Abstract
Vaccination before solid organ transplantation is recommended since post-transplantation immunosuppression is known to impair vaccine responses. However, little is known about post-transplantation seroprotection rates in organ transplant recipients vaccinated pre-transplantation. We aimed to investigate the proportion of transplant recipients vaccinated against hepatitis B virus (HBV) and invasive pneumococcal disease (IPD) pre-transplantation at the time of listing for transplantation with post-transplantation seroprotection. We included 136 solid organ transplant (SOT) recipients vaccinated at the time of listing for transplantation. We investigated post-transplantation antibody concentrations against HBV and IPD. Established antibody thresholds were used to define seroprotection. The proportions of SOT recipients with post-transplantation seroprotection were 27.9% (n = 38) and 42.6% (n = 58) against HBV and IPD, respectively. Compared to completing HBV vaccination pre-transplantation, completing post-transplantation vaccination (adjusted odds ratio (aOR): 7.8, 95% CI: 2.5–24.5, p < 0.001) and incomplete vaccination (aOR: 6.3, 95% CI: 1.2–32.6, p = 0.028) were associated with non-response against HBV, after adjustment for confounders. Importantly, patients with seroprotection at the time of listing had lower odds of non-response against HBV (aOR: 0.04, 95% CI: 0.0–0.1, p < 0.001) and IPD (aOR: 0.3, 95% CI: 0.1–0.7, p = 0.007) compared to those without seroprotection. SOT recipients vaccinated pre-transplantation had low post-transplantation seroprotection rates against HBV and IPD. However, SOT recipients with seroprotection at the time of listing had lower odds of non-response, suggesting early vaccination should be a priority.
1. Introduction
Organ transplant recipients receive immunosuppressive therapy to prevent allograft rejection after transplantation, which, in turn, increases susceptibility to and risk of complications from infections [1]. Specifically, the incidence of invasive pneumococcal disease (IPD) is more than seven times higher among solid organ transplant (SOT) recipients than in the general population [2]. While de novo hepatitis B virus (HBV) infection in SOT-recipients is rare, it has been shown to reduce graft survival in liver transplant recipients [3,4]. Both HBV and IPD are vaccine-preventable diseases [5,6]. Unique for these diseases are known antibody concentration thresholds for protection against disease. In transplant recipients, reduced antibody responses are seen after pneumococcal vaccination, and HBV vaccine-response rates are lower in liver transplant recipients vaccinated after transplantation than in candidates vaccinated before [7,8]. It is therefore recommended in several guidelines to vaccinate transplant candidates prior to transplantation [9,10,11]. However, there are limited data to support this strategy [12,13]. In contrast, previous studies have shown response rates between 13.0% and 67.0% to HBV vaccination in patients with chronic liver disease who are on the transplant wait list [14,15]. While some studies suggest higher response rates among transplant candidates when vaccinated against HBV during early stages of liver disease [16], there are limited data on post-transplantation seroprotection rates when transplant candidates are vaccinated against HBV and IPD pre-transplantation [17,18,19,20]. Furthermore, previous studies were either conducted more than two decades ago, evaluated heterogeneous vaccination schedules, or included few participants [18,19,20]. Thus, there are important gaps in the knowledge of the effect of pre-transplantation vaccination.
This study was conducted to provide information to guide vaccination strategies. We aimed to determine the proportion of transplant recipients vaccinated against HBV and IPD pre-transplantation at the time of listing for transplantation with post-transplantation seroprotection. Furthermore, we determined risk factors for responses below the protective threshold (non-response).
2. Methods
2.1. Study Design and Population
We conducted a prospective cohort study including adult SOT recipients (≥18 years) vaccinated at the time of listing for transplantation between February 2020 and October 2022 before undergoing liver, lung, or heart transplantation. In Denmark, all liver and lung transplantations and around half of heart transplantations are performed at Copenhagen University Hospital, Rigshospitalet, where transplant candidates are assessed at a centralized pre-transplantation vaccination clinic at Department of Infectious Diseases. This is a structured vaccination program based on serology, exposures, and vaccination history for HBV and IPD, with >95% adherence of transplant candidates at our center [21].
