Abstract
Background: Many human activities release harmful substances, contaminating the air, water, and soil. Since exposure to environmental pollutants is currently unavoidable, it is important to verify how these compounds may influence individual immune responses to vaccines. Methods: This review was conducted in accordance with the PRISMA statement. The protocol was registered on the PROSPERO platform with the following ID: CRD42024582592. We evaluated all observational, semi-experimental, and experimental studies written in both Italian and English that reported possible effects of exposure to environmental pollutants on the production of vaccine-induced antibodies. Results: Forty-two studies were included. The effects of pollutants were examined mainly in terms of antibody production in relation to mumps, measles and rubella, diphtheria and tetanus, hepatitis A and B, Haemophilus influenzae type B, influenza, tuberculosis, pertussis, Japanese encephalitis, poliomyelitis, and COVID-19 vaccines. Perfluorinated compounds were the most studied pollutants. Conclusions: Correlations between exposure to pollutants and reductions in antibody production were found in quite all the selected studies, suggesting that pollution control policies could contribute to increase the efficacy of vaccination campaigns. However, the heterogeneity of the examined studies did not allow us to perform a meta-analysis, and the literature on each type of vaccine or pollutant is still too limited to generate robust evidence. In order to confirm the findings of the present systematic review, and in the perspective of establishing possible exposure limit values for each type of pollutant, further research in this field is required.
1. Introduction
Being the most efficient way to stop the spread, morbidity, and mortality of several infectious diseases, vaccination is considered one of the most important public health achievements of the 20th century [1]. The World Health Organization (WHO) estimates that since 1974, vaccination has prevented 154 million deaths worldwide [2]. This practice consists of a process of active immunization: a healthy subject is exposed to a specific antigen, inducing the development of an adaptive immune response to that antigen [3]. However, numerous variables endogenous (genetics, age, and sex) and exogenous (stress, alcohol, physical activity, diet, and infectious illnesses) may influence the effectiveness and duration of vaccine-induced protection [4].
Environmental pollution affects the entire world population and it is a growing problem. In fact, many human activities release substances harmful to health into the environment, contaminating the main environmental matrices in contact with humans: air [5], water [6], and soil [7]. As scientific and technological progress advances and anthropic activity increases, pollution has begun to spread more and more [8] and it has become a serious public health problem [9]. According to the Lancet Commission on Pollution and Health, which used data from the Global Burden of Disease, Injury and Risk Factors 2019, pollution was responsible for 9 million deaths a year, about one in six deaths worldwide, making it the biggest environmental risk factor for disease and premature death [10]. According to WHO data, more than 99% of the global population breathes air where pollution is greater than the levels indicated by the WHO air quality guidelines, and 4.2 million deaths each year are associated with air pollution [11].
Many kinds of environmental pollutants were studied in order to evaluate the impact of exposure to them on human health: for example, an association is reported in the literature between particulates and cardiovascular diseases [12], but also for other pathologies such as chronic kidney disease [13], impaired cognitive function, preterm births, allergies [14], and diabetes [15], between heavy metals and metabolic diseases such as osteopenia and osteoporosis [16], between pesticides and respiratory system diseases like asthma and infections [17], and between per- and polyfluoroalkyl substances (PFASs) and dysregulation of thyroid function [18]. Pollutants such as particulate matter and polycyclic aromatic hydrocarbons have also been shown to be involved in carcinogenesis processes [19,20].
Since pollution exposure is currently unavoidable, it is reasonable to wonder how these compounds can affect individual immune responses to vaccines, which are likely the most commonly used public health tool worldwide. Therefore, this systematic review aims to provide an overview of the scientific data regarding the potential effects of exposure to environmental pollutants on vaccine efficacy, evaluated in terms of the individual production of vaccine-induced antibodies.
2. Materials and Methods
2.1. Research Strategy
This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement [21]. The protocol was registered on the PROSPERO platform with the following ID: CRD42024582592. The protocol is available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024582592 (accessed on 27 October 2024).
The bibliographic and citation databases PubMed (Medline), Scopus, and Web of Science (Science and Social Science Citation Index) were examined. The following keywords with Boolean operators as AND–OR were used for the query: (“environmental pollution” [MeSH] OR “water pollution” [MeSH] OR “indoor pollution” OR “outdoor pollution” OR “water pollutant*” OR “soil pollutant*” OR “air pollutant*” OR “indoor pollutant*” OR “outdoor pollutant*” OR “environmental toxicants exposure” OR “environmental pollutants exposure” OR “environmental toxicants” OR “environmental pollutants”) AND (“vaccine*” OR “vaccination*” OR “vaccine efficacy” OR “vaccination efficacy” OR “immunization” OR “antibody response to vaccination” OR “humoral immunity” OR “vaccine antibody levels”). The search was conducted from 9 August 2024 to 30 August 2024 All articles published from the inception until 30 August 2024 were included in the research.
