Abstract
Safety data following the COVID-19 booster mRNA vaccine in solid cancer patients are scarce. We prospectively evaluated adverse events after a booster dose of the BNT162b2 vaccine as compared to the mRNA-1273 vaccine in solid malignancy patients who had previously received two doses of ChAdOx1 or heterogenous CoronaVac/ChAdOx1. Data regarding solicited and unsolicited adverse events were collected using questionnaires. The primary endpoint was the difference in incidence and severity of adverse events between BNT162b2 and mRNA-1273 vaccines. A total of 370 subjects were enrolled, including 172 (47%) and 198 (54%) patients receiving booster doses of BNT162b2 and mRNA-1273 vaccines, respectively. The overall incidence of adverse events in the two groups was comparable (BNT162b2 vs. mRNA-1273; 63% vs. 66%, p = 0.6). There was no significant difference in severity, and the majority of adverse events reported were classed as mild to moderate. Tenderness at the injection site was the only reaction that had a statistically higher reported incidence after the mRNA-1273 vaccine than after the BNT162b2 vaccine (56% vs. 41%, p = 0.003). In conclusion, a booster dose of the mRNA vaccine, either BNT162b2 or mRNA-1273, in solid cancer patients previously vaccinated with ChAdOx1 and CoronaVac appears safe, and no new safety concerns were observed.
Keywords:
ChAdOx1; CoronaVac; BNT162b2; mRNA-1273; booster COVID-19 vaccine; safety; adverse events; cancer patients; malignancy 1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has impacted all health care systems, including cancer care. Patients with malignancies are classified as a high-risk group because they are at increased risk of serious complications and death from COVID-19 infection [1,2,3,4]. Many different COVID-19 vaccines have been developed and have been shown to reduce the risk of SARS-CoV-2 infection and the likelihood of developing serious illness. However, new emerging variants, such as B.1.617.2 (delta) and the B.1.1.529 (omicron), which, since 2021, have spread worldwide [5,6], coupled with the waning immunity of the vaccine [7,8,9,10,11], have raised major concerns about the effectiveness of the primary series of vaccines. Several studies show that a booster dose with messenger RNA (mRNA) can enhance protection against SARS-CoV-2 variants of concern [10,12,13,14]. Currently, the Center for Disease Control (CDC) recommends a booster dose with mRNA vaccine for everyone aged 5 years and older [15].
Safety data for the mRNA vaccine booster dose in malignancy patients are limited to studies of individuals primed with two doses of the mRNA vaccine [16,17,18]. A study of two prospective cohorts of solid cancer patients showed that the mRNA vaccine booster dose was well tolerated among all participants. The most common side effect observed after the vaccination was pain at the injection site [16,17]. According to Israel’s study, cancer patients had a statistically lower incidence of headache, muscle pain, and chills than healthy controls, with no serious side effects [17]. Another nationwide survey of more than 25,000 individuals, including solid cancer patients, found adverse events following an mRNA vaccine booster dose were generally mild in severity and generally did not require medical care [18].
However, studies of cancer patients who received a primary vaccine with a viral-vectored vaccine (ChAdOx1) or inactivated virus vaccine (CoronaVac) are lacking despite the fact that many countries deployed these vaccines in the early phase of the COVID-19 pandemic. In Thailand, most cancer patients were immunized with the primary series of two doses of the ChAdOx1 vaccine or heterogenous CoronaVac/ChAdOx1 during the early phase of the vaccination program in June 2021 [19,20,21,22]. The mRNA vaccine booster dose was recommended for late 2021 and, since then, vaccine hesitancy has been seen among many cancer patients. Such hesitation is generally due to a fear of side effects, especially among those undergoing cancer treatment.
As a result, we aimed to obtain safety data for the mRNA COVID-19 vaccine booster dose in solid cancer patients previously vaccinated with two doses of ChAdOx1 or heterogenous CoronaVac/ChAdOx1. We also planned to compare the differences in adverse events between the two types of mRNA vaccine (BNT162b2 and mRNA-1273).