2.2. Pre-Transplantation Serology and Vaccination
Before assessment at the vaccination clinic, the SOT candidates underwent serological screening for HBV surface antibodies (anti-HBs) and pneumococcal antibodies.
Candidates with anti-HBs concentrations above the protective cut-off and/or documented history of complete HBV vaccination were offered a booster vaccine. A full HBV vaccination schedule of three doses of 20 µg recombinant HBV surface antigen administered at 0, 1, and 6 months was offered to candidates without seroprotection or documentation for completed HBV vaccination. The available vaccines were Twinrix® (GlaxoSmithKline, London, UK), Engerix-B® (GlaxoSmithKline, London, UK) and Fendrix® (GlaxoSmithKline, London, UK).
Candidates were vaccinated against IPD based on their vaccination history and pneumococcal serology. The recommended pneumococcal vaccinations were either one 13-valent pneumococcal conjugate vaccine (PCV13) followed by a 23-valent pneumococcal polysaccharide vaccine (PPSV23) after 8 weeks or one dose of 20-valent pneumococcal conjugate vaccine (PCV20). If the patient had previously received PPSV23, this was followed by one dose of PCV13 or PCV20 at least one year after the PPSV23 dose. The available vaccines were Pneumovax 23® (Merck Sharp & Dohme Corp. Rahway, NJ, USA), Prevenar 13® (Pfizer Europe MA EEIG, Brussels, Belgium), and Apexxnar® (Pfizer Europe MA EEIG, Brussels, Belgium).
2.3. Post-Transplantation Serology
Concentrations of anti-HBs and the geometric mean concentration (GMC) of antibodies against 12 representative pneumococcal serotypes were measured after transplantation as a part of routine clinical care. Anti-HBs were analyzed following local instructions, and pneumococcal antibodies were quantified at Statens Serum Institute (SSI) using in-house Luminex technology. If more than one test result was available, we used the earliest serology measured at least three months after transplantation.
2.4. Data Retrieval
Information about vaccination history, including timing of vaccines, was retrieved from the Danish Vaccination Registry (DDV) [22]. DDV has complete national coverage and contains all vaccines administered in Denmark since 2015.
Antibody test results were collected from the Danish Microbiology Database (MiBa), a nationwide database containing all microbiology results in Denmark since 2011 [23], and from medical records. Clinical data including immunosuppressive therapy and acute rejections were extracted from medical records.
The study was conducted following the Declaration of Helsinki. According to Danish legislation, the retrieval of data without collection of informed consent was approved by the institutional review board at the Center for Regional Development (R-20051155).
2.5. Definitions
The WHO has defined an anti-HBs concentration of >10 IU/L as protective against HBV [24]. A pneumococcal antibody concentration of ≥0.35 µg/mL is considered protective against pneumococcal disease from a specific serotype in children [24]. However, since thresholds vary by pneumococcal serotype, and a more conservative cut-off has been suggested for adults, we defined seroprotection against IPD as a GMC of IgG antibodies against 12 representative serotypes of ≥1 µg/mL [20,25].
Completed HBV vaccination pre-transplantation was defined as three doses administered pre-transplantation. Post-transplantation completion was defined as ≥1 of the three doses administered after transplantation. Individuals with seroprotection at the time of listing for transplantation who received one HBV booster vaccination were defined as protected at the time of listing and boosted.
Completed IPD vaccination pre-transplantation was defined as either having seroprotection at the time of listing or having received PCV13 and PPSV23 or one dose of PCV20 administered pre-transplantation. Completed post-transplantation IPD vaccination was defined as ≥1 vaccine administered after transplantation.
The time of completion of a vaccine series was defined as the date of the last vaccine.
2.6. Statistical Analysis
Continuous data were reported as means or medians and compared using Student’s t-test or Mann–Whitney U test, as appropriate. Categorical data were reported as percentages and compared using Pearson’s chi-square test or Fisher’s test, as appropriate.
