PB1F2 from Influenza A Virus Regulates the Interaction between Cytochrome C and Cardiolipin
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Media, and Chemicals
Protein Expression and Purification
2.2. Preparation of Nanodiscs
2.3. Liposome Pull down Assay
2.4. Nanodisc-Based Fluorescence Detection
2.5. SEC (Size Exclusion Chromatography) Analysis
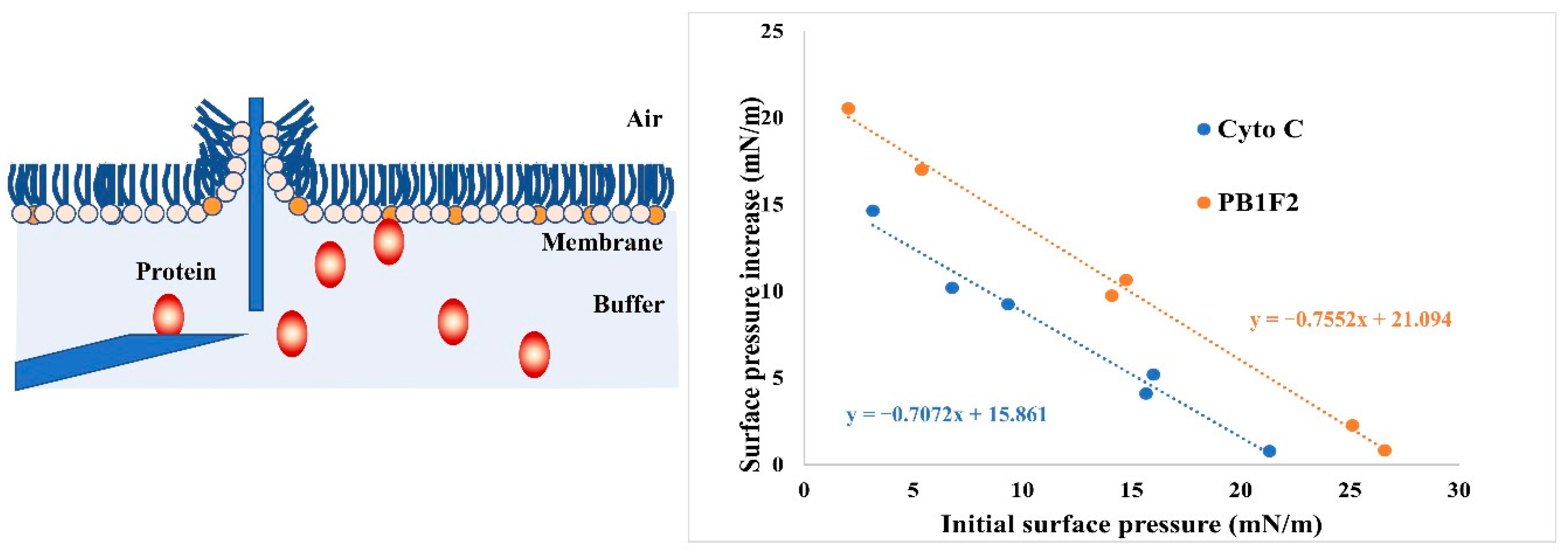
2.6. Binding Affinity Measurements by Using Monolayer Technology
2.7. Statistics and Reproducibility
3. Results
3.1. Interaction of PB1F2 and CytC with Cardiolipins
3.2. PB1F2 Promotes the Dissociation of Cytochrome c from Cardiolipins
3.2.1. Liposome Pulldown
3.2.2. Nanodiscs Assay
3.3. SEC Analysis
3.4. LB Microtrough Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, E.S.; Nucci, N.V.; Fuglestad, B.; Tommos, C.; Wand, A.J. Defining the Apoptotic Trigger. J. Biol. Chem. 2015, 290, 30879–30887. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The Expanding Role of Mitochondria in Apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar]
- Ascenzi, P.; Polticelli, F.; Marino, M.; Santucci, R.; Coletta, M. Cardiolipin Drives Cytochrome c Proapoptotic and Antiapoptotic Actions. IUBMB Life 2011, 63, 160–165. [Google Scholar] [CrossRef]
- Li, X.-X.; Tsoi, B.; Li, Y.-F.; Kurihara, H.; He, R.-R. Cardiolipin and Its Different Properties in Mitophagy and Apoptosis. J. Histochem. Cytochem. 2015, 63, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Calvo, P.A.; Malide, D.; Gibbs, J.; Schubert, U.; Bacik, I.; Basta, S.; O’Neill, R.; Schickli, J.; Palese, P.; et al. A Novel Influenza A Virus Mitochondrial Protein That Induces Cell Death. Nat. Med. 2001, 7, 1306–1312. [Google Scholar] [CrossRef]
- Chakrabarti, A.K.; Pasricha, G. An Insight into the PB1F2 Protein and Its Multifunctional Role in Enhancing the Pathogenicity of the Influenza A Viruses. Virology 2013, 440, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Philipps, A.; Oehring, H.; Schwarzer, K.; Eitner, A.; Wutzler, P.; Zell, R. Current Knowledge on PB1-F2 of Influenza A Viruses. Med. Microbiol. Immunol. 2011, 200, 69–75. [Google Scholar] [CrossRef]
- Leymarie, O.; Meyer, L.; Tafforeau, L.; Lotteau, V.; Costa, B.D.; Delmas, B.; Chevalier, C.; Le Goffic, R. Influenza Virus Protein PB1-F2 Interacts with CALCOCO2 (NDP52) to Modulate Innate Immune Response. J. Gen. Virol. 2017, 98, 1196–1208. [Google Scholar] [CrossRef]
- Smith, A.M.; Adler, F.R.; McAuley, J.L.; Gutenkunst, R.N.; Ribeiro, R.M.; McCullers, J.A.; Perelson, A.S. Effect of 1918 PB1-F2 Expression on Influenza A Virus Infection Kinetics. PLoS Comput. Biol. 2011, 7, e1001081. [Google Scholar] [CrossRef]
- McAuley, J.L.; Hornung, F.; Boyd, K.L.; Smith, A.M.; McKeon, R.; Bennink, J.; Yewdell, J.W.; McCullers, J.A. Expression of the 1918 Influenza A Virus PB1-F2 Enhances the Pathogenesis of Viral and Secondary Bacterial Pneumonia. Cell Host Microbe 2007, 2, 240–249. [Google Scholar] [CrossRef]
- Zamarin, D.; García-Sastre, A.; Xiao, X.; Wang, R.; Palese, P. Influenza Virus PB1-F2 Protein Induces Cell Death through Mitochondrial ANT3 and VDAC1. PLoS Pathog. 2005, 1, e4. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.T.; Ramos, I.; Hai, R.; Schmolke, M.; García-Sastre, A.; Fernandez-Sesma, A.; Palese, P. The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein. PLoS Pathog. 2011, 7, e1002067. [Google Scholar] [CrossRef] [PubMed]
