Abstract
Patients with Chronic Lymphocytic Leukemia (CLL) have a 29- to 36-fold increased risk of invasive pneumococcal disease (IPD) compared to healthy adults. Therefore, most guidelines recommend vaccination with the 13-valent pneumococcal conjugated vaccine (PCV13) followed 2 months later by the 23-valent polysaccharide vaccine (PPSV23). Because both CLL as well as immunosuppressive treatment have been identified as major determinants of immunogenicity, we aimed to assess the vaccination schedule in untreated and treated CLL patients. We quantified pneumococcal IgG concentrations against five serotypes shared across both vaccines, and against four serotypes unique to PPSV23, before and eight weeks after vaccination. In this retrospective cohort study, we included 143 CLL patients, either treated (n = 38) or naive to treatment (n = 105). While antibody concentrations increased significantly after vaccination, the overall serologic response was low (10.5%), defined as a ≥4-fold antibody increase against ≥70% of the measured serotypes, and significantly influenced by treatment status and prior lymphocyte number. The serologic protection rate, defined as an antibody concentration of ≥1.3 µg/mL for ≥70% of serotypes, was 13% in untreated and 3% in treated CLL patients. Future research should focus on vaccine regimens with a higher immunogenic potential, such as multi-dose schedules with higher-valent T cell dependent conjugated vaccines.
1. Introduction
Patients with chronic lymphocytic leukemia (CLL) are at increased risk of both invasive pneumococcal disease (IPD) and community acquired pneumonia (CAP) caused by Streptococcus pneumoniae [1,2,3]. IPD carries a high case-fatality rate of 15% according to the latest analysis of the European Centre for Disease Prevention and Control (ECDC) [4]. A recent study showed that patients with CLL have a 29- to 36-fold increased risk of invasive pneumococcal disease in comparison with the general population (15/100.000 per year) [3]. Although pneumococcal vaccinations are advised and readily available, vaccination responses are diminished [5,6,7], because CLL and its therapy cause profound immune dysfunction, for example, due to hypogammaglobinemia, CLL-mediated T cell dysfunction, and medication induced immunosuppression [8]. More advanced disease stage, lower baseline IgG antibodies and prior treatment have been shown to negatively influence the humoral response to pneumococcal vaccination in CLL patients [7]. International guidelines therefore recommend vaccination at an early stage of disease, preferably before initiation of treatment [9]. The pneumococcal vaccination schedule recommended by the European Conference on Infections in Leukemia (ECIL) 7 guideline consists of Prevenar13, a 13-valent pneumococcal conjugated vaccine (PCV13), followed 2 months later by Pneumovax23, a 23-valent polysaccharide vaccine (PPSV23) [9,10]. Previous studies have only investigated responses to either PCV13 or PPSV23 alone [5,7,11,12,13,14,15,16,17]. Therefore, knowledge about the immunogenicity of this sequential vaccination schedule in CLL patients is lacking. While responses to PPSV23 alone are poor [13,16,18], immunogenicity of the 7-valent pneumococcal conjugated vaccine (PCV7) and PCV13 are better but still significantly impaired compared with healthy individuals [5,7,14]. Our aim was to assess the immunogenicity of the sequential vaccination schedule of PCV13 followed by PPSV23 in treatment naive and treated CLL patients.
2. Materials and Methods
2.1. Study Design and Population
As part of routine care, one dose of PCV13 followed two months later by one dose of PPSV23 were administered to adult CLL patients naive to pneumococcal vaccination in one of seven participating hospitals. According to the current local standard of care for immunosuppressed individuals, the response to vaccination was assessed by measuring serotype-specific pneumococcal immunoglobulin G (IgG) antibody concentrations prior to and eight weeks after the complete vaccination schedule (Figure 1). Written informed consent was given by all participants before the start of the study to collect their clinical data and laboratory results for research purposes. Permission from the medical ethics committee was granted.
Figure 1.
Vaccination and measurement schedule. Patients were retrospectively included when the vaccination and measurement schedule was completed. According to the current local standard of care for immunosuppressed individuals, prior to vaccination, blood was collected to determine serotype-specific pneumococcal antibodies. Post: collection of serum 8 weeks post-immunization; Pre: collection of serum prior to vaccination.