Seroprotection rates were reported as proportions. Logistic regression models with vaccine seroprotection against either HBV or IPD as the dependent variable were used to explore risk factors for vaccine non-response. In adjusted models, variables were tested one at a time, adjusting for age, sex, and transplant type. Furthermore, a sensitivity analysis of the logistic regression models, including serology status at the time of listing for transplantation, was performed for the adjusted models.
3. Results
3.1. Cohort Characteristics
We included 136 SOT recipients with pre-transplantation vaccination and a post-transplantation HBV and IPD serology test result (Figure 1). Of those, 87 (64.0%) were liver, 34 (25.0%) were lung, and 15 (11.0%) were heart transplant recipients (clinical characteristics shown in Table 1).
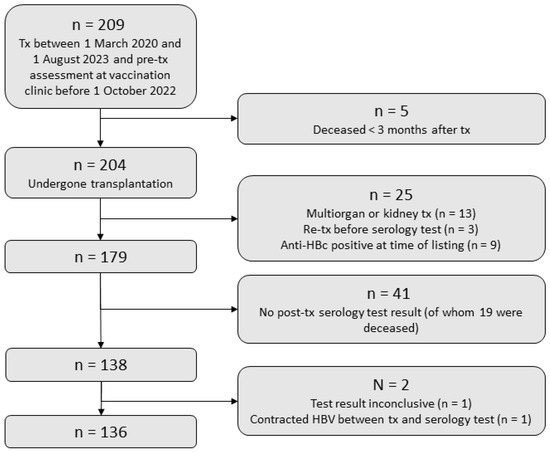
Figure 1.
Study flowchart. Tx, transplantation; Anti-HBc, hepatitis B core antibodies; HBV, hepatitis B virus; IPD, invasive pneumococcal disease.

Table 1.
Patient characteristics at time of listing for transplantation.
HBV serology at the time of listing for transplantation was available for 122 (89.7%) SOT recipients, and of those, 23 (18.9%) had existing seroprotection against HBV (Figure 2). IPD serology from the time of listing for transplantation was available for 132 (97.1%) SOT recipients, and of those, 43 (32.6%) had existing seroprotection against IPD (Figure 2).
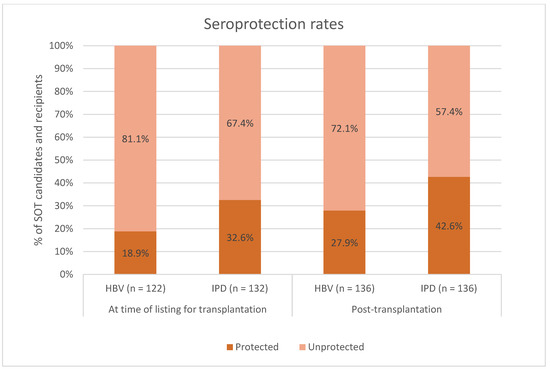
Figure 2.
Seroprotection rates among SOT candidates and recipients vaccinated against hepatitis B virus (HBV) and invasive pneumococcal disease (IPD) at time of listing for transplantation. Dark orange: percentage of SOT candidates and recipients with seroprotection. Light orange: percentage of SOT candidates and recipients without seroprotection. X-axis: seroprotection rates at the time of listing for transplantation (two columns on the left) and seroprotection rates after transplantation (two columns on the right). Y-axis: percentage of SOT candidates and recipients. SOT, solid organ transplant.
3.2. Hepatitis B Virus
Post-transplantation protective anti-HBs concentrations were present in 38 (27.9%) of 136 SOT recipients (Figure 2). Among individuals with post-transplantation seroprotection, 18 (56.2%) had seroprotection at the time of listing for transplantation, while among those without post-transplantation seroprotection, 5 (5.6%, p < 0.001) had seroprotection at the time of listing for transplantation. A higher proportion of the SOT recipients with post-transplantation seroprotection had completed their vaccination schedule pre-transplantation compared to those without post-transplantation seroprotection: 19 (50.0%) vs. 22 (22.4%, p < 0.001) (clinical characteristics between SOT recipients with and without seroprotection against HBV shown in Table 2).

Table 2.