- Bruns, K.; Studtrucker, N.; Sharma, A.; Fossen, T.; Mitzner, D.; Eissmann, A.; Tessmer, U.; Röder, R.; Henklein, P.; Wray, V.; et al. Structural Characterization and Oligomerization of PB1-F2, a Proapoptotic Influenza A Virus Protein. J. Biol. Chem. 2007, 282, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Röder, R.; Bruns, K.; Sharma, A.; Eissmann, A.; Hahn, F.; Studtrucker, N.; Fossen, T.; Wray, V.; Henklein, P.; Schubert, U. Synthesis of Full Length PB1-F2 Influenza A Virus Proteins from ‘Spanish Flu’ and ‘Bird Flu’. J. Pept. Sci. 2008, 14, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Richard, C.-A.; Péchoux, C.; Da Costa, B.; Bertho, N.; Mazerat, S.; Delmas, B.; Chevalier, C. Amyloid Assemblies of Influenza A Virus PB1-F2 Protein Damage Membrane and Induce Cytotoxicity. J. Biol. Chem. 2016, 291, 739–751. [Google Scholar] [CrossRef]
- Chevalier, C.; Leymarie, O.; Sedano, L.; Da Costa, B.; Richard, C.-A.; Maisonnasse, P.; Réfregiers, M.; Jamme, F.; Le Goffic, R. PB1-F2 Amyloid-like Fibers Correlate with Proinflammatory Signaling and Respiratory Distress in Influenza-Infected Mice. J. Biol. Chem. 2021, 297, 100885. [Google Scholar] [CrossRef]
- Chevalier, C.; Al Bazzal, A.; Vidic, J.; Février, V.; Bourdieu, C.; Bouguyon, E.; Le Goffic, R.; Vautherot, J.-F.; Bernard, J.; Moudjou, M.; et al. PB1-F2 Influenza A Virus Protein Adopts a β-Sheet Conformation and Forms Amyloid Fibers in Membrane Environments. J. Biol. Chem. 2010, 285, 13233–13243. [Google Scholar] [CrossRef]
- Miodek, A.; Vidic, J.; Sauriat-Dorizon, H.; Richard, C.-A.; Le Goffic, R.; Korri-Youssoufi, H.; Chevalier, C. Electrochemical Detection of the Oligomerization of PB1-F2 Influenza A Virus Protein in Infected Cells. Anal. Chem. 2014, 86, 9098–9105. [Google Scholar] [CrossRef]
- Leymarie, O.; Jouvion, G.; Hervé, P.-L.; Chevalier, C.; Lorin, V.; Lecardonnel, J.; Da Costa, B.; Delmas, B.; Escriou, N.; Le Goffic, R. Kinetic Characterization of PB1-F2-Mediated Immunopathology during Highly Pathogenic Avian H5N1 Influenza Virus Infection. PLoS ONE 2013, 8, e57894. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Ichinohe, T.; Sasaki, O.; Otera, H.; Kawabata, S.; Mihara, K.; Koshiba, T. Influenza A Virus Protein PB1-F2 Translocates into Mitochondria via Tom40 Channels and Impairs Innate Immunity. Nat. Commun. 2014, 5, 4713. [Google Scholar] [CrossRef]
- Henkel, M.; Mitzner, D.; Henklein, P.; Meyer-Almes, F.-J.; Moroni, A.; DiFrancesco, M.L.; Henkes, L.M.; Kreim, M.; Kast, S.M.; Schubert, U.; et al. The Proapoptotic Influenza A Virus Protein PB1-F2 Forms a Nonselective Ion Channel. PLoS ONE 2010, 5, e11112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Wang, J.; Zhu, L.; Wang, J. Composition-dependent Membrane Disruption by the Proapoptotic Protein PB 1F2 from HK 97 Influenza A Virus. FEBS Lett. 2018, 592, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nagao, S.; Hirota, S. Characterization of the Cytochrome c Membrane-Binding Site Using Cardiolipin-Containing Bicelles with NMR. Angew. Chem. Int. Ed. 2016, 55, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, J.H.; Powell, G.L.; Marsh, D. Cytochrome c -Induced Increase of Motionally Restricted Lipid in Reconstituted Cytochrome c Oxidase Membranes, Revealed by Spin-Label ESR Spectroscopy. Biochemistry 1998, 37, 11579–11585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergstrom, C.L.; Beales, P.A.; Lv, Y.; Vanderlick, T.K.; Groves, J.T. Cytochrome c Causes Pore Formation in Cardiolipin-Containing Membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Shalaeva, D.N.; Lyamzaev, K.G.; Chernyak, B.V. Does Oxidation of Mitochondrial Cardiolipin Trigger a Chain of Antiapoptotic Reactions? Biochem. Mosc. 2018, 83, 1263–1278. [Google Scholar] [CrossRef]
- McClelland, L.J.; Mou, T.-C.; Jeakins-Cooley, M.E.; Sprang, S.R.; Bowler, B.E. Structure of a Mitochondrial Cytochrome c Conformer Competent for Peroxidase Activity. Proc. Natl. Acad. Sci. USA 2014, 111, 6648–6653. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–Initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–Dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Elmer-Dixon, M.M.; Bowler, B.E. Electrostatic Constituents of the Interaction of Cardiolipin with Site A of Cytochrome c. Biochemistry 2018, 57, 5683–5695. [Google Scholar] [CrossRef]
- Barayeu, U.; Lange, M.; Méndez, L.; Arnhold, J.; Shadyro, O.I.; Fedorova, M.; Flemmig, J. Cytochrome c Autocatalyzed Carbonylation in the Presence of Hydrogen Peroxide and Cardiolipins. J. Biol. Chem. 2019, 294, 1816–1830. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, G.; Chai, X.; Zhu, Q.; Sun, P.; Jiang, B.; Zhou, X.; Zhang, X.; Liu, M. NMR Reveals the Conformational Changes of Cytochrome C upon Interaction with Cardiolipin. Life 2021, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Hoop, C.L.; DeLucia, M.; Kodali, R.; Kagan, V.E.; Ahn, J.; van der Wel, P.C.A. Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c. Biophys. J. 2015, 109, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer-Stenner, R. Relating the Multi-Functionality of Cytochrome c to Membrane Binding and Structural Conversion. Biophys. Rev. 2018, 10, 1151–1185. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, F.; Milazzo, L.; Howes, B.D.; Piro, M.C.; Fiorucci, L.; Polticelli, F.; Ascenzi, P.; Coletta, M.; Smulevich, G.; Santucci, R. The Key Role Played by Charge in the Interaction of Cytochrome c with Cardiolipin. J. Biol. Inorg. Chem. 2017, 22, 19–29. [Google Scholar] [CrossRef]
- Wang, X.; Mu, Z.; Li, Y.; Bi, Y.; Wang, Y. Smaller Nanodiscs Are Suitable for Studying Protein Lipid Interactions by Solution NMR. Protein J. 2015, 34, 205–211. [Google Scholar] [CrossRef]
- Hagn, F.; Etzkorn, M.; Raschle, T.; Wagner, G. Optimized Phospholipid Bilayer Nanodiscs Facilitate High-Resolution Structure Determination of Membrane Proteins. J. Am. Chem. Soc. 2013, 135, 1919–1925. [Google Scholar] [CrossRef]
- Wan, C.; Wu, B.; Song, Z.; Zhang, J.; Chu, H.; Wang, A.; Liu, Q.; Shi, Y.; Li, G.; Wang, J. Insights into the Molecular Recognition of the Granuphilin C2A Domain with PI(4,5)P2. Chem. Phys. Lipids 2015, 186, 61–67. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, B.; Yu, C.; Zhao, H.; Zhou, Y.; Wang, J.; Zhu, L. Structural Determinants for Light-Dependent Membrane Binding of a Photoswitchable Polybasic Domain. ACS Synth. Biol. 2021, 10, 542–551. [Google Scholar] [CrossRef]
