Abstract
The coronavirus disease 2019 (COVID-19) pandemic has threatened global health and prompted the need for mass vaccination. We aimed to assess the efficacy and effectiveness of COVID-19 vaccines to prevent mortality and reduce the risk of developing severe disease after the 1st and 2nd doses. From conception to 28 June 2021, we searched PubMed, Cochrane, EBSCO, Scopus, ProQuest, Web of Science, WHO-ICTRP, and Google Scholar. We included both observational and randomized controlled trials. The pooled vaccine efficacy and effectiveness following vaccination, as well as their 95 percent confidence intervals (CI), were estimated using the random-effects model. In total, 22 of the 21,567 screened articles were eligible for quantitative analysis. Mortality 7 and 14 days after full vaccination decreased significantly among the vaccinated group compared to the unvaccinated group (OR = 0.10, ([95% CI, 0.04–0.27], I2 = 54%) and (OR = 0.46, [95% CI, 0.35–0.61], I2 = 0%), respectively. The probability of having severe disease one or two weeks after 2nd dose decreased significantly (OR = 0.29 [95% CI, 0.19–0.46], I2 = 25%) and (OR = 0.08 [95% CI, 0.03–0.25], I2 = 74%), respectively. The incidence of infection any time after the 1st and 2nd doses diminished significantly (OR = 0.14 [95% CI, 0.07–0.4], I2 = 100%) and (OR = 0.179 [95% CI, 0.15–0.19], I2 = 98%), respectively. Also, incidence of infection one week after 2nd dose decreased significantly, (OR = 0.04, [95% CI (0.01–0.2], I2 = 100%). After meta-regression, the type of vaccine and country were the main predictors of outcome [non-mRNA type, ß = 2.99, p = 0.0001; country UK, ß = −0.75, p = 0.038; country USA, ß = 0.8, p = 0.02]. This study showed that most vaccines have comparable effectiveness, and it is purported that mass vaccination may help to end this pandemic.
Keywords:
SARS-CoV-2; efficacy; effectiveness; COVID-19 vaccine; systematic review; meta-anlysis; mortality 1. Introduction
Three novel coronaviruses have been discovered until the writing of this review. The first virus, named severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), was discovered in China in 2002 and caused severe acute respiratory syndrome. That same year, it led to more than 8000 infections and a 10% case fatality ratio (CFR) [1]. The second virus emerged in Saudi Arabia in 2012 and was called Middle East respiratory syndrome (MERS-CoV), with more than 2500 cases and a CFR of about 33% [2,3]. Following the appearance of SARS-CoV-1 and MERS-CoV, many vaccines were developed with live-attenuated, DNA-based, and recombinant viral vectors vaccines [4,5,6]. However, the development of clinical trials to test these postulated vaccines was abandoned when the outbreaks subsided due to the limited number of infections [7,8].
Late in 2019, a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan city, China [9]. The emerging virus causes a disease called coronavirus disease 2019 (COVID-19). Even though the great majority of SARS-CoV-2 infected patients have mild to moderate symptoms, the illness killed a considerable number of patients [10]. A hyperinflammatory process is known as “cytokine storm” is thought to be the cause of much of the serious disease associated with SARS-CoV-2 infection [11]. On 11 February 2022, 407.6 million got SARS-CoV-2 infection with 5.8 million deaths [12]. The CFR varied across countries from less than 0.1% to more than 25% [13].
The world is engaged in a fierce war against COVID-19; The United States Food and Drug Administration (FDA) [14] has granted Paxlovid from Pfizer an emergency use license for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (≥12 years of age) who are at high risk of progressing to severe COVID-19. Paxlovid is only accessible by prescription and should be started as soon as possible following a COVID-19 diagnosis and within five days of the onset of symptoms.
The World Health Organization (WHO) has approved nine vaccines for emergency use up to December 2021. These include two RNA vaccines, Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2); three non-replicating viral vectors, Janssen (Johnson & Johnson) (Ad26.COV2.S), Oxford/AstraZeneca (AZD1222), and Serum Institute of India Covishield (Oxford/AstraZeneca formulation); two protein subunits (NVX-CoV2373 and NovaVax); and inactivated virus techniques, Sinopharm (Beijing) BBIBP-CorV (Vero Cells) and Sinovac (CoronaVac) [15]. The vaccination process against COVID-19 started in December 2020 with the Pfizer-BioNTech, Moderna mRNA vaccines, and the Astra Zeneca/Oxford Chad Ox vaccines, as well as the Chinese Sinovac, inactivated SARS-CoV-2 and Russian Sputnik V adenovirus vaccines, and hundreds of vaccines at different stages of development and different mechanisms, including protein subunits with adjuvant, non-replicating viral vectors, RNA, virus-like-particles (VLP), DNA, inactivated, and live-attenuated virus [16,17]. On 23 August 2021, the FDA has approved the Pfizer-BioNTech vaccine to protect from COVID-19 for people above 16 years old. The vaccine’s previous emergency use authorization will continue for 12- to 15-year-olds [18]. Recently, Pfizer-BioNTech was approved for use among children aged 5–11 years [19]. In total, 61.7% of the population in the world has got at least one dose of COVID-19 vaccine. About 10.32 billion doses have been provided worldwide, and about 26.74 million are now administered each day [19]; however, only 10.6% of people living in low-income countries have received at least one dose. Until February 2022, less than 12% of Africans were fully vaccinated [20], while more than 62% of the population in Asia [21], 62% in South America, 63% in North America [22], and 70% in Europe were fully vaccinated [23].
The characteristics of an ideal vaccine are that it can be produced at a large scale with the lowest possible cost, that it is safe, easy to store and distribute, induces strong protection, has long-lasting neutralizing of antibody and T cell responses, and is equally suitable for any age and sex. Moreover, with the emergence of many variants of the COVID-19 virus, the vaccine also needs to be technically modifiable to deal with these emerging variants [24].
The major determinants of vaccine acceptance are vaccine safety and efficacy [25]. Most COVID-19 vaccines have mild side effects, such as pain at the site of injection, tiredness, headache, fever, or shivering for 1–2 days after vaccination. Very rare side effects include allergic reactions and blood clotting problems, the latter affecting a small number of people who had the Oxford/AstraZeneca vaccine [26]. Vaccine efficacy is defined as the degree to which a vaccine prevents disease, and possibly, also its transmission under ideal and controlled circumstances; this is determined by comparing a vaccinated group with a placebo group in a randomized controlled trial (RCT). Vaccine effectiveness also refers to how well the vaccine performs in the real world based on observational studies [27]. The aim of this systematic review and meta-analysis was to shed light on different studies evaluating the efficacy and effectiveness of COVID-19 vaccines after phase III trials.
2. Materials and Methods
This study was conducted in accordance with the Preferred Reporting Items of the Systematic Review and Meta-Analysis (PRISMA) checklist [28]. All steps were performed with strict compliance to the Cochrane Handbook of Systematic Review and Meta-Analysis [29]. Supplementary Table S1. Reference [1] is cited in Supplementary Materials.
Inclusion and exclusion criteria
All studies that met the following criteria were included:
- Reported COVID-19 vaccine efficacy (RCTs) or effectiveness (observational studies).
- Had a comparator group receiving either a placebo or another vaccine.
- The intervention group were either partially vaccinated (received only one dose of COVID-19 vaccine) or fully vaccinated.
- No restriction regarding country, race, gender, or age.
- We excluded abstract only letters to the editor, reviews, conference reports, study protocols, author responses, case reports, case series, and surveillance studies with no control group, in addition to any studies that had unreliable data for extraction or duplicates.
Studied outcomes
Primary outcomes:
- Efficacy and effectiveness of COVID-19 vaccines to prevent COVID-19 mortality.
Secondary outcomes:
- Efficacy and effectiveness of the vaccine to prevent severe disease.
- Efficacy and effectiveness of the vaccine to prevent SARS-CoV-2 infection within 7, 14, 21, and 28 days of the 1st dose.
- Efficacy and effectiveness of the vaccine to prevent SARS-CoV-2 infection any time after the 1st dose.
- Efficacy and effectiveness of the vaccine to prevent SARS-CoV-2 infection within 7 and 14 days of the 2nd dose, and 7,14 days after the 2nd dose.
- Efficacy and effectiveness of the vaccine to prevent SARS-CoV-2 infection any time after the 2nd dose.