Serology for HBV surface antibodies.
In a univariable logistic regression model, the odds ratio (OR) for non-response was 9.2 ([95% CI: 3.0–27.6], p < 0.001) when completing the HBV vaccination schedule after transplantation and 6.9 ([95% CI: 1.4–34.0], p = 0.017) when never completing vaccination compared to SOT recipients who completed vaccination pre-transplantation. Furthermore, SOT recipients with a longer time from assessment at the vaccination clinic to transplantation had an OR of 0.7 ([95% CI: 0.6–0.9], p = 0.008) for non-response per 3 months of increase in time from assessment at the vaccination clinic to transplantation.
In a multivariable model, adjusting for age, sex, and type of transplanted organ, the adjusted OR (aOR) for non-response was 7.8 ([95% CI: 2.5–24.5], p < 0.001) when completing HBV vaccination schedule after transplantation and 6.3 ([95% CI: 1.2–32.6], p = 0.028) when never completing vaccination compared to SOT recipients who completed vaccination pre-transplantation. SOT recipients with a longer time from assessment at the vaccination clinic to transplantation had an aOR of 0.8 ([95% CI: 0.6–1.0], p = 0.043) for non-response per 3 months of increase in time from assessment to transplantation (Table 3).

Table 3.
Risk factors for non-response to HBV vaccination.
A sensitivity analysis was performed including only the 122 participants with HBV serology at time of listing. In a univariable logistic regression model, SOT recipients with seroprotection against HBV at time of listing had an OR of 0.1 ([95% CI: 0.0–0.1], p < 0.001) for non-response compared to those without seroprotection at time of listing.
In a multivariable model adjusted for age, sex, and transplant type, SOT recipients with seroprotection against HBV at time of listing had an aOR of 0.04 ([95% CI: 0.0–0.1], p < 0.001) for post-transplantation HBV non-response compared to those without seroprotection at time of listing (Table 4).

Table 4.
Sensitivity analysis. Risk of non-response to vaccination among SOT recipients with serology at time of listing for transplantation and post-transplantation serology.
3.3. Invasive Pneumococcal Disease
Protective antibody concentrations against IPD after transplantation were present in 58 (42.6%) of 136 SOT recipients (Figure 2). Among individuals with post-transplantation seroprotection, 25 (43.9%) had seroprotection at the time of listing for transplantation, while among those without post-transplantation seroprotection, 18 (24.0%, p = 0.026) had seroprotection at the time of listing for transplantation. A larger proportion of the SOT recipients with post-transplantation seroprotection had completed their vaccination schedule pre-transplantation compared to those without post-transplantation seroprotection: 44 (75.9%) vs. 52 (66.7%, p = 0.008) (clinical characteristics between SOT recipients with and without seroprotection against IPD shown in Table 5).

Table 5.
Serology for pneumococcal antibodies.
In a univariable logistic regression model, male SOT recipients had an odds ratio (OR) for non-response of 0.5 ([95% CI: 0.2–0.9], p = 0.028) compared to female SOT recipients. No statistically significant risk factors for non-response after vaccination against IPD were found in the multivariable logistic regression model (Table 6).

Table 6.
Risk factors for non-response to pneumococcal vaccination.
A sensitivity analysis was performed including only the 132 participants with IPD serology at the time of listing. In a univariable logistic regression model, SOT recipients with seroprotection against IPD at the time of listing had an OR of 0.4 ([95% CI: 0.2–0.9], p = 0.017) for post-transplantation IPD non-response compared to those without seroprotection at the time of listing.
In a multivariable model adjusted for age, sex, and type of transplanted organ, SOT recipients with seroprotection against IPD at the time of listing had an aOR of 0.3 ([95% CI: 0.1–0.7], p = 0.007) for non-response compared to those without seroprotection at time of listing (Table 4).
4. Discussion
In this cohort study, we included 136 solid organ transplant recipients who were referred for pre-transplantation vaccination, including HBV and IPD, at the time of listing for transplantation. Post-transplantation seroprotection rates for HBV and IPD were 27.9% and 42.6%, respectively. Risk factors for HBV non-response were post-transplantation completed HBV vaccination, incomplete HBV vaccination, and shorter time from assessment at the vaccination clinic to transplantation. Importantly, having seroprotection at the time of listing for transplantation was associated with lower odds of non-response for both HBV and IPD.