2.2. Data Collection
Clinical data were collected according to a standardized electronic case report form (Castor EDC, Amsterdam, The Netherlands). We retrospectively collected the last measured serum IgG antibody level, lymphocyte number, hemoglobin level and platelet level and demographic parameters. Furthermore, we recorded CLL specific parameters from the electronic patient files: Binet and RAI stage, time since CLL diagnosis, past or ongoing treatment and past intravenous immunoglobulins (IVIG) administration.
2.3. Laboratory Methods and Outcomes
Prior to, and eight weeks after vaccination, we quantified serum IgG antibody concentrations against five pneumococcal serotypes shared across both PCV13 and PPSV23 (6B, 9V, 14, 19F, 23F), and against four serotypes unique to PPSV23 (8, 15B, 20, 33F), by a quantitative multiplex immunoassay (Luminex technology), as described previously [19,20,21,22]. This assay identifies serotype-specific anti-capsular polysaccharide IgG antibodies to the pneumococcal serotypes 6B, 8, 9V, 14, 15B, 19F, 20, 23F and 33F. The primary outcome was the serologic response rate (SRR), defined as a ≥4-fold increase among at least 70% of the serotype-specific anti-pneumococcal IgG antibodies, i.e., six or more out of nine serotypes. Secondary outcomes were serologic protection rates (SPR), defined as an antibody concentration of ≥1.3 µg/mL for ≥70% of all measured serotypes, in accordance with the definition by the American Academy of Allergy, Asthma & Immunology (AAAAI) [23]. In addition, since most previous studies used the World Health Organization (WHO) cut-off for protection in infants (≥0.35 µg/mL), we also analyzed our data using this less stringent cut-off to be able to compare our results with previous studies [24]. Other outcomes of interest were factors associated with the SRR and SPR after the completed vaccination schedule.
2.4. Statistical Analysis
Univariable and multivariable logistic regression models were used to identify factors associated with the SRR and SPR after the completed vaccination schedule, using the clinical variables of interest: age, sex, time since diagnosis, baseline laboratory measurements (hemoglobin level, total IgG, platelet level, lymphocyte number) and treatment status, and performing a stepwise backward selection method based on p-value of <0.1. An α significance level of 0.05 was applied. Means and standard deviations are presented for normally distributed data, and medians and interquartile ranges for non-normally distributed data. Serotype-specific antibody concentrations were non-normally distributed and therefore always presented as medians with interquartile ranges. Wilcoxon signed-rank test was used to compare pre- and post-vaccination antibody concentrations. To compare groups, a Student's t-test or a Mann-Whitney U test was performed. Statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 28.0 (IBM Corp, Armonk, NY, USA).
3. Results
From February 2021 through September 2022, a total of 143 CLL patients were included. Patient baseline characteristics are summarized in Table 1a. Participants had a mean age of 66 years (SD 9.2), and 34% were female. Median time since CLL diagnosis was three years (IQR 1.0–8.5). Most participants were classified as CLL Binet stage A (72%) and RAI stage 0 (53%). Of the 143 patients, 105 (73.4%) were treatment naive and 38 (26.6%) had previously received or were currently receiving CLL therapy. Of the 38 treated patients, 11 received a single-agent therapy; 14 patients received multiple sequential therapies or combinations of therapies without chemotherapy; 13 patients received chemotherapy and either rituximab, ibrutinib and/or venetoclax (Table 1b). In total, 28 of the included patients had rituximab in their treatment regimen, of whom 9 less than 12 months prior to vaccination. Seven patients received a combination of ibrutinib and venetoclax less than one month prior the first vaccination. Eight patients received IVIG, of whom two before the first vaccination only, four both before and after, and two only after the vaccination series. Prior to vaccination, the median serotype-specific pneumococcal IgG antibody concentrations in the overall cohort ranged between 0.06 µg/mL (serotype 6B and 9V) and 0.26 µg/mL (serotype 14) for the shared serotypes, and between 0.12 µg/mL (serotype 8) and 0.40 µg/mL (serotype 33F) for the PPSV23 unique serotypes (Table S1). There was a significant difference in pre-vaccination serotype-specific antibody concentrations between treated and untreated CLL patients for serotypes 19F, 23F, 8 and 20 (higher in treatment naive group).

Table 1.