2.2. Inclusion and Exclusion Criteria
We evaluated studies written in both Italian and English language which reported possible effects of exposure to environmental pollutants on immune responses to vaccination. Studies that reported no original data, including reviews, systematic reviews, case studies, proceedings, qualitative investigations, book chapters, editorials, and opinion articles, were omitted. All observational, semi-experimental, and experimental studies on humans reporting original data on the studied issue were included. In addition, we performed citation chaining, examining the reference lists of the recovered studies in order to identify further articles on the focus of the present systematic review. Any article that did not meet the inclusion criteria was excluded. The PICOS model was employed for structuring the research question as follows:
- Population: all people (individuals of all gender, age, ethnicity and health conditions) vaccinated against any vaccine-preventable disease.
- Intervention: exposure to environmental pollutants.
- Control: age-, gender- and condition-matched not vaccinated or vaccinated but differently exposed to pollutant(s).
- Outcomes: effects of exposure to environmental pollutants on vaccine-induced immune response, assessed through vaccine-induced antibody levels.
- Study: observational studies and semi-experimental and experimental studies on humans.
All studies that did not satisfy the inclusion criteria were excluded.
The references of the chosen articles were all transferred to the Zotero citation management software (RRID:SCR_013784, version 6.0.36) in order to evaluate the significance of each article and eliminate any duplicates.
Initially, titles and abstracts were evaluated by three researchers (J.D.P., K.V. and F.L.) to independently verify the potentially qualifying papers. Three content experts (C.P., F.G., and F.V.) helped with the reviewing and evaluating process. Subsequently, two researchers (J.D.P. and K.V.) assessed the full text of each included article independently. The group discussed and resolved any disagreements on the chosen papers.
2.3. Risk of Bias Assessment
Forty-one observational studies were obtained at the end of the evaluation and selection process.
The quality of the observational studies was evaluated using the Newcastle–Ottawa scale (NOS) modified for cohort, case-control, and cross-sectional studies. This scale was used to compute the total rating. Each study was reviewed separately by two authors (J.D.P. and K.V.) and disagreements were resolved by the authors debating with one another. The final rating for each article was determined by taking the mean value of the authors’ scores. The scale has different numbers of questions and scores for different studies: for case-control and cohort studies, 8 questions for a maximum score of 9, and for cross-sectional studies, 6 questions with a maximum score of 7. In particular, the three categories assessed were selection, comparability, and outcome, and a score was given to each based on the type of study: for cross-sectional studies, 3 points for selection, 2 for comparability, and 2 for outcome, with a maximum total score of 7; and for case-control and cohort studies, 4 points for selection, 2 for comparability, and 3 for outcome, with a maximum total score of 9. A score of 7 to 9 points denotes good quality (low risk of bias), 5 to 6 points denotes fair quality (moderate risk of bias), and 0 to 4 points denotes poor quality (high risk of bias) [22].
The quality assessment was entered into the data extraction table. Additionally, the following details were listed in this table for each article: author, publication year, country, presence of a sponsor, study design, sample size and main population characteristics, vaccination features, type of pollutant(s), exposure assessment, main findings, and quality of the included studies.
3. Results
Figure 1 shows the flow chart of the review process.
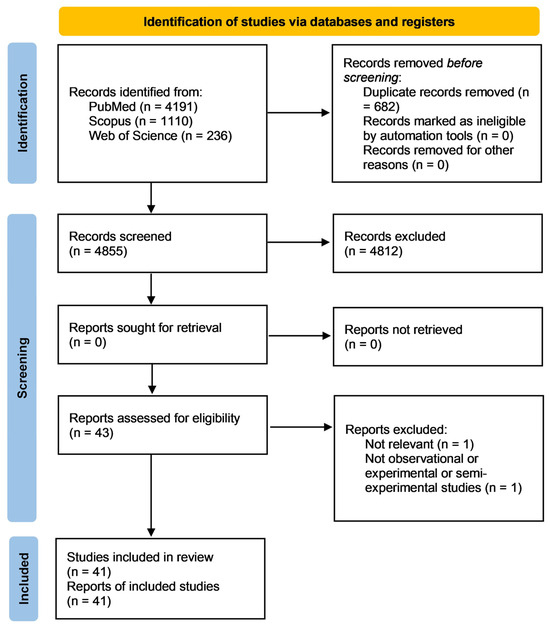
Figure 1.
Prisma flow chart describing the research strategy.
Using the search query, 5537 records were found in the three databases: PubMed (4191), Scopus (1110), and Web of Science (236). After removing the duplicates (682 studies), the title and abstract of 4855 articles were evaluated and the full texts of 43 records were screened. Out of these, two were eliminated due to non-compliance with the eligibility requirements. Upon ending the process, the systematic review included 41 articles.
The data extracted from the included studies are summarized in the Supplementary Table S1 (characteristics of the included studies) and in Table 1 (main results and results of the quality assessment of the included studies).

Table 1.
Main results and quality assessment of the included studies.