The post-transplantation HBV seroprotection rate of 27.9% is low, but it is comparable with previous studies of SOT candidates vaccinated against HBV pre-transplantation and demonstrating post-transplantation anti-HBs seroprotection rates from 8.0% to 74.5% [7,17,19]. We found that completion of post-transplantation HBV vaccination or never completing vaccination were associated with higher odds of non-response, and similar findings have been reported before [19]. Thus, Arslan et al. found that liver transplant candidates who completed HBV vaccination pre-transplantation were more likely to elicit detectable HBV antibodies than candidates who completed post-transplantation vaccination [19]. These results support pre-transplantation vaccination of SOT candidates, and underline the importance of not only initiating but completing the schedule prior to transplantation.
Furthermore, we found an association between longer time from assessment at the vaccination clinic to transplantation and lower risk of HBV non-response. This could be due to increased likelihood of completing the vaccination schedule before transplantation. Another explanation could be an association between longer time from assessment at the vaccination clinic to transplantation and less severe organ disease, which has been described to be associated with lower risk of non-response in liver transplant recipients [19].
Interestingly, we found the presence of protective anti-HBs concentrations at the time of listing for transplantation to be strongly associated with lower odds of post-transplantation non-response. This has not previously been described, mainly due to exclusion of patients with seroprotection in many of the previous studies [7,14,15,17,19]. However, one study including kidney transplant patients with pre-transplantation seroprotection against HBV found a post-transplantation seroprotection rate of 86% among those negative for hepatitis B core antibodies [26]. This study and our findings indicate that pre-transplantation seroprotection may be an important factor when aiming to achieve seroprotection after transplantation.
The SOT recipients included in our study had a seroprotection rate of 18.9% against HBV at the time of listing, most likely due to low rates of prior HBV vaccination. Our findings suggest that vaccinating even earlier in the course of the disease might be important to ensure post-transplantation seroprotection, and perhaps, vaccination should take place before the candidate is listed for transplantation. A suggestion for further investigations is double-dose HBV vaccination, which, in a randomized controlled trial, had an OR for seroprotection of 2.48 compared to a single dose when administered to 107 non-responding patients with human immunodeficiency virus (HIV) [27].
The post-transplantation seroprotection rate against IPD was 42.6%, which seems to be low. However, it is difficult to compare with previous studies in SOT recipients due to heterogenous vaccination schedules and different definitions of a positive vaccine response [18,20,28,29]. One trial included kidney transplant candidates and recipients vaccinated against IPD with either a single or double dose of PCV13 and PPSV23 administered with 12 weeks interval. This study found seroprotection rates of 33% among the wait list patients, of whom 51.7% underwent kidney transplantation during the study, and 16% among the transplant recipients 18 months after vaccination [30]. We did not investigate pre-transplantation seroprotection rates after vaccination, but previous studies of vaccination against IPD in liver transplant candidates have reported the waning of antibodies to or below baseline concentrations after transplantation [18,20]. The seroprotection rate against IPD at the time of listing for transplantation was 32.6%, which is higher than for HBV, probably due to IPD vaccination prior to listing for transplantation being more common than HBV vaccination. We found that seroprotection against IPD at the time of listing for transplantation was associated with lower odds of IPD non-response, which supports the recommendation of pre-transplantation vaccination and suggest that vaccination early during the work-up before listing for transplantation may improve the chance of obtaining post-transplantation seroprotection.
The strengths of this study include established protective antibody thresholds for both vaccines and a well-described cohort with detailed data on vaccination history, immunosuppressive therapy, serology, and comorbidities. Our study also has possible limitations. Information on pre-transplantation serology after vaccination was not available, and therefore, we could not investigate the proportion of patients who were seroprotected after vaccination but lost their antibodies after transplantation. While antibodies are important for evaluating seroprotection, our study did not include measuring cellular immunity. Future studies to build on the present study should include pre-transplantation serology after vaccination, more detailed immunology, and, optimally, kidney transplant recipients, who were not included in this study.