Baseline characteristics of included CLL patients. (a) Baseline characteristics. In total, four patients deviated from the protocol and had PPSV23 less than 8 weeks after PCV13 (range 28–49 days after PCV13). (b) Treatment specification for all treated CLL patients. Percentages are calculated for the overall treated population. Combinations of therapies were not necessarily given together in the same treatment regimen. BTK: Bruton’s tyrosine kinase inhibitor; IgG: Immunoglobulin G; IQR: interquartile range; IVIg: intravenous immunoglobulin G; SD: standard deviation.
3.1. Antibody Concentrations after Complete Vaccination
Serotype-specific antibody concentrations prior to and after the complete vaccination schedule are presented in Figure 2 and Table S1. On average, a statistically significant increase was observed for all serotype-specific antibody concentrations after vaccination compared with prior antibody levels (p < 0.001). However, some patients did not generate measurable antibody concentrations against certain serotypes (Figure 2). Participants without a CLL treatment history obtained significantly higher post-vaccination antibody concentrations compared with treated patients (Table 2; Figure 2).
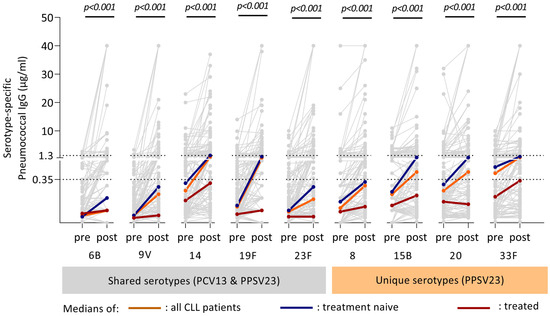
Figure 2.
Antibody dynamics of CLL patients after the combined vaccination schedule (PCV13/PPSV23). Antibody concentrations against the five shared serotypes (PCV13/PPSV23) (serotypes highlighted with the grey bar below) and four serotypes unique to PPSV23 (orange bar below serotypes) for each individual CLL patient are shown in the figure. Each grey line indicates the antibody dynamics of one patient from pre- to post-immunization. Median antibody concentration and gradient between pre- and post-vaccination titer are indicated for the overall group (thick orange line), the treatment naive individuals (blue) and treated individuals (red). The upper dotted line indicates 1.3 μg/mL, cut-off for serologic protection, and the lower dotted line indicates 0.35 μg/mL according to the WHO threshold for protection. p-values are calculated to determine the median difference between pre- and post-immunization concentration for each serotype, using the Wilcoxon Signed Rank test for related samples. Pre: antibody concentration prior to vaccination; Post: antibody concentration eight weeks post-immunization.

Table 2.
Comparison of pre- and post-immunization antibody concentrations (µg/mL) in CLL patients, stratified by treatment status. Fold change between pre- and post-immunization titers stratified by treatment group are indicated. Differences in pre- and post-immunization concentrations (µg/mL) between groups are calculated using the Mann Whitney U test for unrelated samples.
The relative difference in post-vaccination titers between those two groups was most pronounced for the shared serotypes (Table 2).
3.2. Serologic Response and Protection
The overall SRR was 15/143 (10.5%), with better responses in untreated CLL patients (14/105; 13.3%) vs. treated CLL patients (1/38; 2.6%) (Table 3a). The overall SPR increased from 2/143 (1.4%) before vaccination to 15/143 (10.5%) after both vaccinations (Table 3a). None of the patients who received IVIG or rituximab in the 12 months prior to vaccination or during the vaccination schedule obtained a sufficient serologic response. The one treated CLL patient with a serologic response had not recently received chemotherapy (five years prior to the vaccination schedule). Large heterogeneity was observed in the immunogenicity per serotype. Serologic response rates were lowest for serotype 20 (16%) and highest for serotype 9V (40%), whereas the serologic protection rates were highest for serotype 14 and 33F (44% and 40%, respectively), and lowest for serotype 6B (16%) (Table 3b).

Table 3.
Serologic response and protection rates after the sequential pneumococcal vaccination schedule in CLL patients. (a) Serologic response rate (SRR), defined as a ≥4-fold increase for at least 70% of the serotype-specific anti-pneumococcal IgG antibodies, and serologic protection rate (SPR), defined as an antibody concentration of ≥1.3 µg/mL for at least 70% of all measured serotypes, in accordance with the definition by the AAAAI, stratified by treatment status. As a comparison serologic protection defined by the WHO cut-off of 0.35µg/mL is indicated. (b) Percentages of patients obtaining serologic response and serologic protection in the overall CLL cohort prior to and after the PCV13/PPSV23 vaccination schedule, stratified by serotype.