Eight of the 41 articles included in the present review reported cross-sectional studies [28,33,37,42,46,50,56,61], while the others reported cohort studies [23,24,25,26,27,29,30,31,32,34,35,36,38,39,40,41,43,44,45,47,48,49,51,52,53,54,55,57,58,59,60,62,64].
Most of the articles included studies carried out in the United States [24,31,33,37,38,41,43,46,47,51,53,54,57,58,59,62], followed by Denmark [26,29,36,39,43,49,55,63], and China [30,35,42,56,61].
Heavy metals, polychlorinated biphenyls (PCBs), and per- and polyfluoroalkyl substances (PFASs) were the most studied pollutants. To a lesser extent, the included studies evaluated the effect of pesticides [27,28,38,53,57], particulate matter [56,60], and phthalates [52]. The most common method used for assessing exposure to studied pollutants was human biomonitoring using serum or blood samples, with the exception of one study [56] that evaluated the exposure to environmental pollution through monitoring stations of air pollutant concentrations.
Most of the included studies evaluated the exposure to environmental pollution of the general population, while occupational exposure was the subject of just three studies: two evaluated the influence of pesticides [27,28] and did not show statistically significant relationships with hepatitis B vaccination, while the third assessed the role of PFAS on COVID-19 vaccination [58] and showed a significant inverse association between exposure to PFAS and the reduction of vaccine efficacy.
The majority of the vaccinations under investigation were hepatitis B, rubella, measles, mumps, diphtheria, and tetanus [23,33,35,36,39,40,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57,61,62,63]. In addition, a smaller number of studies considered vaccines against Haemophilus influenzae type B (HiB) [30,32,47,48,50], influenza [34,41], Bacillus Calmette-Guérin (BCG) [38], hepatitis type A [37,54], Japanese encephalitis [42], polio [42], and COVID-19 [56,58,59,60] vaccinations.
Many articles included in this review showed evidence of the interference that different classes of environmental pollutants exert on vaccine efficacy [25,26,29,31,32,33,34,35,36,38,40,42,43,44,45,46,47,48,49,50,51,52,53,56,57,58,60,61,62,63]. Some research has shown how PFAS exposure can negatively influence the antibody titers related to influenza vaccines containing the A/H3N2 strain [34], diphtheria and tetanus vaccines [31,36,39], the rubella vaccine in adults, [46] and, overall, the measles, mumps, and rubella (MMR) vaccine and the diphtheria, tetanus, and pertussis (DTP) vaccine [63]. However, conflicting results emerged: in one study, a statistically significant decrease emerged only for diphtheria but not for tetanus [44]; in another, the decrease in antibody titers related to measles was evident at a first follow up performed on 9-month-old children but not in a second one at the age of 2 years [49]. In other studies, only some PFASs (e.g., perfluorooctanoic acid, PFOA) [43] showed negative associations with diphtheria, tetanus, and HiB antibody titers while others did not (e.g., perfluorooctane sulfonate, PFOS) [50]. In another investigation, increased exposure to PFASs led to a decrease in rubella and mumps antibodies in the same participants and, at the same time, an increase in measles antibodies [61]. However, other studies which analyzed the role of PFASs did not yield any statistically significant results [41,54,55,59].
Additionally, a lot of research has been done on heavy metals, which are ubiquitous and have been shown to have a negative effect on vaccine-induced antibody concentration [42]. Lead exposure has been shown in various studies to lower the antibody titer of the vaccinations under investigation: tetanus [48] and measles, mumps, and rubella [40]; conversely, in another, there was a rise in the titers of the tetanus antibodies [51]. In one case, it was shown that a reduction in lead exposure resulted in an increase in the titers of hepatitis B antibodies [35]. With regards to mercury, highly variable results emerged: it was shown that as exposure increased, antibody titers for pertussis, diphtheria, and measles decreased, but in the same children with a better nutritional status, a smaller decrease was registered [47]. A similar situation can be seen in another paper on children who had high levels of methylmalonic acid, low folate, and high homocysteine: when mercury exposure increased by one percentage point, an increase in rubella antibody titer of 0.24% emerged, while in the absence of the previously listed factors a 0.18% decrease was found [33]. The results for arsenic, which is a metalloid, are also quite patchy: increases in exposure lead to increases in hepatitis A antibodies [37], a decrease in mumps antibodies [45], and in measles antibodies [62]. Finally, one study produced no significant results [24].
With regard to PCBs, an inverse relationship between exposure and immune response was reported with regard to rubella and mumps [25], diphtheria and tetanus [26,29], and measles [32]. Other studies, however, have not shown statistically significant associations [23,30].
Regarding pesticide exposure, some research has shown a reduction in BCG vaccine antibody levels for tuberculosis [38], tetanus, and diphtheria [57] associated to the increasing levels of pesticides. Further, in one study, a direct proportionality between pesticide exposure and measles antibody titer was found [53], while in another no significance was found [28].
In addition, the effect of particulate matter exposure on COVID-19 vaccination was evaluated and the studies in field reported possible negative interactions [56,60].
Considering the group of phthalates, the contribution of the exposure to these substances was evaluate in one article, which showed a reduction in the antibody titer related to the hepatitis B vaccine [52].