Operation cases definition
Confirmed cases are persons who had a positive nucleic acid amplification test (NAAT), a person with a positive SARS-CoV-2 rapid diagnostic antigen test [30] and fulfill probable or suspected criteria of WHO case definitions, or a positive SARS-CoV-2 antigen (by rapid diagnostic test) asymptomatic patient but in close contact to probable or confirmed case [31].
Severe COVID-19: Adult patients categorized as having severe COVID-19, if matching one of the following criteria: oxygen saturation less than 90% in room air, a respiratory rate more than 30 breaths per minute, or had signs of severe respiratory distress [32].
Critical COVID-19: Adult patients with acute severe acute respiratory distress, septic shock, or any life-threatening condition needing critical care admission or mechanical ventilation [32].
Test negative cases control design: The best study design to detect risk factors of severe COVID-19 illness. In this study type, symptomatic COVID-19 patients are tested using a polymerase chain reaction (PCR) test, then categorized into cases (test positive patients) and controls (test-negative patients) [33].
Search methods for identification of studies
Electronic searches: The following databases were searched: Scopus, EBSCO, MEDLINE central/PubMed, Cochrane Central Register for Clinical Trials (CENTRAL), WHO International Clinical Trials Registry Platform (ICTRP), Web of Science (WOS), ProQuest Coronavirus database, and Google scholar. Search terms were determined and approved after the consultation with PubMed. The following keywords were used in our search, after adapting according to each database search strategy, (‘coronavirinae’ OR ‘coronaviridae infection’ OR ‘coronavirus disease 2019’ OR ‘coronavirus’ OR ‘coronavirus infection’) AND (Vaccin * efficacy * OR Vaccin * effective * OR Vaccin * immune *). We searched these databases to compile all studies on COVID-19 vaccination that were available up to 28 June 2021.
Searching other resources: In addition to searching the grey literature, a manual search of studies by checking reference lists of all eligible papers was undertaken to ensure that we did not miss any relevant study.
3. Data Collection and Extraction
Two independent reviewers searched the included databases (S.H. and R.M.G.). After which, all citations were exported to Endnote 20 to remove duplicates. Title and abstract screening was conducted by (R.A., A.A., N.H., D.M., and O.A.R.) and disagreement was resolved by (R.M.G). The inter-reviewer agreement was substantial (K = 0.8). Two reviewers (R.M.G. and S.H.) delineated accepted papers eligible for full-text screening. The two reviewers then extracted data related to patient characteristics and outcomes (authors, year of publication, country, inclusion, and exclusion criteria, when the study was conducted, study design, sample size, type of vaccine and number of doses, time point of analysis, primary and secondary outcomes). The Supplementary Materials of eligible articles were also reviewed for relevant data and the extracted data were checked and confirmed by a third author (Y.E.).
4. Data Analysis
Assessment of publication bias: We assessed publication bias through a visual inspection of the funnel plot and Egger’s test.
Quality assessment:
The risk of bias was assessed using two different tools according to the type of study: Cochrane risk of bias for randomized controlled trials (RoB2) [34] and the National Heart, Lung, and Blood Institute’s quality assessment tool for cohort, cross-sectional, and case-control studies [35]. D.M. and N.H. reviewed the quality of the studies and any disagreement was resolved by R.M.G. and S.H.
Effect size measurement:
We used the random-effects model to study the proposed outcomes due to the significant heterogeneity. The effect size was reported as odds ratio (OR) and 95% confidence interval level.
Assessment of heterogeneity:
- Visual inspection of the forest plot was carried out to analyze the consistency of intervention effects across the included studies. If the same intervention effect is estimated, there should be an overlap between the confidence intervals for each effect estimate on the forest plot. However, if the overlap is weak, or there are outliers, statistical heterogeneity is likely to be present.
- Statistical test for variation: heterogeneity was assessed by inspecting the forest plots to detect overlapping confidence intervals (CIs) and the I2 statistic used to denote levels of heterogeneity as defined in the Cochrane Handbook for Systematic Reviews of Interventions [30].
Heterogeneity was classified as follows:
- 0% to 40%: might not be important.
- 30% to 60%: may represent moderate heterogeneity.
- 50% to 90%: may represent substantial heterogeneity.
- 75% to 100%: considerable heterogeneity.
Sensitivity analysis: To conduct a sensitivity analysis, we recalculated the results of our meta-analysis K times, leaving out one study each time. This analysis also provides a classification for what is considered influential. We ordered studies in the plot via I2. Here we identified the studies with the highest heterogeneity as well as the final heterogeneity when these studies were removed.
Subgroup analysis: The included studies were divided into subgroups based on research design (RCT and observational), and the study outcomes were compared between the subgroups of studies.
Meta-regression: The outcome variable was the effect estimate (COVID-19 vaccine efficacy and effectiveness). The explanatory variables were study design (RCT/observational), type of vaccine (mRNA/not mRNA), and country where the study was conducted.
Statistical analysis: we used Review Manager (RevMan) version 5.4 and RStudio Desktop 2022.02.0+443 (meta package).
5. Results
5.1. Study Selection Process
A total of 21,567 articles were found after searching nine different databases. Out of these, 8088 articles were excluded either because they were duplicates as found by Endnote X8 or because they were published before 2019. The title and abstract screening of 13,479 papers resulted in exclusion of 13,284 irrelevant papers and 195 manually found duplicates. A total of 78 articles were screened for eligibility. Finally, 22 papers were deemed eligible for the meta-analysis (Figure 1). List of papers rejected and causes of rejection are provided as Supplementary Table S2. References [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] are cited in Supplementary Materials.
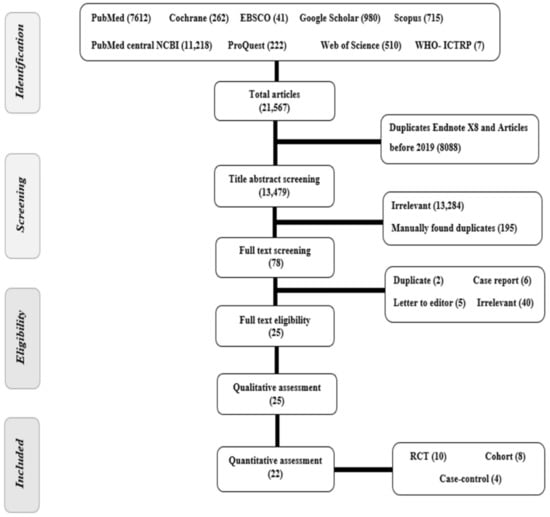
Figure 1.
PRISMA flow chart of studies screened and a Summary of included studies in the qualitative analysis.
In total, 25 studies were included in this review, 11 studies were RCTs [6,30,36,37,38,39,40,41,42,43,44] and 14 studies were observational studies [14,45,46,47,48,49,50,51,52,53,54,55,56,57] with total sample sizes ranged from 268 [48], to 6,538,911 subjects [49]. Among the included studies, two studies were conducted across countries [30,39]. We found that studies assessed the efficacy or effectiveness of vaccination after the 1st dose that ranged from 60 to 94.1% [14,30,37,40,43,45,46,47,48,50,52,53,54,56,57], while 18 studies assessed the efficacy or effectiveness of vaccination after 2nd dose that ranged from 21.1 to 100% [6,14,36,38,39,41,42,43,44,46,47,49,50,51,52,53,55,56]. Effectiveness or efficacy of BNT162b2 (Pfizer vaccine) was reported in 15 studies [14,39,42,45,46,47,48,49,50,51,52,54,55,56,57] while 6 studies addressed the AstraZeneca vaccine [38,41,43,52,54,57], 6 studies addressed the Moderna vaccine [14,37,51,53,55,56], and the Johnson and Johnson vaccine was studied in one study [30] (Table 1).

Table 1.
Summary of included studies in the quantitative analysis.
5.2. Publication Bias
We assessed publication bias of mortality associated with COVID-19 (primary outcome) through visual inspection of a funnel plot (Figure 2) and by conducting Egger’s test (t = 0.844, p = 0.43). We found that data was symmetric with low risk of publication bias.