We used a stringent cut-off for serologic protection of 1.3 µg/mL, as defined by the AAAAI, as mentioned before. Using the less stringent WHO cut-off for infants (0.35 µg/mL), the overall SPRs were higher, and increased from 12/143 (8.4%) to 40/143 (28%): 4/38 (10.5%) in treated CLL patients versus 36/105 (34.3%) in untreated CLL patients (Table 3a).
3.3. Factors Associated with Serologic Response and Protection
In multivariable analysis, treatment status was significantly associated with the SRR, with treated patients having a lower serologic response (OR 0.07 (0.01–0.67), p = 0.01). Additionally, a lower lymphocyte count (associated with treatment status) prior to vaccination was associated with a lower SRR (OR 0.96 (0.92–0.99), p = 0.02) (Table 4). Other factors, such as age, sex, prior hemoglobin level, platelet count, and total IgG level were not significantly associated with the SRR. In a multivariable analysis with the SPR as the dependent variable, only a lower lymphocyte level prior to vaccinations was a significantly associated factor (OR 0.97 (0.94–1.00), p = 0.05). The time-interval between PCV13 and PPSV23 was modestly but significantly associated with both the SRR and SPR in our cohort, OR 1.02 (IQR 1.00–1.05) p = 0.04 and OR 1.03 (1.00–1.06), respectively, with longer intervals associated with better responses.

Table 4.
Factors associated with the SRR and SPR in sequentially vaccinated CLL patients. Variables significantly associated with the SRR and SPR as dichotomous outcomes determined by univariable and multivariable logistic regression analyses. CI: confidence interval; ns: not significant; OR: odds ratio; ref: reference category in analysis; SPR: serologic protection rate; SRR: serologic response rate.
4. Discussion
This study among patients with CLL constitutes the largest CLL cohort to date describing the immunogenicity of the internationally recommended sequential PCV13 combined with PPSV23 vaccination schedule. The serologic response and protection induced by this combined schedule is impaired in previously treated CLL patients as well as in treatment naive patients. Nevertheless, CLL patients naive to treatment obtained consistently higher antibody concentrations than treated CLL patients. This is in accordance with previous research, where both vaccines were investigated separately, instead of sequentially [7,14,16,17]. In our study, the observed response to the combined vaccination schedule is much lower than we recently observed in healthy individuals (82%) and other immunocompromised groups (44–58% in patients receiving immunosuppressive drugs for autoimmune diseases), highlighting the severity of immune suppression associated with CLL [21,25]. Similar poor responses were observed after influenza and recombinant hepatitis B vaccination in CLL patients [26,27]. Despite a relatively small number of treated CLL patients in our study, the negative impact of treatment on vaccination response was clear, with better responses in untreated patients (SRR 13.3% vs. 2.6%), which has also been confirmed by others [6]. This emphasizes the importance of vaccinating early in the disease course of CLL. With a 29- to 36-fold higher incidence of IPD in CLL patients compared with healthy individuals, the low vaccination response observed is of concern [3]. No data exist on vaccine effectiveness in CLL patients, i.e., real-world protection against pneumococcal disease after vaccination. Such studies would require a very large sample size and, more importantly, would be unethical given the already existing guidelines that recommend vaccination in this patient group. Based on the poor immunogenicity of the PCV13/PPSV23 vaccination schedule in this study, vaccine effectiveness is likely to be low.
We recently studied the immunogenicity of sequential PCV13/PPSV23 vaccination in various groups of immunocompromised patients (ICP) [21,22,25,28]. In addition to the absence of a PPSV23 booster response after PCV13 vaccination, PPSV23 even seemed to result in a diminished response (hypo response) for several serotypes common to both PCV13 and PPSV23. As we found a modest but positive effect of a longer time interval between the vaccinations, the observed hypo response induced by PPSV23 could potentially be avoided by prolonging the time-interval between the vaccinations.