5. Conclusions
In conclusion, post-transplantation seroprotection rates for both HBV and IPD were low. Risk factors for HBV non-response were completing vaccination after transplantation, having incomplete vaccination, and shorter duration from assessment at the vaccination clinic to transplantation. For both HBV and IPD, we found that patients with seroprotection at the time of listing for transplantation had lower odds of non-response compared to those without seroprotection. Thus, to improve post-transplantation seroprotection rates, we recommend that early vaccination during the pre-transplantation work-up should be a priority. Furthermore, increased attention to follow-up serology and the use of a booster vaccination may be warranted.
6. Summary of Key Findings
- ⇒
- Post-transplantation seroprotection rates for both hepatitis B virus (HBV) and invasive pneumococcal disease (IPD) were 27.9% (n = 38) and 42.6% (n = 58) against HBV and IPD, respectively;
- ⇒
- Risk factors for HBV non-response were completed HBV vaccination post-transplantation, incomplete HBV vaccination, and shorter time from assessment at the vaccination clinic to transplantation;
- ⇒
- SOT recipients with seroprotection at the time of listing for organ transplantation had lower odds of non-response against HBV and IPD.
Author Contributions
L.B.H., conceptualization, methodology, investigation, formal analysis, and writing—original draft; S.R.H., conceptualization, methodology, formal analysis, data curation, writing—review and editing, and supervision; A.H., Z.B.H., L.F.L., N.E.W., L.D.H., C.E., S.B., J.G.H., I.H.M.M., P.S.K., P.N.B., F.G., M.P. and A.R., investigation and writing—review and editing; S.D.N., conceptualization, methodology, formal analysis, data curation, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research scholarship to L.B.H. from Rigshospitalet, Copenhagen University Hospital. The funding source was not involved in any part of the work.
Institutional Review Board Statement
The study was approved by the Regional Scientific Ehtics Committee of the Capital Region of Denmark(R-20051155).
Informed Consent Statement
The retrieval of data without collection of informed consent was approved by the institutional review board at the Center for Regional Development (R-20051155).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
L.B.H., S.R.H., A.H., L.F.L., N.E.W., L.D.H., C.E., S.B., J.G.H., I.H.M.M., P.S.K., P.N.B., M.P. and A.R. declare no conflicts of interest. Z.B.H. received research grants from Independent Research Fund (grant nr. 3162-00031B), Helen Rudes Foundation, and the Danish Cancer Society (Grant number KBVU-MS R327-A19137) not related to this work. F.G. is an advisor at Pfizer, Abbott, Ionis, Alnylam, Bayer, Astra-Zeneca, AdjuCor, and FineHeart and speaker at Novartis, all outside the current work. S.D.N. has received unrestricted research grants from the Novo Nordisk Foundation, Lundbeck Foundation, and the Independent Research Fund Denmark and reports advisory board activity for Gilead Sciences and GlaxoSmithKline/ViiV Healthcare, all unrelated to this manuscript.