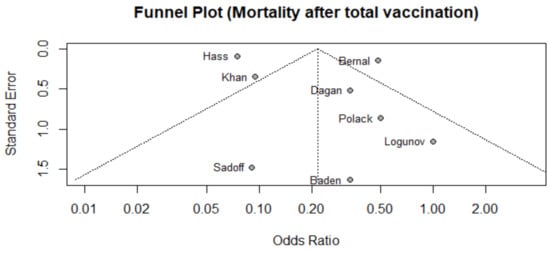
Figure 2.
Funnel plot of studies that reported mortality after 2nd dose among vaccinated population.
6. Quality Assessment
The quality assessment for the RCTs is presented in the summary of the risk of bias graph (Figure 3). The quality assessment of observational studies is included in Supplementary Table S3. References [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in Supplementary Materials.
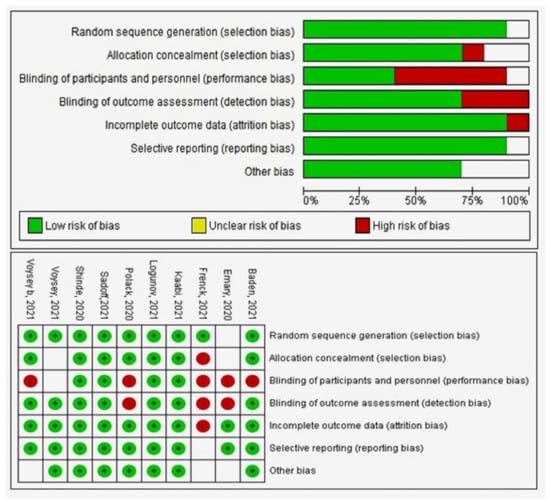
Figure 3.
Quality assessment of RCT studies.
6.1. Primary Outcome
Mortality after 7 and 14 Days after 2nd Dose
- A total of six studies (4 RCTs and 2 observational studies) assessed mortality due to COVID-19, 7 days after the 2nd dose of vaccination. Mortality among vaccinated subjects decreased significantly (OR = 0.14, [95% CI, 0.05–0.41], I2 = 63%). After conducting sensitivity analysis, the study of Logunov et al. [40] was removed, heterogeneity decreased to 54%; and the odds ratio became 0.10 [95% CI, 0.04–0.27], p < 0.001 (Figure 4A). It is important to note that in the study of Khan et al. [51] deaths due to COVID-19 were not separated from other causes of death.
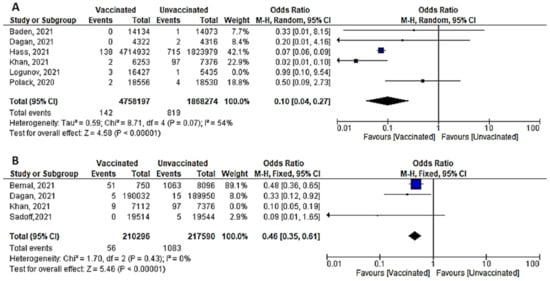 Figure 4. (A) Mortality 7 days after the 2nd dose of COVID-19 vaccination. (B) Mortality 14 days after 2nd dose of COVID-19 vaccination.
Figure 4. (A) Mortality 7 days after the 2nd dose of COVID-19 vaccination. (B) Mortality 14 days after 2nd dose of COVID-19 vaccination. - Four studies addressed mortality 14 days after 2nd dose, all of which were observational, except for the study by Sadoff et al. [30]. The overall mortality among vaccinated population decreased significantly (OR = 0.34, [95% CI, 0.26–0.44], I2 = 85%). After conducting a leave-one-out sensitivity analysis, the study of Khan et al. [51] was omitted. The observed heterogeneity dropped to 0% with an odds ratio of 0.46 [95% CI, 0.35–0.61], p < 0.001 (Figure 4B).
- Main findings: COVID-19 vaccination significantly decreased deaths 7 and 14 days after 2nd dose.
6.2. Secondary Outcomes
Severe COVID-19 Infection 7 Days after the 1st and 2nd Doses
- Seven papers studied the incidence of severe COVID-19 infection 7 days after the 2nd dose. In total, 213 of 480,7683 vaccinated people developed severe COVID-19, compared to 3298 out of 191,5476 unvaccinated subjects. The odds ratio of developing severe COVID-19 among vaccinated subjects was 0.08 [95% CI, 0.03–0.25], I2 = 74%. After subgrouping the included studies into RCTs and observational, the heterogeneity dropped to 0%. The difference between observational and interventional studies was not significant (p = 0.46). The odds ratio of severe COVID-19 among vaccinated population in RCTs was 0.14 (95% CI, 0.03–0.75), I2 = 30%, p < 0.001 while in observational studies was 0.06 [0.02–0.24], I2 = 85%, p < 0.001. (Figure 5A).
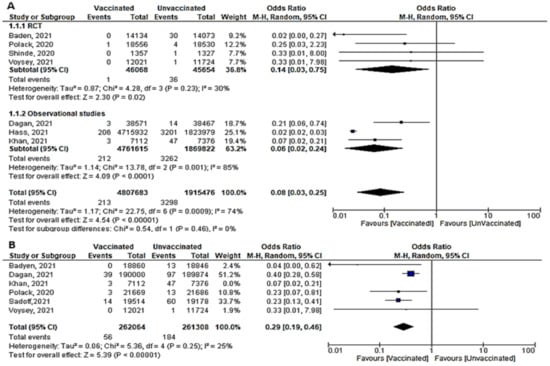 Figure 5. (A) Severe COVID-19 infection 7 days after 2nd dose of vaccination. (B) Severe COVID-19 infection 7 days after 1st dose of vaccination.
Figure 5. (A) Severe COVID-19 infection 7 days after 2nd dose of vaccination. (B) Severe COVID-19 infection 7 days after 1st dose of vaccination. - One week after the 1st dose, the odds ratio of developing severe COVID-19 after vaccination was OR = 0.21, [95% CI, 0.11–0.40], I2 = 61%. After omitting the study of Khan et al., [51] the odds ratio was 0.29 [95% CI, 0.19–0.46], I2 = 25% p < 0.001. (Figure 5B)
- Main findings: COVID-19 vaccination significantly decreased severe COVID-19 after vaccination with either 1 or 2 doses
6.3. Efficacy and Effectiveness of COVID-19 Vaccine in Reducing Infection Incidence after the 1st Dose
6.3.1. Cases Reported within 7 Days of 1st Dose (Total Cases, Symptomatic and Asymptomatic)
- Two studies evaluated the efficacy and effectiveness of the COVID-19 vaccines in reducing SARS-CoV-2 infection (symptomatic and asymptomatic) within 7 days of the 1st dose. Dagan et al., [46] reported that 1965 of 596,618 vaccinated subjects got infection compared to 2362 of 596,618 unvaccinated subjects, OR = 0.83 [95% CI, 0.78–0.88]. 2362 of 596,618 unvaccinated subjects, OR = 0.83 [95% CI, 0.78–0.88]. Hall et al. [50] highlighted that the incidence of SARS-CoV-2 was 140 out of 20,641 unvaccinated subjects; it was lower than cases reported among unvaccinated (977 out of 2683), with OR = 0.01 [95% CI, 0.01–0.01]. Due to the significant heterogeneity, we could not pool the findings of these two outcomes.
- Bernal et al. [52] reported that 346 out of 864 vaccinated subjects versus 8988 out of 24,706 unvaccinated subjects got symptomatic SARS-CoV-2 infection, OR = 1.17 [95% CI, 1.02–1.34]. However, Dagan et al. [46] found that the COVID-19 vaccine had a protective effect, with an OR of 0.78 [95% CI, 0.72–0.84]. Among 596,618 vaccinated individuals, about 1103 subjects developed symptomatic SARS-CoV-2 infection, while among 596,618 unvaccinated individuals, about 1419 subjects developed symptomatic SARS-CoV-2 infection. Due to the significant heterogeneity, we could not pool the findings of these two outcomes.
- No studies reported asymptomatic cases within 7 days of the 1st dose.