The T-cell independent PPSV23 directly stimulates B cells and might stimulate pre-existing antigen-specific memory B cells towards terminal differentiation into antibody-secreting cells [29]. It is suggested that this leads to depletion of the pre-existing pneumococcal-specific memory B cell pool., instead of stimulating the formation of pneumococcal-specific immunological memory [29,30], which precludes the potential of a booster effect upon revaccination. However, the currently most frequently circulating pneumococcal serotypes are mainly covered by PPSV23 [3] and to a lesser extent by PCV13, which means that PPSV23 is still important.
Unlike PPSV23, pneumococcal conjugated vaccines, such as PCV13, stimulate the formation of immunological memory, through the addition of a tetanus toxoid carrier protein, which involve CD4+ T-cells in the immune response, leading to the formation of memory B cells. Therefore, potent booster responses after repeated PCV13 vaccination were demonstrated in hematological stem cell transplant recipients [28]. In addition, PCV13 has been demonstrated to have higher clinical effectiveness against pneumococcal disease compared to PPSV23 [31], possibly because high affinity IgG antibodies are formed by the involvement of T-cells in the immune response [32]. Recently, higher valent pneumococcal conjugated vaccines with broader serotype coverage, such as PCV20 and the 24-valent ASP3772, have been introduced [33,34]. The potential of the mentioned booster effect could mean that multiple doses with these higher valent PCVs could result in better serologic protection and ultimately better vaccine effectiveness. This has already been shown in recent COVID-19 research, where both CLL patients naive to treatment, as well as those treated with ibrutinib, were able to produce sufficient antibody concentrations after vaccination with multiple doses of COVID-19 mRNA vaccine, despite very low initial response rates in treated CLL patients after the standard regimen of two doses [35]. In addition, the mentioned 24-valent ASP3772 pneumococcal conjugate candidate vaccine incorporates a multi antigen-presenting system (MAPS) [34]. This MAPS consists of a fusion protein of two highly conserved pneumococcal proteins, which induced enhanced post-immunization responses in ASP3772-vaccinated healthy individuals, via induction of a B- and Th1 cell response, as well as an additional Th17 cell response, which mediates resistance to mucosal colonization of S. pneumoniae. Together this might induce development of protective T cell memory [34,36].
A major limitation of this pneumococcal vaccine immunogenicity study and of immunogenicity studies in ICP in general is that the measured immune response does not necessarily translates into real-world protection, also called vaccine effectiveness. However, very large studies are required to investigate this, and such studies are not feasible, nor ethical, to conduct in relatively small patient populations. Thus, studies that prove that a certain vaccine is immunogenic in an ICP group often constitute the best level of evidence that can be acquired.
Although no clear correlate of protection exists in pneumococcal vaccination studies, an antibody concentration of 1.3 µg/mL is currently the most accepted cut-off for protection [23]. We prefer this AAAAI cut-off for protection (1.3 µg/mL), since adults often have baseline antibody levels exceeding the WHO cut-off of 0.35 μg/mL [23,25,26]. Furthermore, it has been suggested that the WHO threshold for serologic protection, might lead to an overestimation of protection against certain serotypes, even in children [37]. Using the cut-off of 1.3 μg/mL, recent studies showed an overall serologic protection of 82% after the sequential PCV/PPSV23 vaccination in healthy adults [21,22]. This is much higher than the 10.5% that we found in this study, which raises the question whether vaccination in CLL patients is at all relevant. In our opinion, the 29- to 36-fold increased risk of IPD in CLL patients [3], probably offsets the 8-fold lower serologic protection rate, favoring vaccination for all CLL patients, regardless of treatment status.
Moreover, serologic protection is only part of the story, since absence of antibody responses might not preclude the induction of a cellular immune response, which was observed in a recent COVID-19 vaccination study showing that patients with hematologic malignancies were able to mount robust T cell responses in the absence of humoral immunity [38]. Although only pneumococcal-specific B cell memory is formed after PCV13 vaccination, a potential role for ASP3772 vaccines in development of pneumococcal-specific T cell memory is suggested [34]. Furthermore, knowledge about the role of NK cells after pneumococcal immunization is lacking. This emphasizes the need for future research into T and NK cell immunity induced by vaccines in CLL patients.