References
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia, M.C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus Recommendations for Use of Maintenance Immunosuppression in Solid Organ Transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022, 42, 599–633. [Google Scholar] [CrossRef] [PubMed]
- Rezahosseini, O.; Møller, D.L.; Sørensen, S.S.; Perch, M.; Gustafsson, F.; Gelpi, M.; Knudsen, J.; Helleberg, M.; Rasmussen, A.; Nielsen, S.D.; et al. An Observational Prospective Cohort Study of Incidence and Outcome of Streptococcus pneumoniae and Hemophilus influenzae Infections in Adult Solid Organ Transplant Recipients. Microorganisms 2021, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, W.S.; Martin, P.; Bhamidimarri, K.R. Hepatitis B Virus Infection and Organ Transplantation. Gastroenterol. Hepatol. 2018, 14, 33–40. [Google Scholar]
- Skalski, M.; Patkowski, W.; Grąt, M.; Zieniewicz, K.; Wróblewski, T.; Hołówko, W.; Krawczyk, M. Utilization of Donors with Hepatitis B Core Antibodies in Liver Transplantation. Ann. Transpl. 2015, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Guo, G.N.; Li, C. The Impact of Hepatitis B Vaccination in the United States, 1999–2018. Hepatology 2022, 75, 1566–1578. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Loinaz, C.; de Juanes, J.R.; Gonzalez, E.M.; López, A.; Lumbreras, C.; Gómez, R.; Gonzalez-Pinto, I.; Jiménez, C.; Garcia, I.; Fuertes, A. Hepatitis B Vaccination Results in 140 Liver Transplant Recipients. Hepatogastroenterology 1997, 44, 235–238. [Google Scholar]
- Dendle, C.; Stuart, R.L.; Mulley, W.R.; Holdsworth, S.R. Pneumococcal Vaccination in Adult Solid Organ Transplant Recipients: A Review of Current Evidence. Vaccine 2018, 36, 6253–6261. [Google Scholar] [CrossRef]
- Dansk Selskab for Infektionsmedicin and Dansk Transplantationsselskab. Vaccination Af Voksne Kandidater Og Recipienter Til Solid Organtransplantation (2022). Available online: https://www.infmed.dk/guidelines#vaccination_af_voksne_kandidater_og_recipienter_til_solid_organtransplantation_2022.pdf (accessed on 2 August 2023).
- Bahakel, H.; Feldman, A.G.; Danziger-Isakov, L. Immunization of Solid Organ Transplant Candidates and Recipients: A 2022 Update. Infect. Dis. Clin. N. Am. 2023, 37, 427–441. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D. AST ID Community of Practice. Vaccination of Solid Organ Transplant Candidates and Recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Wang, L.; Verschuuren, E.A.M.; Paap, D.; Rondaan, C.; Raveling-Eelsing, E.; Westra, J.; Bos, N.A. Prophylactic Vaccination with a Live-Attenuated Herpes Zoster Vaccine in Lung Transplant Candidates. J. Heart L. Transpl. 2020, 39, 1445–1454. [Google Scholar] [CrossRef]
- Sokal, E.M.; Ulla, L.; Otte, J.B. Hepatitis B Vaccine Response before and after Transplantation in 55 Extrahepatic Biliary Atresia Children. Dig. Dis. Sci. 1992, 37, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tajes, S.; Pocurull, A.; Lens, S.; Mariño, Z.; Olivas, I.; Soy, G.; Alonso, A.; Vilella, A.; Forns, X. Efficacy of an Accelerated Double-Dose Hepatitis B Vaccine Regimen in Patients with Cirrhosis. J. Viral. Hepat. 2021, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, P.R.; Bacchella, T.; Freitas, A.C.; Osaki, K.T.; Lopes, M.H.; Freire, M.P.; Machado, M.C.C.; Abdalala, E. Double-Dose Hepatitis B Vaccination in Cirrhotic Patients on a Liver Transplant Waiting List. Braz. J. Infect. Dis. 2008, 12, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Domingo, I.; Pascasio Acevedo, J.M.; Alcalde Vargas, A.; Ramos Cuadra, A.; Ferrer Ríos, M.T.; Sousa Martin, J.M.; Sayago Mota, M.; Giráldez Gallego, A.; Suárez Artacho, G. Response to Vaccination against Hepatitis B Virus with a Schedule of Four 40-Μg Doses in Cirrhotic Patients Evaluated for Liver Transplantation: Factors Associated with a Response. Transpl. Proc. 2012, 44, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Bayas, J.M.; Bruni, L.; Díez, C. Solid Organ Transplantation and Response to Vaccination. Vaccine 2007, 25, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- McCashland, T.M.; Preheim, L.C.; Gentry-Nielsen, M.J. Pneumococcal Vaccine Response in Cirrhosis and Liver Transplantation. J. Infect. Dis. 2000, 181, 757–760. [Google Scholar] [CrossRef]
