Abstract
Non-typhoidal Salmonella (NTS) infection and invasive non-typhoidal Salmonella (iNTS) infection cause a significant global health and economic burden. This systematic review aims to investigate the reported economic burden of NTS and iNTS infection, identify research gaps, and suggest future research directions. Data from PubMed and Embase databases up to April 2022 were reviewed, and articles were screened based on predefined criteria. Cost data were extracted, categorized into direct medical costs (DMCs), direct non-medical costs (DNMCs), and indirect costs (ICs), and converted into US dollars (year 2022). Data primarily originated from high-income countries (37 out of 38), with limited representation from Africa and resource-limited settings. For inpatients, DMCs were the primary cost driver for both NTS and iNTS illnesses, with estimates ranging from USD 545.9 (Taiwan, a region of China) to USD 21,179.8 (Türkiye) for NTS and from USD 1973.1 (Taiwan, a region of China) to USD 32,507.5 (United States of America) for iNTS per case. DNMCs and ICs varied widely across studies. Although study quality improved over time, methodological differences persisted. This review underscores the lack of economic data on NTS and iNTS in resource-limited settings. It also highlights the need for economic burden data in resource-limited settings and a standardized approach to generate global datasets, which is critical for informing policy decisions, especially regarding future vaccines.
1. Introduction
Non-typhoidal Salmonella (NTS) infection, including invasive non-typhoidal Salmonella (invasive NTS, abbreviated iNTS), causes significant global morbidity and mortality, with a large economic burden impacting society [1,2]. While NTS infections mostly result in self-limited diarrheal enterocolitis with low case fatality, risk factors such as malnutrition, extremes of age (<5 years and ≥70 years), HIV, malaria, and sickle-cell disease increase susceptibility to iNTS infection [2,3]. iNTS disease occurs when the NTS organisms invade normally sterile sites, leading to sepsis, meningitis, pneumonia, arthritis, and osteomyelitis [2,4,5]. Studies have shown that approximately 6% of diarrheal NTS cases progress to bloodstream infections [6,7,8,9]. With a higher fatality rate than non-invasive infection, iNTS infection is a major cause of morbidity and mortality, especially in sub-Saharan Africa [2,10,11]. Majowicz et al. estimated 93.8 million cases of NTS gastroenteritis (95% UI 61.8–131.6), accounting for 155,000 (39,000–303,000) deaths worldwide in 2006 [12]. In 2010, the Global Burden of Disease (GBD) study estimated that NTS caused 4.84 million (3.82–5.94) DALYs [13] and 81,300 (61,800–101,700) deaths [14]. WHO estimates from the Foodborne Disease Burden Epidemiology Reference Group (FERG, 2007–2015) showed that non-typhoidal Salmonella enterica and invasive non-typhoidal Salmonella enterica were responsible for 4.38 million (3.24–7.18) and 3.9 million (2.4–5.8) DALYs, respectively [15]. Furthermore, foodborne DALYs caused by NTS and iNTS were the largest (4.07 million) among the 22 foodborne enteric diseases globally [15]. More recently, the GBD 2019 estimates report 594,000 (486,000–718,000) cases of iNTS disease, resulting in 79,000 (43,000–124,000) deaths and 6.11 million (3.32–9.71) DALYs globally [16]. In children under 5, NTS (36% deaths) and iNTS (45% deaths) had a greater impact than typhoid fever (17% deaths) and paratyphoid fever (2% deaths) [16]. Most iNTS cases occur in Sub-Saharan Africa, with incidence rates exceeding 100 cases per 100,000 person-year in population at risk [17]. The 2017 GBD report estimated a case-fatality rate of 14.5% (9.2–21.1), with higher rates in children, the elderly, and people living with HIV infection [2]. Other studies report even higher case fatality rates of 20% in Mali, Bangladesh, and Vietnam [17].
Various prevention strategies have been implemented to control NTS disease, targeting animal production, food industries, consumers, and national surveillance [18,19]. Despite these efforts, significant under-reporting persists due to barriers to healthcare access and insufficient attention from public health authorities at national and global levels [1,2,20,21,22]. Additionally, the lack of point-of-care biomarkers and rapid diagnostic tools, such as serological or PCR-based tests, make the clinical diagnosis of NTS and iNTS infection challenging, particularly in low-resource settings such as Africa, where blood culture facilities are limited [11,23]. The absence of an available vaccine against NTS infections exacerbates these challenges, contributing to a significant public health burden.