We conclude that, although the sequential PCV13/PPSV23 vaccination is still relevant for CLL patients and should be recommended, we should focus future research efforts on multiple dosed higher valent pneumococcal conjugated vaccines, such as the recently introduced PCV20 and the 24-valent pneumococcal candidate vaccine ASP3772 that has been announced [33,39].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11071201/s1, Table S1: Serologic response in µg/mL in the overall CLL cohort. Serologic response in µg/mL in the overall CLL cohort (n = 143). Median serotype-specific pneumococcal antibody concentrations prior to and after the sequential PCV13/PPSV23 vaccination schedule are calculated.
Author Contributions
Conceptualization, A.G., K.D.H. and H.M.G.G.; methodology, A.G., H.M.G.G., K.D.H., H.M.V.d.S., F.D.B., S.K., D.I., D.T.R., H.P.J.V. and A.P.K.; formal analysis, S.H.; investigation, S.H., H.M.G.G., I.M.J.K., H.M.V.d.S., F.D.B., S.K., D.I., D.T.R., H.P.J.V. and A.P.K.; resources, A.G., K.D.H., H.M.V.d.S., F.D.B., S.K., D.I., D.T.R., H.P.J.V. and A.P.K.; data curation, S.H., A.G., H.M.G.G., I.M.J.K., H.M.V.d.S., F.D.B., S.K., D.I., D.T.R. and H.P.J.V.; writing—original draft preparation, S.H.; writing—review and editing, A.G., K.D.H., H.M.G.G., H.M.V.d.S., F.D.B., S.K., D.I., D.T.R., H.P.J.V. and A.P.K.; visualization, S.H. and I.M.J.K.; supervision, A.G. and K.D.H.; project administration, H.M.G.G., S.H. and I.M.J.K.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a public research grant of The Netherlands Organisation for Health Research and Development (ZonMw), grant number 522004005. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Amsterdam University Medical Centers, Location AMC (protocol code NL65687.018.18 date of approval 25 June 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
For original data of de-identified individual participant data, please contact Abraham Goorhuis (a.goorhuis@amsterdamumc.nl).
Acknowledgments
The authors would like to thank all patients and collaborating centers.
Conflicts of Interest
A.P. Kater reports being part or ad boards from Abbvie, Genentech, AstraZeneca, BMS, Janssen and from LAVA outside the submitted work; he also receives research funding from Abbvie, Genentech, AstraZeneca, BMS, Janssen. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Backhaus, E.; Berg, S.; Andersson, R.; Ockborn, G.; Malmström, P.; Dahl, M.; Nasic, S.; Trollfors, B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Lindström, V.; Aittoniemi, J.; Lyytikäinen, O.; Klemets, P.; Ollgren, J.; Silvennoinen, R.; Nuorti, J.P.; Sinisalo, M. Invasive pneumococcal disease in patients with haematological malignancies before routine use of conjugate vaccines in Finland. Infect. Dis. 2016, 48, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garrido, H.M.; Knol, M.J.; Heijmans, J.; van Sorge, N.M.; Sanders, E.A.M.; Klümpen, H.J.; Grobusch, M.P.; Goorhuis, A. Invasive pneumococcal disease among adults with hematological and solid organ malignancies: A population-based cohort study. Int. J. Infect. Dis. 2021, 106, 237–245. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease—Annual Epidemiological Report for 2017, in in Annual Epidemiological Report on Communicable Diseases in Europe. European Centre for Disease Prevention and Control. 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2017 (accessed on 7 June 2023).