6.3.2. Cases Reported within 14 Days of 1st Dose:
- Four studies assessed the efficacy and effectiveness of COVID-19 vaccines within 14 days of the 1st dose. In total, 3909 of 637,142 vaccinated subjects developed SARS-CoV-2 infection (symptomatic or asymptomatic) within 2 weeks after the 1st dose, compared to 5087 of 614,989 unvaccinated subjects. The odds ratio of getting SARS-CoV-2 infection decreased significantly (OR = 0.17 ([95% CI, 0.02–1.72], I2 = 100%). After we sub-grouped the included studies into RCTs and observational, the heterogeneity decreased to 94%. The difference between observational and interventional studies was significant (p = 0.001). The odds ratio of getting infection with SARS-CoV-2 in the RCTs was 0.79 [95% CI, 0.48–1.3], p = 0.36 while that of observational studies was 0.05 [95% CI, 0.02–0.15], p < 0.001. (Figure 6A).
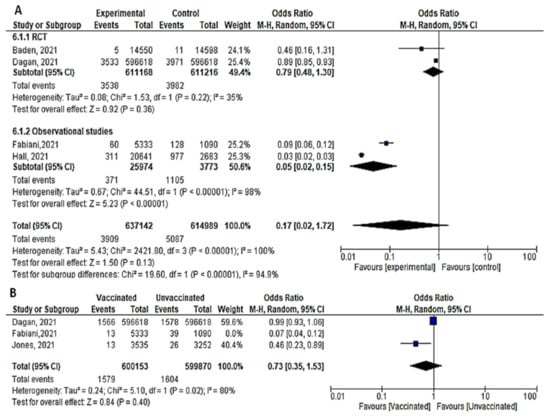 Figure 6. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 14 days of 1st dose of vaccination. (B) Incidence of asymptomatic SARS-CoV-2 within 14 days of 1st dose of vaccination.
Figure 6. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 14 days of 1st dose of vaccination. (B) Incidence of asymptomatic SARS-CoV-2 within 14 days of 1st dose of vaccination. - Bernal et al. [52] reported 958 symptomatic cases out of 1154 vaccinated subjects compared to 89 out of 8988 unvaccinated subjects, OR = 83.84 [95% CI, 68.07–103.26]. On the other hand, the protective effect of the COVID-19 vaccine was addressed by Dagan et al. [46] 1967 out of 596,618 vaccinated subjects versus 2393 out of 596,618 unvaccinated subjects OR = 0.82, [95% CI, 0.77–0.87]. On the same line, Fabiani et al. [47] reported that 47 out of 5333 vaccinated subjects versus 89 out of 1090 unvaccinated subjects developed symptomatic diseases, OR = 0.1, [95% CI, 0.07–0.14]. The results could not be pooled due to the significant heterogeneity.
- Three studies addressed the efficacy and effectiveness of vaccination in reducing the risk of having asymptomatic COVID-19 within 14 days of the 1st dose (OR = 0.23 [95% CI, 0.06–1.63], I2 = 97%). After conducting a leave-one-out sensitivity analysis, the study by Fabiani et al., [47] was omitted. The probability of getting asymptomatic infection did not reduce significantly. (OR = 0.73 [95% CI, 0.35–1.53], I2= 80%), p < 0.02. (Figure 6B).
- Main finding: incidence of SARS-CoV-2 infection in observational studies decreased significantly after vaccination within 14 days of 1st dose.
6.3.3. Cases Reported within 21 Days of 1st Dose
- Five studies assessed the efficacy and effectiveness of COVID-19 vaccines within 21 days of the 1st dose. In total, 4621 of 642,572 vaccinated subjects developed SARS-CoV-2 infection (symptomatic and asymptomatic) within 3 weeks of the 1st dose, compared to 6318 out of 620,356 unvaccinated subjects. The odds ratio of having infection was 0.19 [95% CI, 0.03–1.17], I2 = 100%. After subgrouping the included studies into RCTs and observational, the heterogeneity dropped to 0%. The difference between observational and interventional studies was insignificant (p = 0.33). The odds ratio of infection with COVID-19 in the RCTs was 0.45 [95% CI, 0.31–0.65], p < 0.001, while the OR in observational studies was 0.13 [95% CI, 0.01–1.49], p < 0.001 (Figure 7).
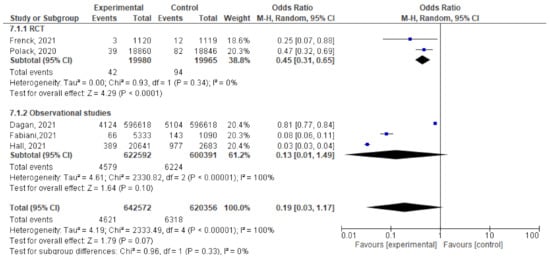 Figure 7. Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 21 days after the 1st dose of vaccination.
Figure 7. Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 21 days after the 1st dose of vaccination. - For Dagan et al. [46], the odds ratio of catching symptomatic SARS-CoV-2 after vaccination was 0.73 [95% CI, 0.69–0.77]. Of the 596,618 vaccinated subjects, 2250 developed symptomatic SARS-CoV-2 infection, compared to 3079 out of 596,618 unvaccinated subjects. Fabiani et al. [47] reported that 51 out of 53,333 vaccinated subjects developed symptomatic disease compared to 97 out of 1090 unvaccinated subjects, OR = 0.1 (95% CI, 0.07–0.14). The results could not be pooled due to the significant heterogeneity.
- Only the study by Fabiani et al. [47] addressed the effectiveness of COVID-19 against asymptomatic infection three weeks after the 1st dose. In total, 15 out of 5333 vaccinated subjects developed asymptomatic SARS-CoV-2 compared to 46 out of 1090 unvaccinated subjects, OR = 0.06 [95% CI, 0.04–0.12].
- Main findings: incidence of SARS-CoV-2 infection within 21 days of the 1st dose of vaccination reduced significantly.
6.3.4. Cases Reported within 28 Days of 1st Dose
- The total number of cases (symptomatic and asymptomatic) reported 28 days after the 1st dose was reported in two studies; Dagan et al. [46] documented 4405 confirmed cases of 596,618 vaccinated individuals compared to 5775 confirmed cases of 596,618 unvaccinated subjects. Hall et al. [50] diagnosed 427 cases of 20,641 vaccinated subjects, and 977 cases of 2683 unvaccinated subject. The odds ratios were 0.76 [95% CI, 0.73–0.79] and 0.04 [95% CI, 0.03–0.4], respectively. The research team could not pool the odds ratios due to the significant heterogeneity.
- No studies reported asymptomatic or symptomatic cases within 28 days of the 1st dose.
6.3.5. Cases Reported Any Time after 1st Dose
- Among 777,171 vaccinated subjects with a single dose of COVID-19 vaccine, 8246 got SARS-CoV-2 infection (symptomatic or asymptomatic) compared to 58,261 of 1,104,745 unvaccinated subjects (OR = 0.14 [95% CI, 0.07–0.4], I2 = 100%). After subgrouping based on the study design, the heterogeneity dropped to 0%, the odds ratio of RCTs was 0.14 [95% CI, 0.07–0.27], p < 0.001 and of observational studies was 0.15 [95% CI, 0.06–0.4] p < 0.001. The difference between subgroups was not significant, p = 0.88. (Figure 8)
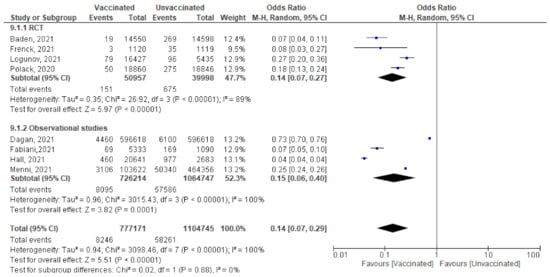 Figure 8. Incidence of all SARS-CoV-2 cases after 1st dose of vaccination.
Figure 8. Incidence of all SARS-CoV-2 cases after 1st dose of vaccination. - Main findings: incidence of SARS-CoV-2 infection any time after the 1st dose decreased significantly among vaccinated compared to unvaccinated.
6.4. Efficacy/Effectiveness of the 2nd Dose
6.4.1. Cases Reported within 7 days of 2nd Dose
- Six studies reported COVID-19 cases within 7 days of vaccination; two were RCTs and four were observational studies. The number of cases reported decreased significantly among the vaccinated versus non-vaccinated population (OR = 0.06 (95% CI, 0.02–0.21), I2 = 98%). We conducted a meta-regression to explain this substantial heterogeneity, using the type of vaccine and country as predictors [mRNA and non-mRNA]. The output of the developed meta-regression model explained 100.0% of the heterogeneity (variability in our data); [mRNA type, β = −3.33, p = 0.028; country UK, β = −3.7, p = 0.025; country USA, β = −2.03, p = 0.037: Israel was the reference country] (Figure 9A). Meaning that vaccination using mRNA decreases the incidence of SARS-CoV-2 infection by 3.33 compared to other vaccines, and vaccination in developed countries like UK and USA decreased the incidence of infection by 3.7 and 2.03 times less than the reference country, respectively.