No systematic attempt has been made to estimate the per-case cost incurred due to NTS and iNTS diseases [24,25]. This review aims to examine the existing studies on the economic burden of NTS and iNTS infections to date, identify gaps, and suggest future research needs.
2. Materials and Methods
A systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines and its checklist (Supplementary Materials; Table S1) [26]. PubMed and Embase were searched using search strategies constructed with free-texts, MeSH-Terms, and Emtree terms, adapted for each database. The search included publications from inception to April 2022, with the last search conducted on 29 April 2022. Two categories of search terms were used: (1) disease category, including terms related to non-typhoidal Salmonella and invasive non-typhoidal Salmonella, and (2) economic burden category, including terms related to the cost of illness. All search terms within each of the two categories were combined using the “OR” operator, and the two categories were combined using the “AND” operator. No filters or limits were applied, and there were no language restrictions. A detailed list of search strategies is presented in Table 1. Duplicate articles were identified and removed using EndNote software. The initial screening of titles and abstracts was conducted independently by two reviewers (SK, HLK). Studies indicating the cost of NTS or iNTS were selected for a second screening. After the initial screening, the list of studies for full-text review was shared, and discrepancies were resolved through discussion. The two reviewers independently carried out the full-text review of 77 articles using pre-determined inclusion and exclusion criteria (Table 2). The reference lists of the selected studies were further reviewed by the two reviewers. Any inconsistencies between the reviewers were resolved with the assistance of a third researcher (JSL).

Table 1.
Search strategies.

Table 2.
Inclusion and exclusion criteria.
The quality of the selected articles was assessed using a quality assessment tool (Supplementary Materials; Table S2) adapted from the cost-of-illness evaluation checklist proposed by Larg and Moss (2011) [27]. The results of the quality assessment (high, medium, and low) are presented in Table S3.
Relevant data were extracted from each included study using a standardized template in a Microsoft Excel workbook. Data extraction was initially performed by SK and HLK and cross-checked for accuracy. The extracted variables included the title of the article, authors’ names, publication year, cost year (study period), study location, disease indication, serovars, currency of the cost, cost item and description, cost type, cost perspective, cost source, duration of illness (number of visits), and other cost-related information identified in the articles. Cost types were categorized into direct medical costs (DMCs), direct non-medical costs (DNMCs), and indirect costs (ICs). DMC refers to costs incurred by patient management such as hospital stays, physician consultations, laboratory tests, and medications. DMC was further classified into DMC inpatient (DMC IP) and DMC outpatient (DMC OP). When DMCs were not specific to costs by inpatient or outpatient healthcare services, they were categorized as DMC non-specific (DMC NS). DNMC includes costs not directly related to healthcare services, such as transportation, food, and accommodation. IC encompasses expenses borne by patients or their families due to work absenteeism related to the illness. Data costs related to outbreak control and the value of lost lives were extracted but not included in the review results. All cost components were calculated as per-case costs whenever possible, by dividing aggregated costs for a group of patients by the number of patients. All costs were converted to US dollars (USD) and adjusted to 2022 values using the official exchange rate and the GDP Deflator provided by the World Bank [28].
3. Results
The literature search yielded 2839 articles after removing duplicates, of which 77 were identified for full-text review based on their titles and abstracts. Among these 77 articles, 34 met the inclusion and exclusion criteria. Additionally, three articles [29,30,31] and one database [32] were identified through bibliography searches and included in the final review (Figure 1).
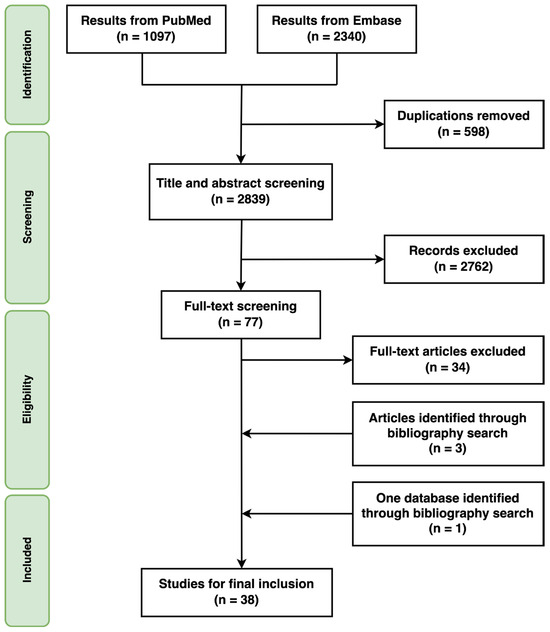
Figure 1.