- Sinisalo, M.; Vilpo, J.; Itälä, M.; Väkeväinen, M.; Taurio, J.; Aittoniemi, J. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine 2007, 26, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Andrick, B.; Alwhaibi, A.; DeRemer, D.L.; Quershi, S.; Khan, R.; Bryan, L.J.; Somanath, P.R.; Pantin, J. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br. J. Haematol. 2018, 182, 712–714. [Google Scholar] [CrossRef]
- Mauro, F.R.; Giannarelli, D.; Galluzzo, C.M.; Vitale, C.; Visentin, A.; Riemma, C.; Rosati, S.; Porrazzo, M.; Pepe, S.; Coscia, M.; et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia 2021, 35, 737–746. [Google Scholar] [CrossRef]
- Riches, J.C.; Gribben, J.G. Understanding the immunodeficiency in chronic lymphocytic leukemia: Potential clinical implications. Hematol. Oncol. Clin. N. Am. 2013, 27, 207–235. [Google Scholar] [CrossRef]
- Mikulska, M.; Cesaro, S.; de Lavallade, H.; Di Blasi, R.; Einarsdottir, S.; Gallo, G.; Rieger, C.; Engelhard, D.; Lehrnbecher, T.; Ljungman, P.; et al. Vaccination of patients with haematological malignancies who did not have transplantations: Guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e188–e199. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Pasiarski, M.; Rolinski, J.; Grywalska, E.; Stelmach-Goldys, A.; Korona-Glowniak, I.; Gozdz, S.; Hus, I.; Malm, A. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients—Preliminary report. PLoS ONE 2014, 9, e114966. [Google Scholar] [CrossRef]
- Sinisalo, M.; Aittoniemi, J.; Oivanen, P.; Käyhty, H.; Olander, R.M.; Vilpo, J. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2001, 114, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, A.M.; Van Velzen-Blad, H.; Claessen, A.M.; Van der Griend, R.; Oltmans, R.; Rijkers, G.T.; Biesma, D.H. The effect of ranitidine on antibody responses to polysaccharide vaccines in patients with B-cell chronic lymphocytic leukaemia. Eur. J. Haematol. 2007, 79, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.; Kättström, M.; Hammarlund, Y.; Roth, D.; Andersson, P.O.; Svensson, M.; Nilsson, I.; Rombo, L.; Cherif, H.; Kimby, E. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine 2018, 36, 3701–3707. [Google Scholar] [CrossRef] [PubMed]
- Lindström, V.; Aittoniemi, J.; Salmenniemi, U.; Käyhty, H.; Huhtala, H.; Itälä-Remes, M.; Sinisalo, M. Antibody persistence after pneumococcal conjugate vaccination in patients with chronic lymphocytic leukemia. Hum. Vaccines Immunother. 2018, 14, 1471–1474. [Google Scholar] [CrossRef]
- Lindström, V.; Aittoniemi, J.; Salmenniemi, U.; Käyhty, H.; Huhtala, H.; Sinisalo, M. Antibody response to the 23-valent pneumococcal polysaccharide vaccine after conjugate vaccine in patients with chronic lymphocytic leukemia. Hum. Vaccines Immunother. 2019, 15, 2910–2913. [Google Scholar] [CrossRef]
- Hartkamp, A.; Mulder, A.H.; Rijkers, G.T.; van Velzen-Blad, H.; Biesma, D.H. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine 2001, 19, 1671–1677. [Google Scholar] [CrossRef]
- Safdar, A.; Rodriguez, G.H.; Rueda, A.M.; Wierda, W.G.; Ferrajoli, A.; Musher, D.M.; O’Brien, S.; Koller, C.A.; Bodey, G.P.; Keating, M.J. Multiple-dose granulocyte-macrophage-colony-stimulating factor plus 23-valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: A prospective, randomized trial of safety and immunogenicity. Cancer 2008, 113, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Wagenvoort, G.H.J.; Vlaminckx, B.J.M.; van Kessel, D.A.; Geever, R.C.L.; de Jong, B.A.W.; Grutters, J.C.; Bos, W.J.W.; Meek, B.; Rijkers, G.T. Pneumococcal conjugate vaccination response in patients after community-acquired pneumonia, differences in patients with S. pneumoniae versus other pathogens. Vaccine 2017, 35, 4886–4895. [Google Scholar] [CrossRef]
- Van Kessel, D.A.; Hoffman, T.W.; van Velzen-Blad, H.; Zanen, P.; Rijkers, G.T.; Grutters, J.C. Response to pneumococcal vaccination in mannose-binding lectin-deficient adults with recurrent respiratory tract infections. Clin. Exp. Immunol. 2014, 177, 272–279. [Google Scholar] [CrossRef]