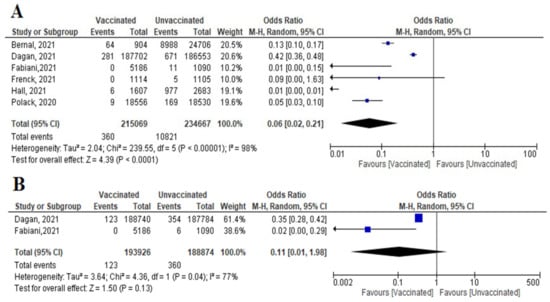 Figure 9. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 7 days of 2nd dose of vaccination. (B) Incidence symptomatic SARS-CoV-2 within 7 days of 2nd dose of vaccination.
Figure 9. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 7 days of 2nd dose of vaccination. (B) Incidence symptomatic SARS-CoV-2 within 7 days of 2nd dose of vaccination. - Incidence of symptomatic SARS-CoV-2 infection did not decrease significantly among vaccinated compared to unvaccinated (OR = 0.11 [95% CI, 0.01–1.98], I2 = 77%), p = 0.13 (Figure 9B).
- No studies reported asymptomatic cases within 7 days of the 2nd dose.
- Main findings: COVID-19 vaccination decreased incidence of SARS-CoV-2 infection within 7 days of 2nd dose. The main predictor of vaccine effectiveness/efficacy were the country where the vaccine was supplied and the type of vaccine.
6.4.2. Cases within 14 Days of 2nd Dose
- Three studies highlighted the number of new cases (symptomatic and asymptomatic) reported within 14 days of 2nd dose. Baden et al. [37] reported no cases among 14,550 vaccinated subjects compared to 19 cases out of 14,598 unvaccinated subjects. Dagan et al. [46] reported 332 confirmed cases out of 187,702 vaccinated subjects and 949 cases out of 186,553 unvaccinated subjects. Hall et al. [50] reported 10 cases out of 1607 vaccinated subjects and 977 cases out of 2683 unvaccinated subjects. Pooling of these findings showed significant reduction in incidence of reported cases (OR = 0.05 [95% CI, 0.0–1.34], I2 = 99%). After conducting leave-one-out sensitivity analysis, the study by Dagan et al. [46] was omitted, the vaccine was effective in reducing the number of cases reported within 14 days of the 2nd dose (OR = 0.01 [95% CI, 0.01–0.02], I2 = 0%), p < 0.001 (Figure 10).
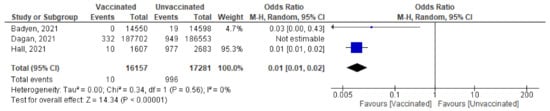 Figure 10. Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 14 days of 2nd dose of vaccination.
Figure 10. Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic within 14 days of 2nd dose of vaccination. - No studies reported asymptomatic or symptomatic cases within 14 days of 2nd dose.
- Main findings: incidence of SARS-CoV-2 infection within 14 days of the 2nd dose decreased significantly after vaccination.
6.4.3. Cases Reported 7 Days after 2nd Dose
- Among 4,935,355 vaccinated subjects, the number of confirmed SARS-CoV-2 infection (symptomatic and asymptomatic) 7 days after 2nd dose was 6576, while 111,923 confirmed cases were reported among 2,041,321 unvaccinated subjects. The OR was 0.04 [95% CI, 0.01–0.2], I2=100, p < 0.001, indicating that COVID-19 vaccines had a protective effect. We were able to explain 100% of this heterogeneity by conducting meta-regression analysis. Vaccine type and country were significant predictors of response; [non-mRNA vaccine (β = 2.99, p = 0.0001): mRNA is the reference type, country UK (β = –0.75, p = 0.038), country USA (β = 0.8, p = 0.02); reference country was Israel] (Figure 11A). In this case, non-mRNA (the highest OR) vaccine and vaccination in the USA increased the risk of SARS-CoV-2 infection by 2.99 and 0.8 times, respectively. On the other hand, vaccination in the UK decreased risk of SARS-CoV-2 infection by 0.75 comparing to reference country (Israel).
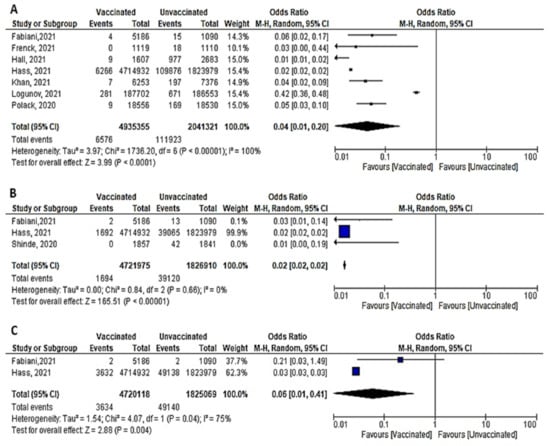 Figure 11. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic after 7 days of 2nd dose of vaccination. (B) Incidence of symptomatic SARS-CoV-2 after 7 days of 2nd dose of vaccination. (C) Incidence of asymptomatic SARS-CoV-2 after 7 days of 2nd dose of vaccination.
Figure 11. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic after 7 days of 2nd dose of vaccination. (B) Incidence of symptomatic SARS-CoV-2 after 7 days of 2nd dose of vaccination. (C) Incidence of asymptomatic SARS-CoV-2 after 7 days of 2nd dose of vaccination. - COVID-19 vaccines were effective in preventing symptomatic COVID-19 infection 7 days after the 2nd dose (OR = 0.02 [95% CI, 0.02–0.02], I2 = 0%), p < 0.001 (Figure 11B).
- Two studies (recruiting 4,720,118 vaccinated subjects and 1,825,069 unvaccinated subjects) assessed asymptomatic SARS-CoV-2 infection after 7 days of vaccination. Fabini et al. [47] diagnosed two cases among vaccinated and non-vaccinated subjects with a statistically non-significant odds ratio (OR = 0.21, [95% CI, 0.03–1.49]). Meanwhile, Hass et al. [49] reported 3632 asymptomatic cases among vaccinated subjects and 49,138 among unvaccinated subjects (OR = 0.03, [95% CI, 0.03–0.03]) (Figure 11C). Vaccination was protective against asymptomatic infection 7 days after the 2nd dose (OR = 0.06 [95% CI, 0.01–0.41), I2 = 75%), p = 0.04.
- Main findings: incidence of SARS-CoV-2 (symptomatic and asymptomatic) reported 7 days after the 2nd dose decreased significantly.
6.4.4. Cases Reported 14 Days after 2nd Dose
- We pooled nine studies that addressed the total number of symptomatic or asymptomatic cases confirmed 14 days after the 2nd dose of vaccination. The total number of cases was 4080 out of 4,832,289 vaccinated subjects and 119,829 out of 1,940,635 unvaccinated subjects. The vaccine had a protective effect against getting SARS-CoV-2 infection 14 days after the 2nd dose (OR = 0.08, [95% CI, 0.02, 0.34], I2 = 100%), p < 0.001. Thus, we carried out a meta-regression analysis to understand the main predictors of this heterogeneity. Again, the type of vaccine and the country were responsible for 88.21% of the heterogeneity; [non-mRNA vaccine β = 3.519, p = 0.004; country Spain β = 2.6256, p = 0.028: Israel was the reference country) (Figure 1A).
- Seven studies reported the incidence of symptomatic infection 14 days after the 2nd dose. Vaccination had a protective effect against symptomatic infection. The odds among vaccinated subjects was (0.10 [95% CI, 0.02–0.54], I2 = 100%), p < 0.001. To further explain this substantial heterogeneity, we performed meta-regression analysis that explained 100% of the heterogeneity using type of vaccine and country as predictors; [non-mRNA type, β = 3.16, p = 0.0044; country Spain β = 2.7, p = 0.0048; Israel is the reference country] (Figure 12B).