2. Materials and Methods
2.1. Study Design and Participants
We conducted a multi-center prospective observational cohort study at King Chulalongkorn Memorial Hospital and Phrapokklao Hospital, Thailand. We enrolled solid cancer patients who had previously been vaccinated with a primary series of two doses of the ChAdOx1 vaccine (AstraZeneca, Cambridge, UK) or heterogenous CoronaVac (Sinovac Biotech, China)/ChAdOx1 (the first dose with CoronaVac and the second dose with ChAdOx1). Enrolled patients then received a third dose using an mRNA vaccine, i.e., either BNT162b2 (Pfizer-BioNTech, New York City, NY, USA) or mRNA-1273 (Moderna, Norwood, MA, USA). The interval between the second and third dose of the vaccine was at least 3 months but not more than 6 months. The study excluded patients with a history of SARS-CoV-2 infection or a life expectancy of less than 6 months. Patient demographics, disease characteristics, and data related to cancer treatments were reviewed and recorded. The type of cancer treatment and history of concurrent corticosteroid use were determined within 4 weeks before the third vaccination.
After vaccination, the patients were asked to self-report adverse events that did not include previous symptoms experienced due to their cancer or cancer treatments using either a paper-based or electronic online questionnaire (Supplementary material for at least 7 consecutive days from the day of injection, which was defined as day 0). Solicited adverse events in the questionnaire were classified into two categories. The first was local adverse effects including pain, tenderness, swelling, and erythema at the injection site. The second comprised systemic adverse effects including fever, headache, myalgia, fatigue, nausea or vomiting, arthralgia, diarrhea, back pain, dizziness, and lymphadenopathy, which is described as the palpation of a lump or mass in the axilla or neck area. Physical examination by a physician at an oncology clinic and review of all imaging performed after the vaccination were also used to determine the incidence of lymphadenopathy. Patients reported unsolicited adverse events themselves. We also gathered additional adverse events of interest including myocarditis, vaccine-induced immune thrombotic thrombocytopenia, and thrombosis by reviewing all laboratory data and medical records.
The severity of adverse events was graded according to the FDA toxicity grading scale for healthy adults and adolescent volunteers enrolled in preventive vaccine clinical trials [23]: grade 1—mild symptoms and does not interfere with activity; grade 2—moderate symptoms and some interference with activity but not requiring medical intervention; grade 3—severe symptoms and prevents daily activity or requires medical intervention; grade 4—life-threatening symptoms requiring an emergency room visit or hospitalization. The details of each grade in each adverse event were described in the questionnaire (Supplementary material).
The study was performed in accordance with the principles of the Declaration of Helsinki, with all patients providing written informed consent. The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (No. 486/64) and the Chanthaburi Research Ethics Committee/Region 6 (CTIREC) (No. 044/64).
2.2. Outcomes
The primary endpoint was the incidence and severity of adverse events following the booster dose with the BNT162b2 as compared to the mRNA-1273 vaccine in solid malignancy patients previously vaccinated with ChAdOx1 or CoronaVac. We also assessed individual risk factors associated with vaccine reactogenicity and their effect on mRNA vaccine type. The secondary endpoints were the onset and duration of adverse events and the frequency with which adverse events interrupted cancer treatment.
2.3. Statistical Analysis
Descriptive statistics including frequencies with percentages and mean with standard deviation were used to describe demographics, disease characteristics, and data on cancer treatments. The difference in baseline characteristics and incidence and severity of adverse events between the BNT162b2 and mRNA-1273 vaccines was analyzed by the Chi-square test. We used the Mann–Whitney U test to compare the distribution of the onset and duration of adverse events between the two types of vaccine. The association between individual risk factors and developing adverse events was evaluated using univariate logistic regression, which was used to calculate the unadjusted odds ratios and corresponding 95% confidence intervals (CIs). The significant factors from the univariate analysis were included in the multivariate analysis to determine significant independent factors, and we used these factors to adjust the impact of mRNA vaccine type. p-values of less than 0.05 were considered statistically significant.