According to the NOS scale, 33 studies presented a good quality [23,24,25,26,27,29,30,31,32,33,34,35,36,38,40,43,44,45,46,47,48,49,51,52,53,54,55,57,58,59,60,61,62,63], seven a fair quality [28,39,41,42,50,56,61], and only one had a poor quality [37].
4. Discussion
The aim of this review was to assess the effects of environmental pollution on human immune responses to vaccination. The available literature suggests that the exposure to pollutants may negatively affect the effectiveness of vaccines in terms of antibody production. In particular, 16 studies aimed to address the relationship between PFC compounds and human responses to vaccination [31,34,36,39,41,43,44,46,49,50,54,55,58,59,61,63]. Among these, only the study by Hollister et al. did not report negative effects of exposure on antibody titers [59]. Due to their hydrophobic and oleophobic characteristics, perfluorinated compounds are largely used by humans, especially as fabric and container components, and, consequently, these compounds enter in the environmental matrices and humans are exposed to them. In particular, they can be easily spread in the air, soil, and water, reaching human tissues through the food chain and leading to negative health outcomes. Indeed, scientific evidence in this field has showed that they can affect the liver, central and peripheral nervous system, and endocrine system [64]. In the last few years, the immunotoxicity of PFCs has gained growing attention. Recently, the mechanism by which PFCs bind IgG has been revealed at the molecular level, providing a theoretical basis for their immunotoxic effect [65]. This research might also contribute to understanding the role of PFCs in lowering antibody titers after vaccination.
Eleven of the examined studies were focused on the possible effects of heavy metals [24,33,35,37,40,42,45,46,47,48,51,62]. Heavy metals are metals and metalloids with an atomic density higher than 4000 kg/m3 and are toxic to human beings even at low concentrations [66]. Individuals exposed to high amounts of heavy metals may develop gastrointestinal, renal, and cardiovascular diseases, tumors, and osteoporosis. Heavy metals can contaminate soil and water sources through industrial discharges and agricultural runoff. Many countries have established regulations for heavy metals that limit their concentrations in food to reduce their consumption. Significant but not always consistent evidence is available regarding the effects of heavy metals on human immune function, with some studies reporting immunostimulation and consequent hypersensitivity, allergies, and autoimmunity development, and others reporting immunosuppression and increased infection and cancer risk [67]. Our findings may be in line with the latter results. Indeed, all of the included studies showed that these pollutants may alter the immune response to vaccines. Among these, the only study involving adult individuals was the only one reporting higher antibody titers related to exposure to arsenic [37]. Interestingly, two of these studies reported worse effects of exposure to mercury in children who showed nutritional susceptibility, suggesting that nutrition could mediate the interaction between this metal and the immune response [33,47].
Furthermore, six out of eight studies investigating the effects of children’s or maternal exposure to PCBs [25,26,29,32,38,55] reported significant reductions in children’s immune responses to vaccines. PCBs can reach humans through the ingestion of contaminated foods, inhalation of contaminated air, and even dust ingestion or dermal contact. Environmental and occupational exposure to high concentrations of PCBs has been associated with several adverse outcomes, such as cardiovascular diseases and cancers, neurological deficits, dementia, and immune system dysfunctions. In addition, the bioaccumulation of PCBs can reduce fertility, with effects on the reproductive system that can be passed to offspring. Our finding highlights the need of further investigating the effects of prolonged exposure to low concentrations of PCBs [68].
The effects of pesticides were explored by four studies [27,28,53,57]. Two of these studies were performed on adults occupationally exposed and reported no differences in antibody titers between exposed and non-exposed individuals [27,28]. However, Baranska et al. reported that exposed workers genetically characterized by interleukin-1 gene polymorphism presented a lower immune response, demonstrating the relevance of the genetic variability in the evaluation of the effects determined by environmental exposure and the possibility of high-risk individuals [28]. Prahl et al. [53] and Hammel et al. [57] focalized their attention on children exposed to pesticides and their immune responses, reporting, respectively, higher and lower antibody levels in exposed children. The difference between age classes, which was also reported by Pilkerton et al. for PFCs [46], deserves attention in light of the definition of limit values for exposure to such compounds for individuals of different ages. Moreover, as also reported by Cardenas et al. [37], for arsenic, some pollutants may exert immune dysregulation, which can lead to the detection of increased antibody levels. This aspect should be considered when interpreting the results of similar studies.
The studies by Zhang et al. and Kogevinas et al. analyzed the interaction between air pollutants and immune responses to vaccines [56,60] and both of them showed an inverse relationship between exposure to pollution and anti-vaccine Ig levels. Finally, Wen et al. assessed and verified the negative effects of phthalates on vaccine effectiveness.
Taken together, all this evidence highlights the negative effects of environmental pollution on the immune system and suggests that it can also reduce the effectiveness of active immunization. Besides the technical, economic, political, and demographic issues that can reduce the effectiveness of vaccination programs [69], this aspect can contribute to hindering the control of vaccine-preventable infectious diseases and should be considered by health institutions.