- Garcia Garrido, H.M.; Vollaard, A.; D’Haens, G.R.; Spuls, P.I.; Bemelman, F.J.; Tanck, M.W.; de Bree, G.J.; Meek, B.; Grobusch, M.P.; Goorhuis, A. Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy. Vaccines 2022, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garrido, H.M.; Schnyder, J.L.; Haydari, B.; Vollaard, A.M.; Tanck, M.W.T.; de Bree, G.J.; Meek, B.; Grobusch, M.P.; Goorhuis, A. Immunogenicity of the 13-valent pneumococcal conjugate vaccine followed by the 23-valent pneumococcal polysaccharide vaccine in people living with HIV on combination antiretroviral therapy. Int. J. Antimicrob. Agents 2022, 60, 106629. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130 (Suppl. S3), S1–S24. [Google Scholar] [CrossRef]
- WHO. Recommendations for the Production and Control of Pneumococcal Conjugate Vaccines; WHO Expert Committee on Biological Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Van Aalst, M.; Garcia Garrido, H.M.; van der Leun, J.; Meek, B.; van Leeuwen, E.M.M.; Löwenberg, M.; D’Haens, G.R.; Ponsioen, C.Y.I.; Grobusch, M.P.; Goorhuis, A. Immunogenicity of the Currently Recommended Pneumococcal Vaccination Schedule in Patients with Inflammatory Bowel Disease. Clin. Infect. Dis. 2020, 70, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.P.; Trubiano, J.A.; Barr, I.; Leung, V.; Slavin, M.A.; Tam, C.S. Ibrutinib may impair serological responses to influenza vaccination. Haematologica 2017, 102, e397–e399. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, C.; Ali, M.A.; Cohen, J.I.; Tian, X.; Soto, S.; Ahn, I.E.; Gaglione, E.M.; Nierman, P.; Marti, G.E.; Hesdorffer, C.; et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood 2021, 137, 185–189. [Google Scholar] [CrossRef]
- Garcia Garrido, H.M.; Haggenburg, S.; Schoordijk, M.C.E.; Meijer, E.; Tanck, M.W.T.; Hazenberg, M.D.; Rutten, C.E.; de Bree, G.J.; Nur, E.; Meek, B.; et al. Immunogenicity of a 5-dose pneumococcal vaccination schedule following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2022, 97, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Papadatou, I.; Tzovara, I.; Licciardi, P.V. The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects. Vaccines 2019, 7, 13. [Google Scholar] [CrossRef]
- Stein, K.E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 1992, 165 (Suppl. S1), S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Love, B.L.; Finney, C.J.; Gaidos, J.K.J. Effectiveness of Conjugate and Polysaccharide Pneumococcal Vaccines for Prevention of Severe Pneumococcal Disease Among Inflammatory Bowel Disease Patients. J. Crohns Colitis 2021, 15, 1279–1283. [Google Scholar] [CrossRef]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Essink, B.; Sabharwal, C.; Cannon, K.; Frenck, R.; Lal, H.; Xu, X.; Sundaraiyer, V.; Peng, Y.; Moyer, L.; Pride, M.W.; et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18 Years. Clin. Infect. Dis. 2022, 75, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Haggenburg, S.; Hofsink, Q.; Lissenberg-Witte, B.I.; Broers, A.E.C.; van Doesum, J.A.; van Binnendijk, R.S.; den Hartog, G.; Bhoekhan, M.S.; Haverkate, N.J.E.; Burger, J.A.; et al. Antibody Response in Immunocompromised Patients with Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19. JAMA Oncol. 2022, 8, 1477–1483. [Google Scholar] [CrossRef]
- Moffitt, K.L.; Gierahn, T.M.; Lu, Y.J.; Gouveia, P.; Alderson, M.; Flechtner, J.B.; Higgins, D.E.; Malley, R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 2011, 9, 158–165. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Boerenkamp, L.S.; Pothast, C.R.; Dijkland, R.C.; van Dijk, K.; van Gorkom, G.N.Y.; van Loo, I.H.M.; Wieten, L.; Halkes, C.J.M.; Heemskerk, M.H.M.; Van Elssen, C. Increased CD8 T-cell immunity after COVID-19 vaccination in lymphoid malignancy patients lacking adequate humoral response: An immune compensation mechanism? Am. J. Hematol. 2022, 97, E457–E461. [Google Scholar] [CrossRef] [PubMed]
- Chichili, G.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Malinoski, F.J.; Sebastian, S.; Siber, G.R.; Malley, R. 1242. Safety and Immunogenicity of Novel 24-Valent Pneumococcal Vaccine in Healthy Adults. Open Forum Infect. Dis. 2020, 7 (Suppl. S1), S640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).