Study selection.
Among the 38 studies, 4 articles contained data related to the economic burden of iNTS [30,33,34,35]. Evidence for NTS disease came from 11 countries: Australia, Canada, Hong Kong SAR, China (hereinafter Hong Kong), the Netherlands, Poland, Spain, Sweden, Taiwan, a region of China (hereinafter Taiwan), Türkiye, the United Kingdom (UK), and the United States of America (US). Evidence for iNTS was found in three countries: Spain, Taiwan, and the US. The income level of each country, classified by the World Bank (2021), was compared to assess the distribution of data [36]. Only one study [37] was conducted in an upper middle-income country (Türkiye) in 2009, while all other studies were from high-income countries. There were no studies from lower middle-income and low-income countries for both NTS and iNTS diseases. Geographically, no studies were conducted in Africa, and only two studies were conducted in Asia (Taiwan [5,35] and Hong Kong [38]).
Publication years ranged from 1978 to 2021. All studies used economic evaluation methods from various perspectives, such as societal, the healthcare system, and the patient. More than half (n = 25, 66%) of the studies used a societal perspective, encompassing DMC, DNMC, and IC. Eight [5,35,37,39,40,41,42,43] out of thirty-eight studies used the healthcare system perspective, focusing on government and healthcare facility costs. Five studies used the patient perspective, which focused on the medical expenses incurred by the patient and their families, excluding indirect costs [29,33,34,38,44].
Among the 38 studies reviewed, 13 aimed to estimate the economic burden of NTS outbreaks [20,22,37,39,40,41,45,46,47,48,49,50,51], of which five were in hospital settings [37,39,40,41,47]. Todd et al. estimated the economic burden of multiple Salmonella community outbreaks in the UK, US, Canada, Sweden, and Australia across different years [20], and Scharff et al. did so in the US from 1994 to 2009, using national surveillance data [51]. None of the outbreak studies included iNTS cases. Economic evidence related to iNTS was derived from either hospital databases (US and Spain) or national claims databases (US and Taiwan) [30,33,34,35]. A summary of the extracted components from the included studies is provided in Table S3 in Supplementary Materials.
The reported DMC IP was the primary cost driver for the economic burden of NTS and iNTS illnesses, ranging from USD 545.9 (Taiwan) to USD 21,179.8 (Türkiye) for NTS illnesses and from USD 1973.1 (Taiwan) to USD 32,507.5 (US) for iNTS illnesses per case. Considering the significant inflation surge in Türkiye in 2022, around 72% [36], the second highest DMC IP for NTS illness was USD 19,788.7 from the US. The DMC OP reported was lower than the DMC IP, ranging from USD 17.4 (UK) to USD 739.8 (US) for NTS illnesses, with only one study from the US [30] estimating DMC OP for iNTS as USD 469.4 per case. The IC ranged from USD 188.9 in Australia to USD 3028.8 in the UK per case for NTS illnesses. The IC for iNTS illnesses, as reported by Adhikari et al. (2001) from the US, was USD 1768.8 [30]. DNMCs were assessed in 14 studies, ranging from USD 4.3 in the Netherlands to USD 18,261.2 in Türkiye [20,31,37,40,41,45,46,47,49,51,52,53,54,55]. Five studies (13%) did not report costs by components but aggregated total costs [19,39,50,56,57]. Costs reported from each study are presented in Table 3 (NTS illnesses) and Table 4 (iNTS illnesses). When costs could not be categorized into DMC, DNMC, and IC, total costs were presented as total costs (TC) in the tables (all costs are expressed in US dollars at 2022 value).

Table 3.
Healthcare costs by cost type related to non-typhoidal Salmonella diseases (USD in 2022).

Table 4.
Healthcare costs by cost type related to invasive non-typhoidal Salmonella diseases (USD in 2022).