This review shows important limitations. First of all, most of the findings have come from the US and the Netherlands. This result is due to the fact that all the studies performed in this field and published in international journals in the English language were carried out in those countries. We selected only studies in English because most peer-reviewed articles are published in this language, In addition, we also selected articles published in Italian, the language of the authors, but no articles in Italian were included in this review. Previous systematic reviews [70,71] demonstrated that restricting the search strategy to publications in the English language has little influence on the conclusions of systematic reviews on conventional and alternative medicine. To the contrary, other research has highlighted that studies published in languages different from English frequently present methodological limitations [72]. Although the majority of the included studies showed a good quality and reported the same measure of vaccine effectiveness and also the same type of pollutant exposure assessment, it should be considered that they examined the responses to different type of vaccines and the effects of different pollutants. Therefore, our findings should be considered with caution. Nevertheless, the literature regarding each type of vaccine or pollutant is still too scarce to generate robust evidence. In order to confirm our findings, and in the perspective of establishing possible exposure limit values for each type of pollutant, further research in this field is required. The systematic analysis of comparable studies, selected through more specific eligibility criteria, would allow to formulate stronger conclusions.
5. Conclusions
The results of the present systematic review showed that, overall, environmental pollution can negatively influence the human immune response to vaccines. This evidence is of great importance for public health because vaccines are one of the most relevant instruments available to prevent and control infectious diseases. The increasing environmental issues represent a multifaceted threat for population health. In this perspective, pollution control policies assume a fundamental role since they can also contribute to increase the effectiveness of immunization campaigns. However, the examined studies showed a great heterogeneity due to the variability in vaccines and pollutants investigated, but also in the type of populations examined and in immune response indicators evaluated. Furthermore, the individual differences in immune response should be taken in account when considering these data. Thus, it is essential to perform further studies to define the effects of environmental pollution on vaccines effectiveness. In particular, controlled studies focused on specific types of vaccines and pollutants and using the same assessment methods to evaluate immune response in comparable population groups are needed to obtain comparable results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12111252/s1, Table S1: Characteristics of the included studies.
Author Contributions
Conceptualization, C.P., F.V., and F.G.; methodology, K.V., F.L., and J.D.P.; software, K.V. and J.D.P.; validation, C.P., F.V., and F.G.; formal analysis, K.V., J.D.P., and F.L.; data curation, C.P., F.V., and F.G.; writing original draft preparation, C.P., F.V., K.V., J.D.P., and F.G.; writing review and editing, C.P., F.V., and F.G.; resources, G.L.; supervision, C.P., F.V., G.L., and F.G.; project administration, C.P., F.V., and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Italian Ministry of University and Research (PRIN 2017 Grant n. 20177MKB4H).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hammershaimb, E.A.D.; Campbell, J.D. Vaccine Development. Pediatr. Clin. N. Am. 2024, 71, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Vidal, R.M.; Velasco, J.; Carreño, L.J.; Torres, J.P.; Benachi, O.M.A.; Tovar-Rosero, Y.Y.; Oñate, A.A.; O’Ryan, M. Two centuries of vaccination: Historical and conceptual approach and future perspectives. Front. Public Health 2024, 11, 1326154. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.C.; Sarsaiya, S.; Gupta, A. A systematic review on the impact of cement industries on the natural environment. Environ. Sci. Pollut. Res. Int. 2022, 29, 18440–18451. [Google Scholar] [CrossRef]
- Kundu, D.; Dutta, D.; Joseph, A.; Jana, A.; Samanta, P.; Bhakta, J.N.; Alreshidi, M.A. Safeguarding drinking water: A brief insight on characteristics, treatments and risk assessment of contamination. Environ. Monit. Assess. 2024, 196, 180. [Google Scholar] [CrossRef]
- Vašíčková, J.; Hvězdová, M.; Kosubová, P.; Hofman, J. Ecological risk assessment of pesticide residues in arable soils of the Czech Republic. Chemosphere 2019, 216, 479–487. [Google Scholar] [CrossRef]
- Fletcher, C.; Ripple, W.J.; Newsome, T.; Barnard, P.; Beamer, K.; Behl, A.; Bowen, J.; Cooney, M.; Crist, E.; Field, C.; et al. Earth at risk: An urgent call to end the age of destruction and forge a just and sustainable future. PNAS Nexus 2024, 3, 106. [Google Scholar] [CrossRef]
- Rojas-Rueda, D.; Morales-Zamora, E.; Alsufyani, W.A.; Herbst, C.H.; AlBalawi, S.M.; Alsukait, R.; Alomran, M. Environmental Risk Factors and Health: An Umbrella Review of Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 704. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, 535–547. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality, Energy and Health, Type of Pollutants. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (accessed on 6 September 2024).