- Wang, F.; Chen, W.; Abousalham, A.; Yang, B.; Wang, Y. Exploring the Influence of Phospholipid Monolayer Conformation and Environmental Conditions on the Interfacial Binding of Gibberella Zeae Lipase. Int. J. Biol. Macromol. 2019, 132, 1051–1056. [Google Scholar] [CrossRef]
- Lhor, M.; Bernier, S.C.; Horchani, H.; Bussières, S.; Cantin, L.; Desbat, B.; Salesse, C. Comparison between the Behavior of Different Hydrophobic Peptides Allowing Membrane Anchoring of Proteins. Adv. Colloid Interface Sci. 2014, 207, 223–239. [Google Scholar] [CrossRef]
- Thumser, A.E.; Evans, C.; Worrall, A.F.; Wilton, D.C. Effect on Ligand Binding of Arginine Mutations in Recombinant Rat Liver Fatty Acid-Binding Protein. Biochem. J. 1994, 297, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wootan, M.G.; Bass, N.M.; Bernlohr, D.A.; Storch, J. Fatty Acid Binding Sites of Rodent Adipocyte and Heart Fatty Acid Binding Proteins: Characterization Using Fluorescent Fatty Acids. Biochemistry 1990, 29, 9305–9311. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Leymarie, O.; Chevalier, C.; Rebours, E.; Da Costa, B.; Vidic, J.; Descamps, D.; Sallenave, J.-M.; Rauch, M.; Samson, M.; et al. Transcriptomic Analysis of Host Immune and Cell Death Responses Associated with the Influenza A Virus PB1-F2 Protein. PLoS Pathog. 2011, 7, e1002202. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.T.; Grant, A.; Manicassamy, B.; Palese, P. Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon by Binding to MAVS and Decreasing Mitochondrial Membrane Potential. J. Virol. 2012, 86, 8359–8366. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Ren, C.; Yang, S.; Tian, S.; Chen, H.; Jin, M.; Zhou, H. Influenza A Virus Protein PB1-F2 Impairs Innate Immunity by Inducing Mitophagy. Autophagy 2021, 17, 496–511. [Google Scholar] [CrossRef]
- Chanturiya, A.N.; Basañez, G.; Schubert, U.; Henklein, P.; Yewdell, J.W.; Zimmerberg, J. PB1-F2, an Influenza A Virus-Encoded Proapoptotic Mitochondrial Protein, Creates Variably Sized Pores in Planar Lipid Membranes. J. Virol. 2004, 78, 6304–6312. [Google Scholar] [CrossRef]
- Coleman, J.R. The PB1-F2 Protein of Influenza A Virus: Increasing Pathogenicity by Disrupting Alveolar Macrophages. Virol. J. 2007, 4, 9. [Google Scholar] [CrossRef][Green Version]



| Sample | Nanodisc | Cytochrome c | PB1F2 | Buffer |
|---|---|---|---|---|
| ND | + | - | - | ++ 1 |
| ND+Cyto C | + | + | - | + 2 |
| ND+Cyto C+PB1F2 | + | + | + | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J. PB1F2 from Influenza A Virus Regulates the Interaction between Cytochrome C and Cardiolipin. Membranes 2022, 12, 795. https://doi.org/10.3390/membranes12080795
Wang Y, Wang J. PB1F2 from Influenza A Virus Regulates the Interaction between Cytochrome C and Cardiolipin. Membranes. 2022; 12(8):795. https://doi.org/10.3390/membranes12080795
Chicago/Turabian StyleWang, Yujuan, and Junfeng Wang. 2022. "PB1F2 from Influenza A Virus Regulates the Interaction between Cytochrome C and Cardiolipin" Membranes 12, no. 8: 795. https://doi.org/10.3390/membranes12080795
APA StyleWang, Y., & Wang, J. (2022). PB1F2 from Influenza A Virus Regulates the Interaction between Cytochrome C and Cardiolipin. Membranes, 12(8), 795. https://doi.org/10.3390/membranes12080795