To better understand the economic burden relative to each country’s economic context, we calculated the healthcare costs as a percentage of the country’s GDP per capita [28]. For NTS illnesses, the DMCs IP as a percentage of GDP per capita ranged from 0.3% (UK) to 198.4% (Türkiye) (Table 3). This significant variation indicates the differing economic impacts on patients in different countries, with Türkiye experiencing a disproportionately high economic burden due to its lower GDP per capita and exceptionally high inflation rate in 2022 [36]. For iNTS illnesses, the DMCs IP as a percentage of GDP per capita ranged from 0.6% (US) to 42.6% (US) (Table 4). The high percentages for iNTS reflect the severe financial impact of invasive infections, particularly in high-income countries like the US, where healthcare costs are substantial. Countries with national healthcare systems, such as Taiwan and the UK, generally report lower out-of-pocket expenses for patients compared to those without such systems, impacting the overall economic burden experienced by individuals.
Regarding the study quality of the 37 articles, 20 studies were rated as high quality [5,22,30,35,37,38,42,43,44,49,50,53,54,55,57,58,60,61,62,63], 12 as medium quality [29,31,33,34,41,46,47,48,51,52,56,59], and 5 as low quality [19,20,39,40,45]. Study quality was assessed using a set of questions regarding the analytical framework, methodology and data, and analysis and reporting [27]. The quality assessment tool was not applicable to the one database established by the Economic Research Service (ERS) at the U.S. Department of Agriculture (USDA) due to its structure [32]. Detailed information is available in Supplementary Materials (Table S2). It is noteworthy that the quality of cost-of-illness studies has improved during a span of 44 years (1978–2021), particularly in analysis and reporting, despite methods still being varied among the recent studies.
Data on the duration of illness were also collected, as shown in Table 5. Hayes et al. reported the number of outpatient visits for NTS illness management to be 1.6 per case [46]. No other study clearly indicated the number of outpatient visits related to NTS illness management, and none did so for iNTS illness. Hospital bed-days or length of hospitalization was reported to be 7.5 days (95% Confidence Interval 6.0–8.9) in 16 studies for NTS illness per case and 12.6 days (95% CI 8.6–16.5) in three studies for iNTS illness per case. Roberts and Sockett, Herrick et al., and Ailes et al. presented the duration of NTS illness to be 12, 2, and 4 days (median) per case, respectively, without specifying the type of healthcare resource (inpatient or outpatient) [22,49,56].

Table 5.
Number of outpatient visits and hospital bed-days by indication.
4. Discussion
At the global level, the economic burden of NTS and iNTS is predominantly informed by data from a limited number of high-income countries, mainly the US and the UK. Among the 37 articles and 1 database reviewed, 15 included data from the US and 10 from the UK. Notably, no economic evaluation was conducted in the African region, despite the high burden of NTS disease in sub-Saharan Africa [2,11,17,23]. Additionally, there was no evidence from any lower middle-income or low-income countries, highlighting the scarcity of cost data for NTS and iNTS diseases in resource-limited settings, as previously reported [13,14,23]. Direct comparisons of costs across studies are challenging due to the varied approaches and definitions of the costs used. Furthermore, several studies did not separately describe cost categories (DMC, DNMC, IC) and reported combined total costs instead [19,39,50,56,57].
This study has limitations. Although we employed a systematic approach to identifying literature relevant to the economic burden of NTS and iNTS diseases by searching multiple databases with different search terms, there remains a possibility of missing some literature. Costs associated with iNTS diseases may not have been separately estimated and reported if the study did not specifically aim to do so. Additionally, the diverse methods and outcome measures of cost-of-illness studies made it infeasible to conduct a meta-analysis.
To understand the global economic burden of NTS and iNTS, a consensus on standardized methods of economic evaluation across nations is needed to generate reliable data. Efforts to improve diagnostics, understand disease sources, and study routes of transmission should be combined with high-quality primary data to assess the economic burden of NTS and iNTS. A comprehensive understanding of the economic burden is essential for making evidence-based policy decisions at national and global levels.