- Farhadi, Z.; Abulghasem Gorgi, H.; Shabaninejad, H.; Aghajani Delavar, M.; Torani, S. Association between PM2.5 and risk of hospitalization for myocardial infarction: A systematic review and a meta-analysis. BMC Public Health 2020, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Aly, Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J. Am. Soc. Nephrol. 2018, 29, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Debelu, D.; Mengistu, D.A.; Aschalew, A.; Mengistie, B.; Deriba, W. Global Public Health Implications of Traffic Related Air Pollution: Systematic Review. Environ. Health Insights 2024, 18, 11786302241272403. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Memon, A.N.; Sheikh, S.A.; Rouq, F.A.; Usmani, A.M.; Hassan, A.; Arian, S.A. Effect of environmental air pollution on type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 123–128. [Google Scholar]
- Jalili, C.; Kazemi, M.; Taheri, E.; Mohammadi, H.; Boozari, B.; Hadi, A.; Moradi, S. Exposure to heavy metals and the risk of osteopenia or osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1671–1682. [Google Scholar] [CrossRef]
- Keleb, A.; Daba, C.; Asmare, L.; Bayou, F.D.; Arefaynie, M.; Mohammed, A.; Tareke, A.A.; Kebede, N.; Tsega, Y.; Endawkie, A.; et al. The association between children’s exposure to pesticides and asthma, wheezing, and lower respiratory tract infections. A systematic review and meta-analysis. Front. Public Health 2024, 12, 1402908. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Korevaar, T.I.M.; Martin, L.; Sun, Y.; Bibi, Z.; Torres, N.; Coburn-Sanderson, A.; First, O.; Souter, I.; et al. Association between per- and polyfluoroalkyl substances exposure and thyroid function biomarkers among females attending a fertility clinic. Environ. Pollut. 2024, 346, 123513. [Google Scholar] [CrossRef]
- Arif, I.; Adams, M.D.; Johnson, M.T.J. A meta-analysis of the carcinogenic effects of particulate matter and polycyclic aromatic hydrocarbons. Environ. Pollut. 2024, 351, 123941. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 6 September 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Military Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Weisglas-Kuperus, N.; Sas, T.C.; Koopman-Esseboom, C.; van der Zwan, C.W.; De Ridder, M.A.; Beishuizen, A.; Hooijkaas, H.; Sauer, P.J. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr. Res. 1995, 38, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.M.; Wilson, T.J.; Ireland, J.; Jones, A.L.; Gorman, J.S.; Gale, N.L.; Johnson, J.C.; Hewett, J.E. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology 1999, 134, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Weisglas-Kuperus, N.; Patandin, S.; Berbers, G.A.; Sas, T.C.; Mulder, P.G.; Sauer, P.J.; Hooijkaas, H. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ. Health Perspect. 2000, 108, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef]
- Steerenberg, P.; van Amelsvoort, L.; Colosio, C.; Corsini, E.; Fustinoni, S.; Vergieva, T.; Zaikov, C.; Pennanen, S.; Liesivuori, J.; Van Loveren, H. Toxicological evaluation of the immune function of pesticide workers, a European wide assessment. Hum. Exp. Toxicol. 2008, 27, 701–707. [Google Scholar] [CrossRef]
- Baranska, M.; Van Amelsvoort, L.; Birindelli, S.; Fustinoni, S.; Corsini, E.; Liesivuori, J.; Van Loveren, H. Association of pesticide exposure, vaccination response, and interleukin-1 gene polymorphisms. Hum. Exp. Toxicol. 2008, 27, 709–713. [Google Scholar] [CrossRef]
- Heilmann, C.; Budtz-Jørgensen, E.; Nielsen, F.; Heinzow, B.; Weihe, P.; Grandjean, P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health Perspect. 2010, 118, 1434–1438. [Google Scholar] [CrossRef]
- Jusko, T.A.; De Roos, A.J.; Schwartz, S.M.; Lawrence, B.P.; Palkovicova, L.; Nemessanyi, T.; Drobna, B.; Fabisikova, A.; Kocan, A.; Sonneborn, D.; et al. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ. Res. 2010, 110, 388–395. [Google Scholar] [CrossRef]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012, 307, 391–397, Erratum in JAMA 2012, 307, 1142. [Google Scholar] [CrossRef]
- Stølevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; van Loveren, H.; et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef]