We used SPSS version 28.0 (IBM Corp., Armonk, NY, USA) and Stata 15 (StataCeorp LLC, College Station, TX, USA) for the statistical analyses.
3. Results
3.1. Patient Characteristics
From December 2021 to February 2022, 370 solid malignancy patients previously vaccinated with a primary series of two doses of the ChAdOx1 vaccine or heterogenous CoronaVac/ChAdOx1 were recruited for the study. Each subject then received a third dose of an mRNA vaccine. Of these, 172/370 (46.5%) and 198/370 (53.5%) were vaccinated with BNT162b2 and mRNA-1273, respectively. The BNT162b2 group contained more elderly, female, and metastatic stage cancer individuals, but less comorbidity than the mRNA-1273 group. The distribution of cancer types and treatments was also significantly different between the two groups (Table 1). The mRNA-1273 group had a higher rate of concurrent steroid use because that group contained more patients who were being treated with chemotherapy, thus more individuals taking steroids for pre-medication purposes. The interval between the second and the third vaccinations was longer among the patients who were vaccinated with BNT162b2.

Table 1.
Patient demographics and disease characteristics.
3.2. Vaccine Safety
A total of 238 out of 370 patients (64.3%) reported adverse events after receiving a booster dose of the mRNA COVID-19 vaccine. The overall incidence of any adverse events associated with BNT162b2 vaccines was comparable to that of the mRNA-1273 vaccines (62.8% vs. 65.7%, p = 0.57). Although the BNT162b2 group had a higher reported incidence of grade 3 severity adverse events than the mRNA-1273 group, including all adverse events (6.5% vs. 2.3%, p = 0.41), there was no statistically significant difference and the most common reported severity in both groups was mild to moderate. The most common local reaction was pain at the injection site, while the most common systemic reaction was myalgia in both vaccine types. Tenderness at the injection site was the only reaction that had a statistically higher incidence after the mRNA-1273 than after BNT162b2 vaccination (56.1% vs. 40.7%, p = 0.003), as shown in Figure 1 and Table 2. In most solicited adverse events, the median onset was 1 day after vaccination and the median duration was 2 days (Figure 2 and Figure 3). The adverse event that was reported for the longest duration was lymphadenopathy (14 days in one patient).
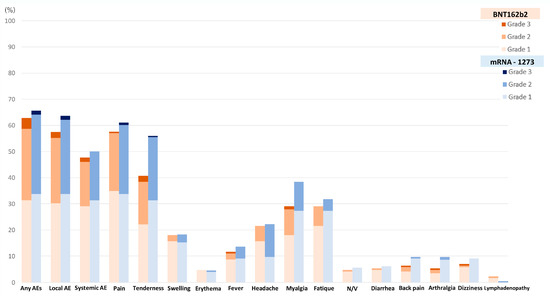
Figure 1.
Incidence of adverse events following the booster dose with the BNT162b2 as compared to the mRNA-1273 vaccine.

Table 2.
Incidence of adverse events following the booster dose with the BNT162b2 as compared to the mRNA-1273 vaccine.
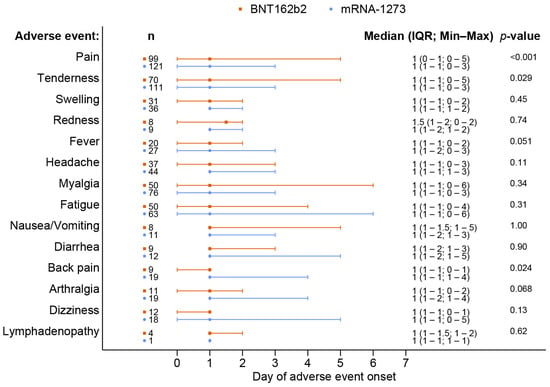
Figure 2.