5. Conclusions
The findings of this review highlight the significant lack of economic evidence for NTS and iNTS in the regions most in need, particularly Africa. This disparity underscores the disproportionate attention and resources concentrated in high-income countries, especially within a limited number of nations. Currently, there are a few Salmonella combination vaccine candidates in early clinical trial phases, including bivalent NTS vaccines (GSK Vaccines Institute for Global Health (GVGH), Boston Children’s Hospital) and trivalent NTS and typhoid vaccines (University of Maryland and Bharat Biotechnology, GVGH, SK Bioscience and International Vaccine Institute) [64]. McLennan et al. emphasized that a vaccine-related strategy with multivalent Salmonella vaccines for control should be applicable in LMIC settings, considering the lack of diagnostic capacity and adequate treatment options [64]. To support the development of NTS and iNTS vaccines by informing investments and policy decisions, a better understanding of the economic burden of NTS and iNTS diseases, especially in endemic regions, is essential. Public health communities must make concerted efforts to identify and address the substantial burden of NTS and iNTS diseases globally.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12070758/s1, Table S1: PRISMA 2020 checklist; Table S2: Quality assessment; Table S3: Summary of the included articles.
Author Contributions
Conceptualization, S.K. and J.-S.L.; methodology, S.K. and J.-S.L.; software, S.K. and H.K.; validation, S.K., H.K. and J.-S.L.; formal analysis, S.K.; investigation, S.K. and H.K.; resources, S.K. and J.-S.L.; data curation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, J.-S.L. and J.-L.E.; visualization, S.K.; supervision, J.-S.L., J.-L.E. and J.H.K.; project administration, S.K. funding acquisition, J.-S.L., J.-L.E. and J.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Wellcome Trust, grant number 222044/Z/20/Z. The APC was funded by the Wellcome Trust.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All crude cost data are extracted from published articles and are publicly accessible. Access to the database searched and included in this review is available at https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses/ (accessed on 2 May 2024) (Economic Research Service, U.S. Department of Agriculture, Washington, D.C., United States of America).
Acknowledgments
We express our sincere gratitude to the dedicated members of the Full Value of Vaccine Assessment of invasive non-typhoidal Salmonella Vaccines (iNOVVEL) consortium for their invaluable expertise and contributions to the work achieved for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Balasubramanian, R.; Im, J.; Lee, J.S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccin. Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H. The global burden of non-typhoidal Salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-J.; Sheen, J.-M.; Yang, Y.-H.; Kuo, K.-C. Increased non-typhoidal Salmonella hospitalizations in transfusion-naïve thalassemia children: A nationwide population-based cohort study. Pediatr. Res. 2021, 91, 1858–1863. [Google Scholar] [CrossRef]
- Phu Huong Lan, N.; Le Thi Phuong, T.; Nguyen Huu, H.; Thuy, L.; Mather, A.E.; Park, S.E.; Marks, F.; Thwaites, G.E.; Van Vinh Chau, N.; Thompson, C.N.; et al. Invasive Non-typhoidal Salmonella Infections in Asia: Clinical Observations, Disease Outcome and Dominant Serovars from an Infectious Disease Hospital in Vietnam. PLoS Negl. Trop. Dis. 2016, 10, e0004857. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Chung, Y.A.; Wu, Y.C.; Fang, C.T.; Chen, P.J. Disease burden from foodborne illnesses in Taiwan, 2012–2015. J. Formos. Med. Assoc. 2020, 119, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Villar, R.; Vugia, D.J.; Rabatsky-Ehr, T.; Farley, M.M.; Pass, M.; Smith, K.; Smith, P.; Cieslak, P.R.; Imhoff, B.; et al. Hospitalizations and deaths due to Salmonella infections, FoodNet, 1996–1999. Clin. Infect. Dis. 2004, 38 (Suppl. 3), S142–S148. [Google Scholar] [CrossRef] [PubMed]
- Varma, J.K.; Molbak, K.; Barrett, T.J.; Beebe, J.L.; Jones, T.F.; Rabatsky-Ehr, T.; Smith, K.E.; Vugia, D.J.; Chang, H.G.; Angulo, F.J. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 2005, 191, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.M.; Thomas, S.; Aspinall, E.J.; Cooke, R.P.; Rogerson, S.J.; Harries, A.D.; Beeching, N.J. A retrospective study of secondary bacteraemia in hospitalised adults with community acquired non-typhoidal Salmonella gastroenteritis. BMC Infect. Dis. 2013, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Uche, I.V.; MacLennan, C.A.; Saul, A. A Systematic Review of the Incidence, Risk Factors and Case Fatality Rates of Invasive Nontyphoidal Salmonella (iNTS) Disease in Africa (1966 to 2014). PLoS Negl. Trop. Dis. 2017, 11, e0005118. [Google Scholar] [CrossRef]