- Gallagher, C.M.; Smith, D.M.; Golightly, M.G.; Meliker, J.R. Total blood mercury and rubella antibody concentrations in US children aged 6-11 years, NHANES 2003–2004. Sci. Total Environ 2013, 442, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Looker, C.; Luster, M.I.; Calafat, A.M.; Johnson, V.J.; Burleson, G.R.; Burleson, F.G.; Fletcher, T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 2014, 138, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Zhang, J.; Guo, P.; Fu, T.; Dai, Y.; Lin, S.L.; Huo, X. Decreased blood hepatitis B surface antibody levels linked to e-waste lead exposure in preschool children. J. Hazard. Mater. 2015, 298, 122–128. [Google Scholar] [CrossRef]
- Mogensen, U.B.; Grandjean, P.; Heilmann, C.; Nielsen, F.; Weihe, P.; Budtz-Jørgensen, E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ. Health 2015, 14, 47. [Google Scholar] [CrossRef]
- Cardenas, A.; Smit, E.; Bethel, J.W.; Houseman, E.A.; Kile, M.L. Arsenic exposure and the seroprevalence of total hepatitis A antibodies in the US population: NHANES, 2003–2012. Epidemiol. Infect. 2016, 144, 1641–1651. [Google Scholar] [CrossRef]
- Jusko, T.A.; De Roos, A.J.; Lee, S.Y.; Thevenet-Morrison, K.; Schwartz, S.M.; Verner, M.A.; Murinova, L.P.; Drobná, B.; Kočan, A.; Fabišiková, A.; et al. A Birth Cohort Study of Maternal and Infant Serum PCB-153 and DDE Concentrations and Responses to Infant Tuberculosis Vaccination. Environ. Health Perspect. 2016, 124, 813–821. [Google Scholar] [CrossRef]
- Kielsen, K.; Shamim, Z.; Ryder, L.P.; Nielsen, F.; Grandjean, P.; Budtz-Jørgensen, E.; Heilmann, C. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J. Immunotoxicol. 2016, 13, 270–273. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, X.; Dai, Y.; Zhang, Y.; Li, W.; Huo, X. Considerable decrease of antibody titers against measles, mumps, and rubella in preschool children from an e-waste recycling area. Sci. Total Environ. 2016, 573, 760–766. [Google Scholar] [CrossRef]
- Stein, C.R.; Ge, Y.; Wolff, M.S.; Ye, X.; Calafat, A.M.; Kraus, T.; Moran, T.M. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environ. Res. 2016, 149, 171–178. [Google Scholar] [CrossRef]
- Lin, X.; Xu, X.; Zeng, X.; Xu, L.; Zeng, Z.; Huo, X. Decreased vaccine antibody titers following exposure to multiple metals and metalloids in e-waste-exposed preschool children. Environ. Pollut. 2017, 220, 354–363. [Google Scholar] [CrossRef]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Timmermann, A.; Budtz-Jørgensen, E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jørgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018. [Google Scholar] [CrossRef]
- Raqib, R.; Ahmed, S.; Ahsan, K.B.; Kippler, M.; Akhtar, E.; Roy, A.K.; Lu, Y.; Arifeen, S.E.; Wagatsuma, Y.; Vahter, M. Humoral Immunity in Arsenic-Exposed Children in Rural Bangladesh: Total Immunoglobulins and Vaccine-Specific Antibodies. Environ. Health Perspect. 2017, 125, 067006. [Google Scholar] [CrossRef] [PubMed]
- Pilkerton, C.S.; Hobbs, G.R.; Lilly, C.; Knox, S.S. Rubella immunity and serum perfluoroalkyl substances: Sex and analytic strategy. PLoS ONE 2018, 13, e0203330. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.; Permar, S.R.; Ortiz, E.; Berky, A.; Woods, C.W.; Amouou, G.F.; Itell, H.; Hsu-Kim, H.; Pan, W. Mercury Exposure and Poor Nutritional Status Reduce Response to Six Expanded Program on Immunization Vaccines in Children: An Observational Cohort Study of Communities Affected by Gold Mining in the Peruvian Amazon. Int. J. Environ. Res. Public Health 2019, 16, 638. [Google Scholar] [CrossRef]
- Di Lenardo, T.Z.; Ward, B.J.; Pillet, S.; Mann, K.; Bornman, R.; Obida, M.; Chevrier, J. Exposure to lead and vaccine-specific IgG titers in South African children participating in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE): A longitudinal study. Environ. Res. 2020, 180, 108794. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Jensen, K.J.; Nielsen, F.; Budtz-Jørgensen, E.; van der Klis, F.; Benn, C.S.; Grandjean, P.; Fisker, A.B. Serum Perfluoroalkyl Substances, Vaccine Responses, and Morbidity in a Cohort of Guinea-Bissau Children. Environ. Health Perspect. 2020, 128, 87002. [Google Scholar] [CrossRef]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Welch, B.M.; Branscum, A.; Geldhof, G.J.; Ahmed, S.M.; Hystad, P.; Smit, E.; Afroz, S.; Megowan, M.; Golam, M.; Sharif, O.; et al. Evaluating the effects between metal mixtures and serum vaccine antibody concentrations in children: A prospective birth cohort study. Environ. Health 2020, 19, 41. [Google Scholar] [CrossRef]
- Wen, H.J.; Guo, Y.L.; Su, P.H.; Sun, C.W.; Wang, S.J. Prenatal and childhood exposure to phthalic acid esters and vaccination antibodies in children: A 15-year follow-up birth cohort study. Environ. Int. 2020, 145, 106134. [Google Scholar] [CrossRef]