Onset of adverse events following the booster dose with the BNT162b2 as compared to the mRNA-1273 vaccine.
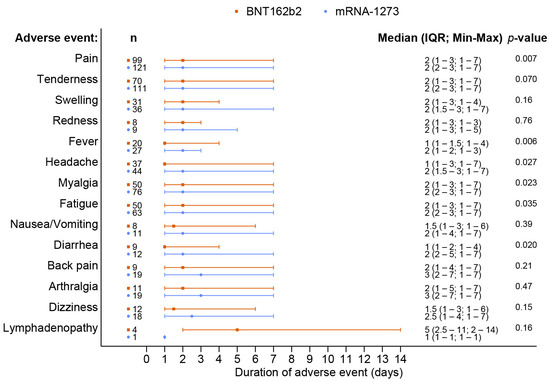
Figure 3.
Duration of adverse events following the booster dose with the BNT162b2 as compared to the mRNA-1273 vaccine.
At the time of data cut off (31 October 2022), no serious adverse events or deaths related to the vaccine were reported. Only one patient who received the BNT162b2 vaccine needed to interrupt their cancer treatment (Ribociclib) with a cessation of only 2 days due to a grade 3 fever. There were also no adverse events of interest, including myocarditis, vaccine-induced immune thrombotic thrombocytopenia, or thrombosis, observed.
Results from the univariate logistic regression analysis revealed significant factors associated with any adverse events, including age, sex, concurrent steroid use, and type of primary vaccine (Table 3). We incorporated these factors into the multivariate regression analysis to evaluate the association between the type of mRNA vaccine and vaccine reactogenicity. While controlling for significant independent factors, we found that the booster dose with the BNT162b2 or mRNA-1273 vaccines resulted in a similar risk of developing any adverse events (BNT162b2 vs. mRNA-1273; OR 1.22; 95% CI, 0.78–1.92; p = 0.38). We also investigated this association in relation to local and systemic reactions, and there was no correlation between the type of booster mRNA vaccine and vaccine reactogenicity (Table 4 and Table 5).

Table 3.
Factors associated with any adverse events following the booster dose with the mRNA vaccine.

Table 4.
Factors associated with local adverse events following the booster dose with the mRNA vaccine.

Table 5.
Factors associated with systemic adverse events following the booster dose with the mRNA vaccine.
4. Discussion
To the best of our knowledge, this is the first prospective study to assess the safety of an mRNA COVID-19 booster vaccine in solid cancer patients previously vaccinated with two doses of ChAdOx1 or heterogenous CoronaVac/ChAdOx1. When we compared our findings with the safety data of the third dose of the mRNA vaccine following ChAdOx1 in a healthy population (the COV-BOOST study) [24], we found similar results as regards the pattern of reactogenicity. Local adverse events were reported more frequently than systemic adverse events, and pain at the injection site, fatigue, myalgia, and headache were common adverse events in both studies. However, the overall incidence of side effects had a higher prevalence in the healthy population [24] as compared to our population.
In cancer patients, studies of adverse events after the COVID-19 vaccine booster dose are limited to individuals primed with two doses of the mRNA vaccine [16,17,18]. A recent study in Italy that evaluated the safety of the third dose of BNT162b2 in 142 solid malignancy patients who had previously received two doses of BNT162b2 found no risk of serious adverse events, with common side effects including pain at the injection site (64.8%), fever (24.6%), arthralgia (17.6%), headache (12%), and myalgia (9.2%) [16]. Comparing the BNT162b group in Italian study with our study, ours reported a higher incidence of headache and myalgia but less local pain, fever, and arthralgia. These differences might be related to the different type of primary vaccine or the differing baseline characteristics of the studies. The patients in the Italian study were all receiving active cancer treatment and 6% had a previously documented SAR-CoV-2 infection [16]. In our study, however, 83% of patients were undergoing treatment and no patients had a history of COVID-19 infection. Moreover, there were more elderly patients and fewer females in the Italian study than in ours. Both younger age and female sex have been linked to higher vaccine reactogenicity [25,26,27].