- Gilchrist, J.J.; MacLennan, C.A. Invasive Nontyphoidal Salmonella Disease in Africa. EcoSal Plus 2019, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.P.; Naghavi, M.P.; Lim, S.P.; Aboyans, V.P.; Abraham, J.M.P.H.; Adair, T.P.; Ahn, S.Y.M.P.H.; AlMazroa, M.A.M.D.; Anderson, H.R.P.; Anderson, L.M.P.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Word Bank. Global Burden of Disease Study 2019 (GBD 2019) Cause, REI, and Location Hierarchies; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020. [Google Scholar] [CrossRef]
- Marchello, C.S.; Birkhold, M.; Crump, J.A.; Martin, L.B.; Ansah, M.O.; Breghi, G.; Canals, R.; Fiorino, F.; Gordon, M.A.; Kim, J.-H.; et al. Complications and mortality of non-typhoidal Salmonella invasive disease: A global systematic review and meta-analysis. Lancet Infect. Dis. 2022, 22, 692–705. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food Agriculture Organization of the United Nations. Interventions for the Control of Non-Typhoidal Salmonella spp. in Beef and Pork: Meeting Report and Systematic Review; Microbiological risk assessment series, 30; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Gomez, T.M.; Motarjemi, Y.; Miyagawa, S.; Käferstein, F.K.; Stöhr, K. Foodborne salmonellosis. World Health Stat. Q. 1997, 50, 81–89. [Google Scholar] [PubMed]
- Todd, E.C.D. Economic Loss from Foodborne Disease and Non-Illness Related Recalls Because of Mishandling by Food Processors. J. Food Prot. 1985, 48, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Todd, E. Epidemiology of foodborne illness: North America. Lancet 1990, 336, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Herrick, R.L.; Buchberger, S.G.; Clark, R.M.; Kupferle, M.; Murray, R.; Succop, P. A markov model to estimate Salmonella morbidity, mortality, illness duration, and cost. Health Econ. 2012, 21, 1169–1182. [Google Scholar] [CrossRef]
- Crump, J.A.; Heyderman, R.S. A Perspective on Invasive Salmonella Disease in Africa. Clin. Infect. Dis. 2015, 61 (Suppl. 4), S235–S240. [Google Scholar] [CrossRef]
- McLinden, T.; Sargeant, J.M.; Thomas, M.K.; Papadopoulos, A.; Fazil, A. Association between component costs, study methodologies, and foodborne illness-related factors with the cost of nontyphoidal Salmonella illness. Foodborne Pathog. Dis. 2014, 11, 718–726. [Google Scholar] [CrossRef]
- McLinden, T.; Sargeant, J.M.; Thomas, M.K.; Papadopoulos, A.; Fazil, A. Component costs of foodborne illness: A scoping review. BMC Public Health 2014, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Larg, A.; Moss, J. Cost-of-Illness Studies: A Guide to Critical Evaluation. Pharmacoeconomics 2011, 29, 653–671. [Google Scholar] [CrossRef] [PubMed]
- GDP Deflator (Base Year Varies by Country); World Bank: Washington, DC, USA, 2023; Available online: https://data.worldbank.org/indicator/NY.GDP.DEFL.ZS (accessed on 2 May 2024).
- Engvall, A.A.; Cerenius, F. Control of Foodborne Diseases in Humans and Animals: Strategies and Approaches at the Animal Production Leve: The Swedish Salmonella Control Programme; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Adhikari, B.; Angulo, F.; Meltzer, M. Economic Burden of Salmonella Infections in the United States. In Proceedings of the American Agricultural Economics Association (New Name 2008: Agricultural and Applied Economics Association), 2004 Annual Meeting, Denver, CO, USA, 1–4 August 2004. [Google Scholar]
- Curtin, L. Economic Study of Salmonella Poisoning and Control Measures in Canada; Food Markets Analysis Division, Marketing and Economics Branch, Agriculture Canada: Halifax, NS, Canada, 1984. [Google Scholar]
- Hoffmann, S.; Maculloch, B.; Batz, M. Cost Estimates of Foodborne Illnesses; Economic Research Service (ERS), U.S. Department of Agriculture (USDA): Washington, DC, USA, 2021. [Google Scholar]
- Trevejo, R.T.; Courtney, J.G.; Starr, M.; Vugia, D.J. Epidemiology of salmonellosis in California, 1990–1999: Morbidity, mortality, and hospitalization costs. Am. J. Epidemiol. 2003, 157, 48–57. [Google Scholar] [CrossRef]
- Gil Prieto, R.; Alejandre, C.G.; Meca, A.Á.; Barrera, V.H.; de Miguel, Á.G. Epidemiology of hospital-treated Salmonella infection Data from a national cohort over a ten-year period. J. Infect. 2009, 58, 175–181. [Google Scholar] [CrossRef]
- Chen, P.L.; Li, C.Y.; Hsieh, T.H.; Chang, C.M.; Lee, H.C.; Lee, N.Y.; Wu, C.J.; Lee, C.C.; Shih, H.I.; Ko, W.C. Epidemiology, disease spectrum and economic burden of non-typhoidal Salmonella infections in Taiwan, 2006–2008. Epidemiol. Infect. 2012, 140, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- World Bank Country and Lending Groups; World Bank: Washington, DC, USA, 2021.
