O. Chronic administration of gabapentin and a gabapentin-carbamazepine combination reversibly suppress testicular function in male Wistar rats (Rattus norvegicus). Pathophysiology 2017, 24, 63–69. [Google Scholar] [CrossRef]
- Valevski, A.; Modai, I.; Zbarski, E.; Zemishlany, Z.; Weizman, A. Effect of amantadine on sexual dysfunction in neuroleptic-treated male schizophrenic patients. Clin. Neuropharmacol. 1998, 21, 355–357. [Google Scholar]
- Drobnis, E.Z.; Nangia, A.K. Introduction to Medication Effects on Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 1–4. [Google Scholar] [CrossRef]
- Massarotti, C.; Scaruffi, P.; Lambertini, M.; Sozzi, F.; Remorgida, V.; Anserini, P. Beyond fertility preservation: Role of the oncofertility unit in the reproductive and gynecological follow-up of young cancer patients. Hum. Reprod. 2019, 34, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).