There are no previous studies that compare adverse events following immunization for the two types of mRNA vaccines (BNT162b2 and mRNA-1273) used as the booster dose in cancer patients. There is, however, one study, involving healthy volunteers, that reported a similar incidence for any grade of local and systemic reaction among different types of booster vaccines [24]. In our study, the overall incidence and severity of adverse events were also comparable between the two mRNA vaccine types. The mRNA-1273 group had a statistically significant higher incidence of tenderness at the injection site and more moderate severity of headache. Several adverse events, including myalgia, fatigue, and back pain, were also reported more frequently, but the lack of statistical significance was likely due to the limited number of patients who experienced the events. In the mRNA-1273 group, no cancer treatment interruption due to vaccine side effects was reported, while one patient who received the BNT162b2 vaccine during treatment with Ribociclib and Letrozole developed a grade 3 fever and needed to interrupt their Ribociclib treatment for 2 days. Developing a fever during cancer treatment is a matter of concern for patients and physicians because it is a critical sign of infection. The incidence of fever associated with vaccine reactogenicity was 11.6% and 13.6% in the BNT162b2 and mRNA-1273 groups, respectively, with a median onset of 1 day after vaccination and a median duration of 1–2 days in our cohort. These findings may help oncologists to educate and reassure cancer patients that adverse events following the booster vaccine rarely affect cancer treatment.
Regarding adverse events of interest, 5 out of 370 patients (1.4%) developed lymphadenopathy after the third vaccination. This occurred after the BNT162b2 vaccine in 4 patients (2.3%) and after the mRNA-1273 vaccine in 1 patient (0.5%). All were affected in the axillary region on the same side as the injection site. Reactive lymphadenopathy, which has been reported in relation to the primary series of the mRNA COVID-19 vaccine [28,29,30,31], is an important reaction after vaccination, because it can confound tumor staging and mimic cancer progression. The incidence of reactive lymphadenopathy following a booster vaccine in malignancy patients has not yet been assessed in a large cohort study. There is a published case series describing one patient following a third dose of BNT162b2, and one case following a third dose of mRNA-1273 [32]. Oncologists and radiologists should be aware of this reaction. A booster vaccination history should be requested from each cancer patient before interpreting imaging.
As a result of the non-randomized study design, imbalances in patient demographics and disease characteristics were present between the two booster vaccine groups. Several baseline characteristics that influence vaccine reactogenicity are documented in the literature [25,26,27,33,34]. We attempted to overcome this bias by using logistic regression analysis. From the univariate analysis, the significant factors associated with any adverse events following booster immunization were age, sex, concurrent steroid use, and type of priming vaccine. We then used these factors to adjust the impact of the mRNA booster vaccine type and found no statistically significant correlation. However, the multivariate regression analysis showed that non-elderly (<65 years) and female sex were associated with a higher risk of adverse events, whereas heterogenous CoronaVac/ChAdOx1 as the priming vaccine was associated with a lower risk of developing any adverse events after booster vaccination. Younger age and female sex have previously been identified as known factors related to an increased risk of COVID-19 vaccine reactogenicity in general and in the cancer population [25,26,27,33,34]. Our results support this finding in booster immunization. No previous studies have assessed the impact of the type of the two primary vaccine doses, including both homologous and heterologous vaccine types, on booster vaccine reactogenicity. Our study is the first to establish this association.
There were a few limitations to our study. First, certain adverse events might overlap with side effects from cancer treatment because the study included patients who were receiving cancer treatment (87%). This could lead to overreporting or underreporting of adverse effects. Second, our study was not a randomized control trial, so differences in patient demographics and disease characteristics were present between the two mRNA vaccine groups. We attempted to correct this bias by using logistic regression analysis, as mentioned above.