- Anil, M.; Helvaci, M.; Ozkalay, N.; Toprak, E.; Anil, A.B.; Dilek, M.; Agus, N. Salmonella typhimurium outbreak in a neonatal unit in Turkey. Indian J. Pediatr. 2009, 76, 629–633. [Google Scholar] [CrossRef]
- Broughton, E.I.; Ip, M.; Coles, C.L.; Walker, D.G. Higher hospital costs and lengths of stay associated with quinolone-resistant Salmonella enterica infections in Hong Kong. J. Public Health 2010, 32, 165–172. [Google Scholar] [CrossRef]
- Barnass, S.; O’Mahony, M.; Sockett, P.N.; Garner, J.; Franklin, J.; Tabaqchali, S. The tangible cost implications of a hospital outbreak of multiply-resistant Salmonella. Epidemiol. Infect. 1989, 103, 227–234. [Google Scholar] [CrossRef]
- Choi, M.; Yoshikawa, T.T.; Bridge, J.; Schlaifer, A.; Osterweil, D.; Reid, D.; Norman, D.C. Salmonella outbreak in a nursing home. J. Am. Geriatr. Soc. 1990, 38, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S.; Keyworth, N.; Gabb, R.; Stein, K. Asymptomatic foodhandlers as the source of nosocomial salmonellosis. J. Hosp. Infect. 1994, 28, 195–208. [Google Scholar] [CrossRef]
- Cummings, P.L.; Kuo, T.; Javanbakht, M.; Shafir, S.; Wang, M.; Sorvillo, F. Salmonellosis Hospitalizations in the United States: Associated Chronic Conditions, Costs, and Hospital Outcomes, 2011, Trends 2000–2011. Foodborne Pathog. Dis. 2016, 13, 40–48. [Google Scholar] [CrossRef]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Estepa, M.; Latasa, P.; Ordóñez-León, G.Y.; Martínez-Avilés, M.; de la Torre, A.; García-Comas, L. Non-Typhi, non-Paratyphi Salmonella-related hospitalisations in Spain: Trends, clinical aspects, risk factors for worse prognosis and hospital costs. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L.; Fontaine, R.E.; Pollard, R.A.; VonAllmen, S.D.; Vernon, T.M.; Gangarosa, E.J. An assessment of patient-related economic costs in an outbreak of salmonellosis. N. Engl. J. Med. 1978, 299, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.; Lyons, R.A.; Warde, C. A large outbreak of salmonellosis and its economic cost. Ir. Med. J. 1991, 84, 65–66. [Google Scholar] [PubMed]
- Spearing, N.M.; Jensen, A.; McCall, B.J.; Neill, A.S.; McCormack, J.G. Direct costs associated with a nosocomial outbreak of Salmonella infection: An ounce of prevention is worth a pound of cure. Am. J. Infect. Control 2000, 28, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Cumberland, P.; Sockett, P.N.; Wheeler, J.; Rodrigues, L.C.; Sethi, D.; Roderick, P.J. The study of infectious intestinal disease in England: Socio-economic impact. Epidemiol. Infect. 2003, 130, 1–11. [Google Scholar] [CrossRef]
- Ailes, E.; Budge, P.; Shankar, M.; Collier, S.; Brinton, W.; Cronquist, A.; Chen, M.; Thornton, A.; Beach, M.J.; Brunkard, J.M. Economic and Health Impacts Associated with a Salmonella Typhimurium Drinking Water Outbreak-Alamosa, CO, 2008. PLoS ONE 2013, 8, e57439. [Google Scholar] [CrossRef]
- Scharff, R.L.; Besser, J.; Sharp, D.J.; Jones, T.F.; Peter, G.S.; Hedberg, C.W. An Economic Evaluation of PulseNet: A Network for Foodborne Disease Surveillance. Am. J. Prev. Med. 2016, 50, S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Suijkerbuijk, A.W.M.; Bouwknegt, M.; Mangen, M.J.; de Wit, G.A.; van Pelt, W.; Bijkerk, P.; Friesema, I.H.M. The economic burden of a Salmonella Thompson outbreak caused by smoked salmon in the Netherlands, 2012–2013. Eur. J. Public Health 2017, 27, 325–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sockett, P.N.; Roberts, J.A. The social and economic impact of salmonellosis. A report of a national survey in England and Wales of laboratory-confirmed Salmonella infections. Epidemiol. Infect. 1991, 107, 335–347. [Google Scholar] [CrossRef]