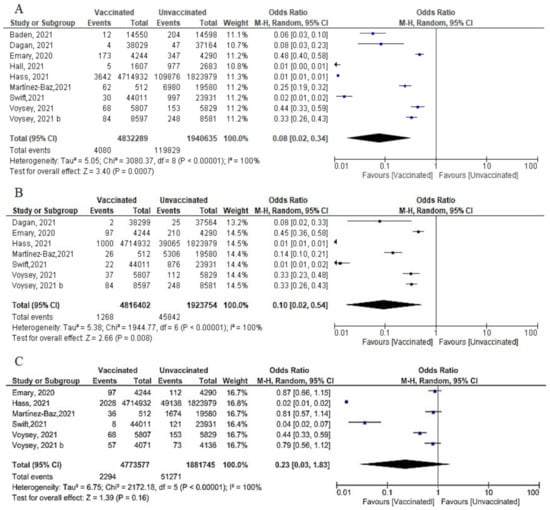 Figure 12. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic after 14 days of 2nd dose of vaccination. (B) Incidence of symptomatic SARS-CoV-2 after 14 days of 2nd dose of vaccination. (C) Incidence of asymptomatic SARS-CoV-2 after 14 days of 2nd dose of vaccination.
Figure 12. (A) Incidence of SARS-CoV-2 infection either symptomatic or asymptomatic after 14 days of 2nd dose of vaccination. (B) Incidence of symptomatic SARS-CoV-2 after 14 days of 2nd dose of vaccination. (C) Incidence of asymptomatic SARS-CoV-2 after 14 days of 2nd dose of vaccination. - Regarding asymptomatic cases after 14 days of the 2nd dose, 2294 of 4,773,577 vaccinated subjects compared to 51,271 of 1,881,745 unvaccinated subjects developed asymptomatic infection. (OR = 0.23 [95% CI, 0.03–1.83], I2 = 100%.), p < 0.001. Meta-regression was performed and explain 96.29% of heterogeneity, using type of vaccine and the country as predictors, [non-mRNA type, β = 2.9, p = 0.033; country Spain β = 3.96, p = 0.016: reference Israel]. (Figure 12C).
- Main findings: incidence of SARS-CoV-2 cases (total cases and asymptomatic cases) two weeks after the 2nd dose was significantly reduced.
6.4.5. Cases Reported 7–14 Days after 2nd Dose
Dagan et al. [46] reported 51 cases out of 108,529 vaccinated subjects and 278 cases out of 107,209 unvaccinated subjects got SARS-CoV-2 infection 7–14 days after the 2nd dose of vaccination OR = 0.18 [95%CI, 0.13–0.24]. Meanwhile, Hall et al. [50] reported 4 cases out of 1607 vaccinated subjects and 977 out of 2683 unvaccinated subjects, OR = 0.0 (95% CI, 0.0–0.01). The result could not be pooled due to substantial heterogeneity I2 = 99%.
6.4.6. All Cases Reported after the 2nd Dose
- A total of 377 confirmed cases out of 228,715 vaccinated subjects, and 2435 cases out of 224,569 unvaccinated subjects were reported after the 2nd dose, (OR = 0.179 [95% CI, 0.15–0.19], I2 = 98%). The test for subgroup differences suggested that there was no statistically significant subgroup effect (p = 0.98), meaning that the type of study did not significantly modify the efficacy and effectiveness of vaccination. Vaccination decreased the number of cases regardless of the study design, although the protective effect was greater in RCT than in observational studies. There was no heterogeneity between the results of the RCT studies, while the heterogeneity of observational studies was I2 = 99% (Figure 13).
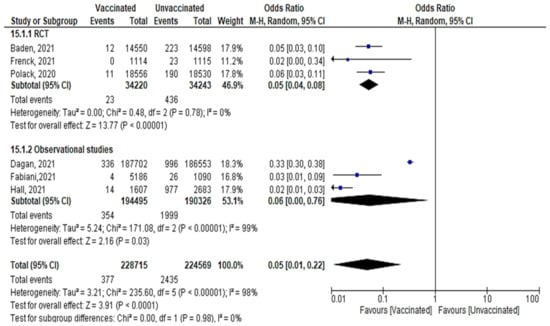 Figure 13. All cases of confirmed SARS-CoV-2 infection reported after 2nd dose of vaccination regardless of the duration.
Figure 13. All cases of confirmed SARS-CoV-2 infection reported after 2nd dose of vaccination regardless of the duration. - Main findings: vaccination against SARS-CoV-2 decreased incidence of infection after the 2nd dose regardless of the duration.
7. Discussion
The aim of vaccine development is to provide a weapon that protects people from getting infected or becoming a source of transmission. By the end of 2020, several COVID-19 vaccines had become available for use across the world, with over 40 different vaccines in human trials, and over 150 in preclinical trials. An updated list of vaccine candidates under evaluation is maintained by the WHO [58]. Although some of the vaccines were approved for emergency use by the FDA in the USA and the respective health departments of other countries across the world, the efficacy and effectiveness should be periodically assessed due to the ongoing antigenic drift. It is worth noting that while vaccinations are still being administered worldwide, the vaccinated population (received at least one dose of vaccine) represents around three-fifth of the entire population [59], with safety and effectiveness representing the main concern and points of hesitation for many people [25]. Another main concern affecting vaccination coverage is COVID-19 vaccine inequity; vaccine supply will have a long-term and severe impact on socioeconomic recovery in low-and lower-middle-income countries (LMIC) unless immediate action is taken to increase supply and provide equal access for all countries. If LMIC had similar vaccination rates as high-income countries (HIC), an acceleration in scaling up manufacturing and providing adequate vaccine doses might have added costs. A high price per COVID-19 vaccine dose in comparison to other vaccines, as well as delivery costs, including those for the health workforce surge, could put a huge strain on fragile health systems, undermining vaccination programs and essential health services, and causing alarming spikes in measles, pneumonia, and diarrhea [60].
Several studies recruited different numbers of participants to study various outcomes with variable endpoints providing different doses of vaccine with variable intervals. Studying the efficacy and effectiveness of several types of COVID-19 vaccines in reducing mortality and severity were the focus of this meta-analysis aiming to provide strong evidence to decision-makers in health policy sectors to deal with the ongoing pandemic.
We reviewed a total of 22 articles to study the desired outcomes, among which 10 were RCTs, 4 were case-control, and 8 were cohort studies. The highest number of recruited subjects in a single study was 6,538,911 [49], while the smallest number was 268 subjects [48].
7.1. Mortality and Severe COVID-19
Based on the findings of this meta-analysis, the mortality related to COVID-19 two weeks after vaccination was significantly decreased (OR = 0.46, [95% CI, 0.35–0.61], I2 = 0%). Similarly, mortality one week after vaccination dropped significantly (OR = 0.10, [95% CI, 0.04–0.27], I2 = 54%). In RCTs, the odds ratio for severe COVID-19 was 0.14 [95% CI, 0.03–0.75], I2 = 30%), whereas in observational studies, the odds ratio was 0.06 [0.02–0.24], I2 = 85%. In the same line, the odds ratio of having severe COVID-19 after the 1st dose was 0.15 [0.10–0.25], I2 = 26%. Analyzing our results, different studies reported a significant reduction in SARS-CoV-2 infection, hospitalizations, and fatalities among those who had been fully vaccinated compared to those who had not been fully vaccinated [61,62,63]. Fiolet et al. [64] recently published a review on different COVID-19 vaccines effectiveness; when the strain was not sequenced, the effectiveness of the mRNA vaccination against hospitalization and mortality was over 87–94%. Similarly, inactivated viral COVID-19 vaccine (CoronaVac) was extremely effective against hospitalization (87.5%) and death (86.3%). In addition, if a breakthrough occurs in a vaccinated individual, the events are usually less severe than in an unprotected person [65]. Similarly, a recently published meta-analysis highlighted that the BNT162b2 and mRNA-1273 vaccines had the best effectiveness in preventing symptomatic COVID-19. The efficacy of comparing different vaccines in preventing serious illness was not different. Moreover, there was no difference in the efficacy of vaccinations to prevent symptomatic COVID-19 among the elderly [66]. Unfortunately, this protective effect wanes with time—5 months or more after vaccination—and vaccine effectiveness decreased against hospitalization and deaths (80.0 and 84.8% with the ChAdOx1-S) and (91.7% and 91.9% with BNT162b2), respectively [67].