5. Conclusions
A booster dose with an mRNA COVID-19 vaccine in solid cancer patients previously vaccinated with ChAdOx1 and CoronaVac appears safe, with no new safety concerns being observed in our study. The overall incidence and severity of adverse events for the BNT162b2 and mRNA-1273 vaccines were found to be comparable. The majority of adverse effects were mild to moderate in severity, with hardly any interruption of cancer treatment being reported (only one patient in our study reported this, i.e., an incidence of less than 0.5%). Our findings will help health care professionals reduce the vaccine hesitancy associated with booster mRNA COVID-19 vaccines in cancer patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11020356/s1, Supplementary Materials S1: questionnaire.
Author Contributions
Conceptualization, P.W., P.S., N.T., S.L., N.W. and N.P.; methodology, P.W., P.S., N.T., S.L. and N.P.; software, P.S. and N.P.; validation, Y.P. and N.P.; formal analysis, P.W., P.S. and N.P.; investigation, P.W., P.S., N.T., S.L., T.S., N.Z. and N.P.; resources, P.W., N.T., S.L., Y.P., N.W., S.T., V.S. and N.P.; data curation, P.W., P.S., S.L., T.S., N.Z. and N.P.; writing—original draft preparation, P.W., P.S. and N.P.; writing—review and editing, N.W., Y.P. and N.P.; visualization, Y.P., V.S., S.T. and N.P.; supervision, Y.P., V.S., S.T. and N.P.; project administration, N.T., S.L. and N.P.; funding acquisition, P.W., N.T., Y.P. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, Grant number RA65/018 to N.P.; and Quality Improvement Fund, King Chulalongkorn Memorial Hospital, The Thai Red Cross Society, Grant number HA-65-3300-C1-067 to N.T.; and Covid vaccine antibody research fund, Phrapokklao Hospital, Grant number PPK_63_001 to P.W.; and the National Research Council (NRCT), Thailand and the Health Systems Research Institute, Grant number RES_64_308-30_074 to Y.P.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (No. 486/64) and the Chanthaburi Research Ethics Committee/Region 6 (CTIREC) (No. 044/64).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
We would like to thank the department of medicine at the King Chulalongkorn Memorial Hospital for providing assistance in polishing our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, L.; Chai, P.; Yu, J.; Fan, X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e415. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Aragones, M.; Tubert, J.E.; et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: Test negative case-control study. BMJ 2021, 375, e068848. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Talisa, V.B.; Shaikh, O.S.; Omer, S.B.; Mayr, F.B. Relative Vaccine SARS-CoV-2 RNA Vaccine Booster Dose Against the Omicron Variant. Clin. Infect. Dis. 2022, 75, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID-19 Vaccine Boosters. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed on 1 October 2021).
- Lasagna, A.; Bergami, F.; Lilleri, D.; Percivalle, E.; Quaccini, M.; Alessio, N.; Comolli, G.; Sarasini, A.; Sammartino, J.C.; Ferrari, A.; et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: A prospective cohort study. ESMO Open 2022, 7, 100458. [Google Scholar] [CrossRef]
- Ligumsky, H.; Dor, H.; Etan, T.; Golomb, I.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Angel, Y.; Henig, O.; Spitzer, A.; et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022, 23, 193–195. [Google Scholar] [CrossRef]
- Auster, O.; Finkel, U.; Dagan, N.; Barda, N.; Laufer, A.; Balicer, R.D.; Ben-Shachar, S. Short-term Adverse Events After the Third Dose of the BNT162b2 mRNA COVID-19 Vaccine in Adults 60 Years or Older. JAMA Netw. Open 2022, 5, e227657. [Google Scholar] [CrossRef]