- Arslan, M.; Wiesner, R.H.; Sievers, C.; Egan, K.; Zein, N.N. Double-Dose Accelerated Hepatitis B Vaccine in Patients With End-Stage Liver Disease. Liver Transpl. 2001, 7, 314–320. [Google Scholar] [CrossRef]
- Eriksson, M.; Käyhty, H.; Lahdenkari, M.; Mäkisalo, H.; Anttila, V.-J. A Randomized, Controlled Trial Comparing the Immunogenicity and Safety of a 23-Valent Pneumococcal Polysaccharide Vaccination to a Repeated Dose 13-Valent Pneumococcal Conjugate Vaccination in Adult Liver Transplant Recipients. Vaccine 2021, 39, 2351–2359. [Google Scholar] [CrossRef]
- Harboe, Z.B.; Hald, A.; Ekenberg, C.; Ete Wareham, N.; Fogt Lundbo, L.; Holler, J.G.; Qvist, T.; Rask Hamm, S.; Bjerrum, S.; Rezahosseini, O.; et al. Implementation of a Vaccination Clinic for Adult Solid Organ Transplant Candidates: A Single-Center Experience. Vaccine 2023, 41, 6637–6644. [Google Scholar] [CrossRef]
- Grove Krause, T.; Jakobsen, S.; Haarh, M.; Mølbak, K. The Danish Vaccination Register. Euro. Surveill. 2012, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Voldstedlund, M.; Haarh, M.; Mølbak, K.; MiBa Board of Representatives. The Danish Microbiology Database (MiBa) 2010 to 2013. Euro. Surveill. 2014, 19, 20667. [Google Scholar] [CrossRef] [PubMed]
- WHO. Correlates of Vaccine-Induced Protection: Methods and Implications Immunization, Vaccines and Biologicals; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-Specific Effectiveness and Correlates of Protection for the 13-Valent Pneumococcal Conjugate Vaccine: A Postlicensure Indirect Cohort Study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Moal, V.; Motte, A.; Vacher-Coponat, H.; Tamalet, C.; Berland, Y.; Colson, P. Considerable Decrease in Antibodies against Hepatitis B Surface Antigen Following Kidney Transplantation. J. Clin. Virol. 2015, 68, 32–36. [Google Scholar] [CrossRef]
- Vargas, J.I.; Jensen, D.; Martínez, F.; Sarmiento, V.; Peirano, F.; Acuña, P.; Provoste, F.; Bustos, V.; Cornejo, F.; Fuster, A.; et al. Comparative Efficacy of a High-Dose vs Standard-Dose Hepatitis B Revaccination Schedule Among Patients With HIV A Randomized Clinical Trial + Visual Abstract + Invited Commentary + Supplemental Content. JAMA Netw. Open 2021, 4, 2120929. [Google Scholar] [CrossRef]
- Sarmiento, E.; Rodríguez-Hernández, C.; Rodríguez-Molina, J.; Fernández-Yánez, J.; Palomo, J.; Anguita, J.; Pérez, J.L.; Lanio, N.; Fernández-Cruz, E.; Carbone, J. Impaired Anti-Pneumococcal Polysaccharide Antibody Production and Invasive Pneumococcal Infection Following Heart Transplantation. Int. Immunopharmacol. 2006, 6, 2027–2030. [Google Scholar] [CrossRef]
- Van Kessel, D.A.; Hoffman, T.W.; Kwakkel-Van Erp, J.M.; Oudijk, E.J.D.; Zanen, P.; Rijkers, G.T.; Grutters, J.C. Long-Term Follow-up of Humoral Immune Status in Adult Lung Transplant Recipients. Transplantation 2017, 101, 2477–2483. [Google Scholar] [CrossRef]
- Larsen, L.; Bistrup, C.; Sørensen, S.S.; Boesby, L.; Svaerke, C.; Nielsen, C.; Somuncu, I. Durability of Antibody Response after Primary Pneumococcal Double-Dose Prime-Boost Vaccination in Adult Kidney Transplant Recipients and Candidates: 18-Month Follow-Up in a Non-Blinded, Randomised Clinical Trial. Vaccines 2022, 10, 1091. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).