7.2. Infection after Vaccination
It is worthy to note that Alagoz et al. [68] hypothesized that if there is a strong adherence to non-pharmacological interventions in the community, the controllable spread of SARS-CoV-2 can be reached sooner than when a substantial part of the population gets vaccinated (e.g., 70–80%). In the current study, COVID-19 vaccines effectively reduced the incidence of symptomatic and asymptomatic infection. On the same lines, the WHO reported that unvaccinated persons account for the great majority of the current SARS-CoV-2 infection [65]. Virus-neutralizing antibodies are principally responsible for the protection provided by presently available vaccinations. These antibodies often inhibit the virus’s binding with its cellular receptor or prevent the virus from undergoing the conformational changes essential for fusion with the cell membrane [69] We found that vaccination against COVID-19 decreased the number of cases reported within a week of the 2nd dose (OR = 0.06 (95% CI, 0.02–0.21), I2 = 98%). Type of vaccine and country where study was conducted were the main predictors of vaccine efficacy and effectiveness. Similarly, the total number of cases diagnosed within 14 days of the 2nd dose decreased significantly, (OR = 0.01 [95% CI, 0.01–0.02], I2 = 0%). In terms of cases reported 7 days after 2nd dose, the total number of cases decreased significantly with vaccination (OR = 0.03 [95% CI, 0.02–0.05], I2 = 73%). About 100% of this heterogeneity was explained by meta-regression (vaccine type and country). Regarding symptomatic cases diagnosed 7 days after the 2nd dose, COVID-19 vaccine was effective in reducing the number of symptomatic cases in comparison to placebo or control group (OR = 0.02 [95% CI, 0.02–0.02], I2 = 0%). The odds ratio of cases reported 14 days after the 2nd dose among vaccinated versus unvaccinated subjects was OR = 0.08, [95% CI, 0.02-0.34], I2 = 100%). Confirmed cases reported after the 1st and 2nd dose regardless of the duration decreased significantly, OR = 0.14 (95% CI, 0.07–0.4) I2 = 100% and 0.18 (95% CI, 0.15–0.19), I2 = 98%, respectively.
In the same vein, many reviews addressed vaccine effectiveness and efficacy. Pormohammad et al. [70] included 25 studies in phase II/III RCTs, the efficacy of mRNA-based and adenovirus-vectored COVID-19 vaccines was 94.6% and 80.2%, respectively. After 3 weeks of vaccinations, the adenovirus-vectored vaccine had the maximum efficacy against receptor-binding domain (RBD) antigen after the 1st and 2nd doses (97.6% and 98.2% respectively). Similarly, a review of phase III studies showed a significant increase in neutralizing antibodies with the 2nd dose of the vaccine [71]. However, it was also advised that when vaccine supply is scarce, countries should vaccinate with a single dose. This may provide better overall protection in the population than vaccinating half the number of individuals with both doses [72].
Many factors can explain the observed difference in efficacy and effectiveness of the COVID-19 vaccines. The Center for Disease Control and Prevention [73] demonstrated that in the real-world, vaccine effectiveness can be affected by several factors, including population host factors (e.g., those who were not included in clinical trials) and virus factors (e.g., variants) as well as programmatic factors (e.g., adherence to dosing schedules or vaccine storage/handling) [74]. Thompson et al. [75] reported that under real-world conditions, complete immunization (14 days after 2nd dose) was 90% effective against SARS-CoV-2 infection, while partial immunization (14 days after 1st dose but before 2nd dose) was 80% effective. In addition, the effectiveness of vaccination varied according to the types of vaccine; Pilishvili et al. [76] stated that vaccine effectiveness for Pfizer–BioNTech and Moderna were 77.6% (95.6% CI, 70.9–82.7) and 88.9% (95.9% CI, 78.7–94.2) after the 1st dose and were 88.8% (95% CI, 84.6–91.8) and 96.3% (95.3–98.4) after the 2nd dose, respectively. Of note, when the SARS-CoV-2 Delta variant became prevalent, the percentage of completely vaccinated people who got SARS-CoV-2 infection grew higher than predicted [63]. The effectiveness of the mRNA vaccine against COVID-19 was 88–100% against Alpha, 76–100% against Beta/Gamma, 47.3–88% against Delta, and 89–100% when the SARS-CoV-2 strain was not sequenced. Oxford/AstraZeneca (AZD1222) was 74.5% effective against Alpha and 67% effective against Delta. CoronaVac was effective against the Alpha/Gamma/D614G strain in 36.8–73.8% of cases [64].
Unfortunately, new data consistently demonstrated that vaccine efficacy against SARS-CoV-2 infection declines with time following immunization [77]. It is worth noting that according to a recently published systematic review and meta-analysis, immunization efficacy against severe COVID-19 infection dropped by around 8% (95% CI, 4–15) during the 6-months period in all age groups. Over the same time, vaccine efficacy against serious illness declined by around 10% (95% CI, 6–15%) in individuals over the age of 50. Vaccine efficacy against symptomatic illness fell by 32% (95% CI, 11–69%) in individuals over the age of 50 [78]. Consequently, WHO has already recommended administering a booster dose of vaccine to people aged 60 years or older as part of the main series to strengthen initial protection [65]. Therefore, people should adhere to public health and social measures even though they have received vaccines to avoid COVID-19 infection and its consequences [79].
7.3. Strengths and Limitations
This systematic review has some limitations. First, there is no evidence of the long-term effectiveness of the vaccine. Due to the urgency of vaccine development, most trials only followed up participants for 28 days after vaccination. Second, this metanalysis cannot give solid evidence on the efficacy and effectiveness of COVID-19 vaccines on the variant strain B.1.351. This variant strain can escape neutralizing relevant antibodies. Consequently, more studies need to be conducted to assess the effectiveness and efficacy of COVID-19 vaccines against variants of concern like delta strain and omicron [64]. Third, due to scarce of literature, we neither included all approved vaccines nor all age groups (elderly, adolescents, and children).
The points of strength in this systematic review are that we did not include preprinted documents, studies that were not peer-reviewed, and studies with missing data. Due to the scarcity of RCTs, observational studies were included, as were retrospective case analyses. Animal studies were excluded, and we did not have lingual restrictions. Our analysis has identified numerous critical components to consider when planning a real-world efficacy trial of COVID-19 vaccinations, such as the appropriate study design, study population, outcome, and period for follow-up. The majority of studies identified were from HICs, frequently utilizing national databases (which may not exist or may be of lower quality in LMICs), and the vast majority assessed mRNA vaccines, which are more prevalent in HICs. These findings highlight the need for pressing for real-world efficacy studies on all licensed COVID-19 vaccines in a variety of LMIC contexts using different study designs.
8. Conclusions
This systematic review and meta-analysis summarized the results of clinical trials related to the COVID-19 vaccines, showing that most vaccines had comparable effectiveness and efficacy. It is believed that vaccination can effectively reduce COVID-19 related deaths and severe cases. The incidence of COVID-19, either symptomatic or asymptomatic, decreased significantly after vaccination by one or two doses. However, in the light of the ongoing appearance of novel variants, the efficacy/effectiveness of vaccination against COVID-19 infection needs to be re-assessed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10030350/s1, Table S1: PRISMA 2020 Checklist. Table S2: Summary of studies not met inclusion criteria of qualitative and quantitative analysis. Table S3: Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
Author Contributions
Conceptualization of research idea, R.M.G.; design and data collection, R.M.G., O.A.R., A.A., R.S. and S.H.N.T.; methodology and data analysis, R.M.G., R.A. and S.H.N.T.; writing—original draft preparation: R.M.G., N.A.H., Y.A.M.E. and D.E.; Writing—review and editing: R.M.G. and S.H.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Memish, Z.A.; Perlman, S.; Van Kerkhove, M.D.; Zumla, A. Middle East respiratory syndrome. Lancet 2020, 395, 1063–1077. [Google Scholar] [CrossRef]
- World Health Organization. Epidemic and Pandemic-Prone Diseases: MERS Situation Update. Available online: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-december-2019.html (accessed on 3 August 2021).