- Luangdilok, S.; Wanchaijiraboon, P.; Pakvisal, N.; Susiriwatananont, T.; Zungsontiporn, N.; Sriuranpong, V.; Sainamthip, P.; Suntronwong, N.; Vichaiwattana, P.; Wanlapakorn, N.; et al. Immunogenicity after a Third COVID-19 mRNA Booster in Solid Cancer Patients Who Previously Received the Primary Heterologous CoronaVac/ChAdOx1 Vaccine. Vaccines 2022, 10, 1613. [Google Scholar] [CrossRef]
- Luangdilok, S.; Wanchaijiraboon, P.; Pakvisal, N.; Susiriwatananont, T.; Zungsontiporn, N.; Sriuranpong, V.; Namkanisorn, T.; Sainamthip, P.; Suntronwong, N.; Vichaiwattana, P.; et al. Immunogenicity and Omicron Neutralization Following a Third COVID-19 Vaccination in Solid Cancer Patients Previously Primed with Two Doses of Chadox1 Vaccine: A Prospective Cohort Study. Lancet 2022. [Google Scholar] [CrossRef]
- Teeyapun, N.; Luangdilok, S.; Pakvisal, N.; Sainamthip, P.; Mingmalairak, S.; Poovorawan, N.; Sitthideatphaiboon, P.; Parinyanitikul, N.; Sriuranpong, V.; Namkanisorn, T.; et al. Immunogenicity of ChAdOx1-nCoV-19 vaccine in solid malignancy patients by treatment regimen versus healthy controls: A prospective, multicenter observational study. Eclinicalmedicine 2022, 52, 101608. [Google Scholar] [CrossRef]
- Wanchaijiraboon, P.; Teeyapun, N.; Pakvisal, N.; Sainamthip, P.; Susiriwatananont, T.; Zungsontiporn, N.; Suntronwong, N.; Vichaiwattana, P.; Klinsawat, W.; Wanlapakorn, N.; et al. Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment. Vaccines 2022, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- FDA US. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials, Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (accessed on 1 October 2021).
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Nachtigall, I.; Bonsignore, M.; Hohenstein, S.; Bollmann, A.; Günther, R.; Kodde, C.; Englisch, M.; Ahmad-Nejad, P.; Schröder, A.; Glenz, C.; et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: A survey among health care workers. BMC Infect. Dis. 2022, 22, 291. [Google Scholar] [CrossRef]
- Rolfes, L.; Härmark, L.; Kant, A.; van Balveren, L.; Hilgersom, W.; van Hunsel, F. COVID-19 vaccine reactogenicity—A cohort event monitoring study in the Netherlands using patient reported outcomes. Vaccine 2022, 40, 970–976. [Google Scholar] [CrossRef]
- Mehta, N.; Sales, R.M.; Babagbemi, K.; Levy, A.D.; McGrath, A.L.; Drotman, M.; Dodelzon, K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin. Imaging 2021, 75, 12–15. [Google Scholar] [CrossRef]
- Lehman, C.D.; D’Alessandro, H.A.; Mendoza, D.P.; Succi, M.D.; Kambadakone, A.; Lamb, L.R. Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J. Am. Coll. Radiol. 2021, 18, 843–852. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Özütemiz, C.; Potter, D.A.; Özütemiz, A.; Steinberger, D. Lymphadenopathy after the Third COVID-19 Vaccine. Curr. Probl. Cancer Case Rep. 2021, 4, 100127. [Google Scholar] [CrossRef]
- So, A.C.P.; McGrath, H.; Ting, J.; Srikandarajah, K.; Germanou, S.; Moss, C.; Russell, B.; Monroy-Iglesias, M.; Dolly, S.; Irshad, S.; et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers 2021, 13, 3573. [Google Scholar] [CrossRef] [PubMed]
- Pakvisal, N.; Sainamthip, P.; Teeyapun, N.; Luangdilok, S.; Wanlapakorn, N.; Yorsaeng, R.; Poovorawan, Y.; Pakvisal, P.; Susiriwatananont, T.; Zungsontiporn, N.; et al. Vaccine-Related adverse events following AZD1222 (ChAdOx1-nCoV-19) COVID-19 vaccine in solid malignancy patients receiving cancer treatment, as compared to age-matched healthy controls. Hum. Vaccines Immunother. 2022, 18, 2094149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).