- Prahl, M.; Odorizzi, P.; Gingrich, D.; Muhindo, M.; McIntyre, T.; Budker, R.; Jagannathan, P.; Farrington, L.; Nalubega, M.; Nankya, F.; et al. Exposure to pesticides in utero impacts the fetal immune system and response to vaccination in infancy. Nat. Commun. 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Blomberg, A.J.; Bind, M.A.; Holm, D.; Nielsen, F.; Heilmann, C.; Weihe, P.; Grandjean, P. Serum vaccine antibody concentrations in adults exposed to per- and polyfluoroalkyl substances: A birth cohort in the Faroe Islands. J. Immunotoxicol. 2021, 18, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.A.G.; Pedersen, H.S.; Weihe, P.; Bjerregaard, P.; Nielsen, F.; Heilmann, C.; Grandjean, P. Concentrations of tetanus and diphtheria antibodies in vaccinated Greenlandic children aged 7–12 years exposed to marine pollutants, a cross sectional study. Environ. Res. 2022, 203, 111712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, S.; Xiao, G.; Zhao, M.; Li, J.; Dong, W.; Hu, J.; Yuan, T.; Li, Y.; Liu, L. The associations between air pollutant exposure and neutralizing antibody titers of an inactivated SARS-CoV-2 vaccine. Environ. Sci. Pollut. Res. Int. 2022, 29, 13720–13728. [Google Scholar] [CrossRef]
- Hammel, S.C.; Nordone, S.; Zhang, S.; Lorenzo, A.M.; Eichner, B.; Moody, M.A.; Harrington, L.; Gandee, J.; Schmidt, L.; Smith, S.; et al. Infants’ diminished response to DTaP vaccine is associated with exposure to organophosphate esters. Sci. Total Environ. 2022, 837, 155782. [Google Scholar] [CrossRef]
- Porter, A.K.; Kleinschmidt, S.E.; Andres, K.L.; Reusch, C.N.; Krisko, R.M.; Taiwo, O.A.; Olsen, G.W.; Longnecker, M.P. Antibody response to COVID-19 vaccines among workers with a wide range of exposure to per- and polyfluoroalkyl substances. Environ. Int. 2022, 169, 107537. [Google Scholar] [CrossRef]
- Hollister, J.; Caban-Martinez, A.J.; Ellingson, K.D.; Beitel, S.; Fowlkes, A.L.; Lutrick, K.; Tyner, H.L.; Naleway, A.L.; Yoon, S.K.; Gaglani, M.; et al. Serum per- and polyfluoroalkyl substance concentrations and longitudinal change in post-infection and post-vaccination SARS-CoV-2 antibodies. Environ. Res. 2023, 239, 117297. [Google Scholar] [CrossRef]
- Kogevinas, M.; Karachaliou, M.; Espinosa, A.; Aguilar, R.; Castaño-Vinyals, G.; Garcia-Aymerich, J.; Carreras, A.; Cortés, B.; Pleguezuelos, V.; Papantoniou, K.; et al. Long-Term Exposure to Air Pollution and COVID-19 Vaccine Antibody Response in a General Population Cohort (COVICAT Study, Catalonia). Environ. Health Perspect. 2023, 131, 47001. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Wang, Y.X.; Sun, Q.; Coull, B.; Sun, Y.; Slitt, A.; Messerlian, C. Red Blood Cell Folate Modifies the Association between Serum Per- and Polyfluoroalkyl Substances and Antibody Concentrations in U.S. Adolescents. Environ. Sci. Technol. 2023, 57, 2445–2456. [Google Scholar] [CrossRef]
- Roh, T.; Regan, A.K.; Johnson, N.M.; Hasan, N.T.; Trisha, N.F.; Aggarwal, A.; Han, D. Association of arsenic exposure with measles antibody titers in US children: Influence of sex and serum folate levels. Environ. Int. 2024, 183, 108329. [Google Scholar] [CrossRef]
- Sigvaldsen, A.; Højsager, F.D.; Paarup, H.M.; Beck, I.H.; Timmermann, C.A.G.; Boye, H.; Nielsen, F.; Halldorsson, T.I.; Nielsen, C.; Möller, S.; et al. Early-life exposure to perfluoroalkyl substances and serum antibody concentrations towards common childhood vaccines in 18-month-old children in the Odense Child Cohort. Environ. Res. 2024, 242, 117814. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, Z.; Zhao, W.; Hu, X.; Wang, H.; Wang, J.; Han, M.; Xu, L.; Sun, H.; Qin, C.; et al. Molecular mechanism of immunotoxicity: Binding interaction between perfluorinated compounds and human immunoglobulin G. Environ. Pollut. 2024, 362, 125032. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- de Gomensoro, E.; Del Giudice, G.; Doherty, T.M. Challenges in adult vaccination. Ann. Med. 2018, 50, 181–192. [Google Scholar] [CrossRef]
- Dobrescu, A.I.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Moher, D.; Fortin, P.; Jadad, A.R.; Jüni, P.; Klassen, T.; Le Lorier, J.; Liberati, A.; Linde, K.; Penna, A. Completeness of reporting of trials published in languages other than English: Implications for conduct and reporting of systematic reviews. Lancet 1996, 347, 363–366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).