- Mubarak, A.; Alturaiki, W.; Hemida, M.G. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J. Immunol. Res. 2019, 2019, 6491738. [Google Scholar] [CrossRef] [PubMed]
- Roper, R.L.; Rehm, K.E. SARS vaccines: Where are we? Expert Rev. Vaccines 2009, 8, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Adney, D.; Wang, L.; van Doremalen, N.; Shi, W.; Zhang, Y.; Kong, W.-P.; Miller, M.; Bushmaker, T.; Scott, D.; de Wit, E.; et al. Efficacy of an Adjuvanted Middle East Respiratory Syndrome Coronavirus Spike Protein Vaccine in Dromedary Camels and Alpacas. Viruses 2019, 11, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilgenfeld, R.; Peiris, M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013, 100, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stratton, C.W.; Tang, Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 January 2022).
- World Health Organization. Estimating Mortality from COVID-19. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed on 2 January 2022).
- Pilishvili, T.; Fleming-Dutra, K.E.; Farrar, J.L.; Gierke, R.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel—33 U.S. Sites, January–March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 753–758. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 2 January 2022).
- Chen, W.-H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/docs/default-source/a-future-for-children/novel-coronavirus_landscape_covid-19.pdf?sfvrsn=4d8bd201_1 (accessed on 2 January 2022).
- Tanne, J.H. COVID-19: FDA approves Pfizer-BioNTech vaccine in record time. BMJ 2021, 374, n2096. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 6 January 2022).
- Africa CDC. COVID-19 Vaccination. Available online: https://africacdc.org/covid-19-vaccination/ (accessed on 2 January 2022).
- Covidvax. Live COVID-19 Vaccination Tracker Asia. Available online: https://covidvax.live/continent/asia (accessed on 2 January 2022).
- The New New York Times. Covid Vaccinations Tracker. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 2 January 2022).
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 2 January 2022).
- Funk, C.D.; Laferrière, C.; Ardakani, A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front. Pharmacol. 2020, 11, 937. [Google Scholar] [CrossRef]
- Abdou, M.S.; Kheirallah, K.A.; Aly, M.O.; Ramadan, A.; Elhadi, Y.A.M.; Elbarazi, I.; Deghidy, E.A.; El Saeh, H.M.; Salem, K.M.; Ghazy, R.M. The coronavirus disease 2019 (COVID-19) vaccination psychological antecedent assessment using the Arabic 5c validated tool: An online survey in 13 Arab countries. PLoS ONE 2021, 16, e0260321. [Google Scholar] [CrossRef]
- National Health Service. Coronavirus (COVID-19) Vaccines Side Effects and Safety. Available online: https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/safety-and-side-effects/ (accessed on 25 December 2021).
- World Health Organization. Vaccine Efficacy, Effectiveness and Protection. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 12 December 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2009; Volume 5. [Google Scholar]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- World Health Organization. WHO COVID-19 Case Definition. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 (accessed on 28 December 2021).
- World Health Organization. COVID-19 Clinical Management: Living Guidance. Available online: https://apps.who.int/iris/handle/10665/338882 (accessed on 12 December 2021).
- Vandenbroucke, J.P.; Brickley, E.B.; Vandenbroucke-Grauls, C.M.J.E.; Pearce, N. A Test-Negative Design with Additional Population Controls Can Be Used to Rapidly Study Causes of the SARS-CoV-2. Epidemiology 2020, 31, 836–843. [Google Scholar] [CrossRef]
- Cochrane. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 21 December 2021).
- National Institution of Health. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 18 May 2021).
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An explora-tory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterol-ogous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and ef-ficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Britton, A.; Jacobs Slifka, K.M.; Edens, C.; Nanduri, S.A.; Bart, S.M.; Shang, N.; Harizaj, A.; Armstrong, J.; Xu, K.; Ehrlich, H.Y.; et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks—Connecticut, December 2020–February 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 396–401. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Fabiani, M.; Ramigni, M.; Gobbetto, V.; Mateo-Urdiales, A.; Pezzotti, P.; Piovesan, C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Eurosurveillance 2021, 26, 2100420. [Google Scholar] [CrossRef]
- Gras-Valentí, P.; Chico-Sánchez, P.; Algado-Sellés, N.; Jiménez-Sepúlveda, N.J.; Gómez-Sotero, I.L.; Fuster-Pérez, M.; Carta-gena-Llopis, L.; Sánchez-Valero, M.; Cerezo-Milán, P.; Martínez-Tornero, I.; et al. Effectiveness of the first dose of BNT162b2 vaccine to preventing COVID-19 in healthcare personnel. Rev. Esp. Salud Publica 2021, 95, 319–325. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infec-tion (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Khan, N.; Mahmud, N. Effectiveness of SARS-CoV-2 Vaccination in a Veterans Affairs Cohort of Patients with Inflammatory Bowel Disease with Diverse Exposure to Immunosuppressive Medications. Gastroenterology 2021, 161, 827–836. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital ad-missions, and mortality in older adults in England: Test negative case-control study. BMJ. 2021, 373, n1088. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Miqueleiz, A.; Casado, I.; Navascués, A.; Trobajo-Sanmartín, C.; Burgui, C.; Guevara, M.; Ezpeleta, C.; Castilla, J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance 2021, 26, 2100438. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effec-tiveness of Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in a Cohort of Healthcare Personnel. Clin. Infect. Dis. 2021, 73, e1376–e1379. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A na-tional prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- McGill University. COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/country/egypt/ (accessed on 21 December 2021).
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/covid-vaccinations (accessed on 7 January 2022).
- World Health Organization. Vaccine Inequity Undermining Global Economic Recovery. Available online: https://www.who.int/news/item/22-07-2021-vaccine-inequity-undermining-global-economic-recovery (accessed on 2 January 2022).
- Griffin, J.B.; Haddix, M.; Danza, P.; Fisher, R.; Koo, T.H.; Traub, E.; Gounder, P.; Jarashow, C.; Balter, S. SARS-CoV-2 infections and hospitalizations among persons aged≥ 16 years, by vaccination status—Los Angeles County, California, May 1–July 25, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1170. [Google Scholar] [CrossRef]
- Havers, F.P.; Pham, H.; Taylor, C.A.; Whitaker, M.; Patel, K.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Zell, E.; Chai, S.J. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults ≥18 years–COVID-NET, 13 states, January 1–July 24, 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Scobie, H.M.; Johnson, A.G.; Suthar, A.B.; Severson, R.; Alden, N.B.; Balter, S.; Bertolino, D.; Blythe, D.; Brady, S.; Cadwell, B. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status—13 US jurisdictions, April 4–July 17, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1284. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2021, 28, 202–221. [Google Scholar] [CrossRef]
- Hasan, S.A.W. Interim Statement on Booster Doses for COVID-19 Vaccination. Available online: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination---update-22-december-2021 (accessed on 2 January 2022).
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Alagoz, O.; Sethi, A.K.; Patterson, B.W.; Churpek, M.; Alhanaee, G.; Scaria, E.; Safdar, N. The impact of vaccination to control COVID-19 burden in the United States: A simulation modeling approach. PLoS ONE 2021, 16, e0254456. [Google Scholar] [CrossRef]
- Speiser, D.E.; Bachmann, M.F. COVID-19: Mechanisms of Vaccination and Immunity. Vaccines 2020, 8, 404. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical dis-tributions. Postgrad. Med. J. 2021. [Google Scholar] [CrossRef]
- Olliaro, P.; Torreele, E.; Vaillant, M. COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe 2021, 2, e279–e280. [Google Scholar] [CrossRef]
- Africa Centers for Diseases Control and Prevention. Responding to COVID-19 in Africa: Finding the Balance (Part IV) and Calls to action. Available online: https://africacdc.org/download/responding-to-covid-19-in-africa-finding-the-balance-part-iv-and-calls-to-action/ (accessed on 4 January 2022).
- Centers for Disease Control and Prevention. COVID-19 Vaccine Effectiveness Research. Available online: https://www.cdc.gov/vaccines/covid-19/effectiveness-research/protocols.html (accessed on 7 January 2022).
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 495. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C. Effectiveness of mRNA COVID-19 vaccine among US health care personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.; Cravioto, A.; Rees, H.; Higgins, J.P.; Boutron, I. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Feikin, D.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.; Huppert, A.; O’Brien, K.; Smith, P.G. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3961378 (accessed on 2 January 2022).
- Ghazy, R.M.; Taha, S.H.N.; Elhadi, Y.A.M. Letter from Egypt. Respirology 2022, 27, 242–244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).