- Santos, A.C.; Roberts, J.A.; Cook, A.J.C.; Simons, R.; Sheehan, R.; Lane, C.; Adak, G.K.; Clifton-Hadley, F.A.; Rodrigues, L.C. Salmonella Typhimurium and Salmonella Enteritidis in England: Costs to patients, their families, and primary and community health services of the NHS. Epidemiol. Infect. 2010, 139, 742–753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffmann, S.; Batz, M.B.; Morris, J.G., Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Sundström, K.; Wahlström, H.; Ivarsson, S.; Sternberg Lewerin, S. Economic effects of introducing alternative Salmonella control strategies in Sweden. PLoS ONE 2014, 9, e96446. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Sockett, P.N. The socio-economic impact of human Salmonella enteritidis infection. Int. J. Food Microbiol. 1994, 21, 117–129. [Google Scholar] [CrossRef] [PubMed]
- van den Brandhof, W.E.; De Wit, G.A.; de Wit, M.A.; van Duynhoven, Y.T. Costs of gastroenteritis in The Netherlands. Epidemiol. Infect. 2004, 132, 211–221. [Google Scholar] [CrossRef]
- Duff, S.B.; Scott, E.A.; Mafilios, M.S.; Todd, E.C.; Krilov, L.R.; Geddes, A.M.; Ackerman, S.J. Cost-effectiveness of a targeted disinfection program in household kitchens to prevent foodborne illnesses in the United States, Canada, and the United Kingdom. J. Food Prot. 2003, 66, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Fyfe, M.; Doré, K.; Buxton, J.A.; Pollari, F.; Henry, B.; Middleton, D.; Ahmed, R.; Jamieson, F.; Ciebin, B.; et al. Increased Burden of Illness Associated with Antimicrobial-Resistant Salmonella enterica Serotype Typhimurium Infections. J. Infect. Dis. 2004, 189, 377–384. [Google Scholar] [CrossRef][Green Version]
- Stephen, D.M.; Barnett, A.G. Using Microsimulation to Estimate the Future Health and Economic Costs of Salmonellosis under Climate Change in Central Queensland, Australia. Environ. Health Perspect. 2017, 125, 127001. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, P.; Spyczak von Brzezinski, M.; Żelazowski, J.; Wojtkiewicz, J.; Jung, S.; Harazny, J.M. Epidemiological Survey and Retrospective Analysis of Salmonella Infections between 2000 and 2017 in Warmia and Masuria Voivodship in Poland. Medicina 2019, 55, 74. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Haywood, P.; Kirk, M.D.; Lancsar, E.; Williamson, D.A.; Glass, K. Cost of Salmonella Infections in Australia, 2015. J. Food Prot. 2019, 82, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.; Hoffmann, S.; White, A.; Ahn, J.W.; McQueen, R.B.; Scallan Walter, E. Cost of hospitalizations for leading foodborne pathogens in the united states: Identification by international classification of disease coding and variation by pathogen. Foodborne Pathog. Dis. 2021, 18, 812–821. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Stanaway, J.; Grow, S.; Vannice, K.; Steele, A.D. Salmonella Combination Vaccines: Moving Beyond Typhoid. Open Forum Infect. Dis. 2023, 10, S58–S66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).