Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solution Preparation
2.2. Animals and Embryo Production
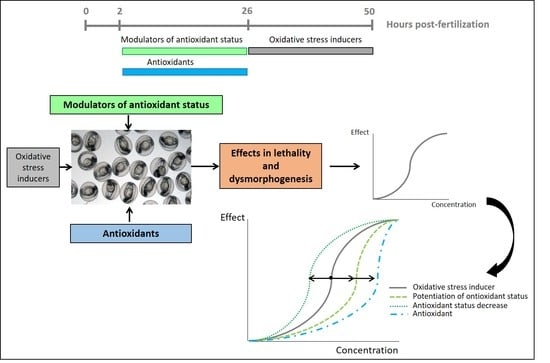
2.3. Exposure of Zebrafish Embryos to Oxidative Stress Related Compounds
2.4. Pre-Exposure of the Embryos to Modulators of Antioxidant Status + Exposure to OS Inducers
2.5. Pre-Exposure of the Embryos to Antioxidant Compounds + Exposure to OS Inducer
2.6. Data Evaluation
3. Results
3.1. Characterization of the Effects of Oxidative Stress Related Compounds in Zebrafish Embryos
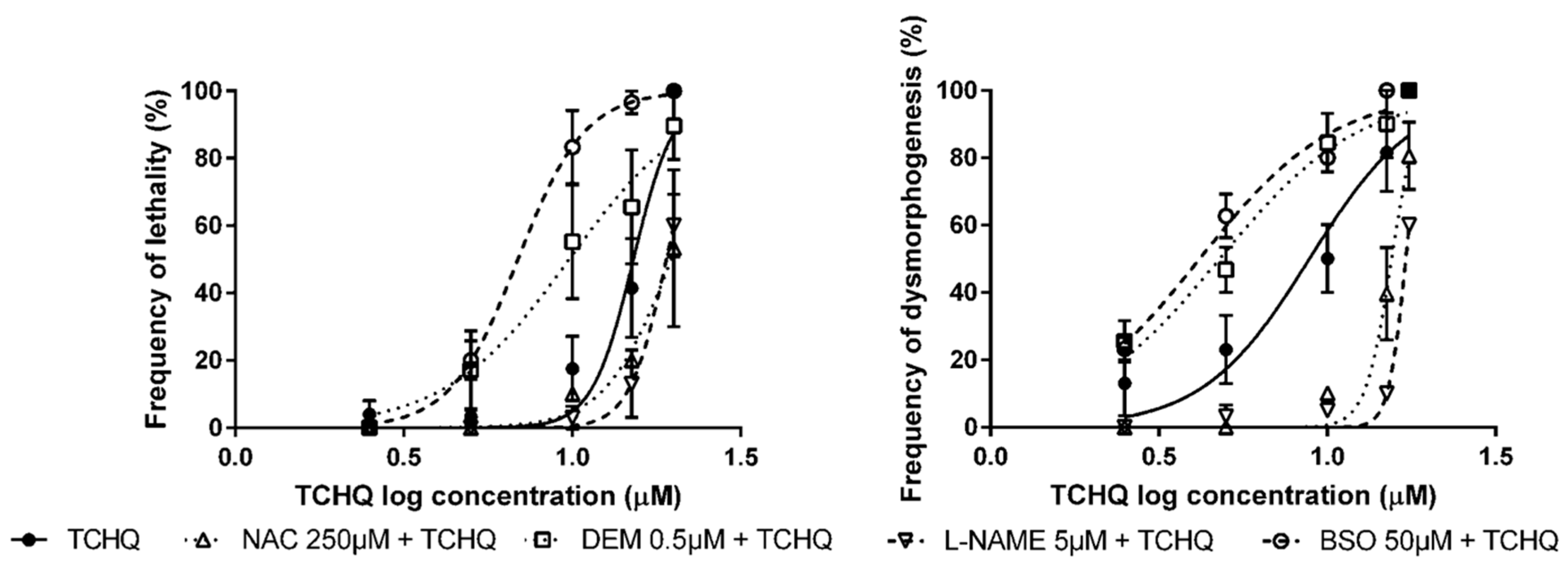
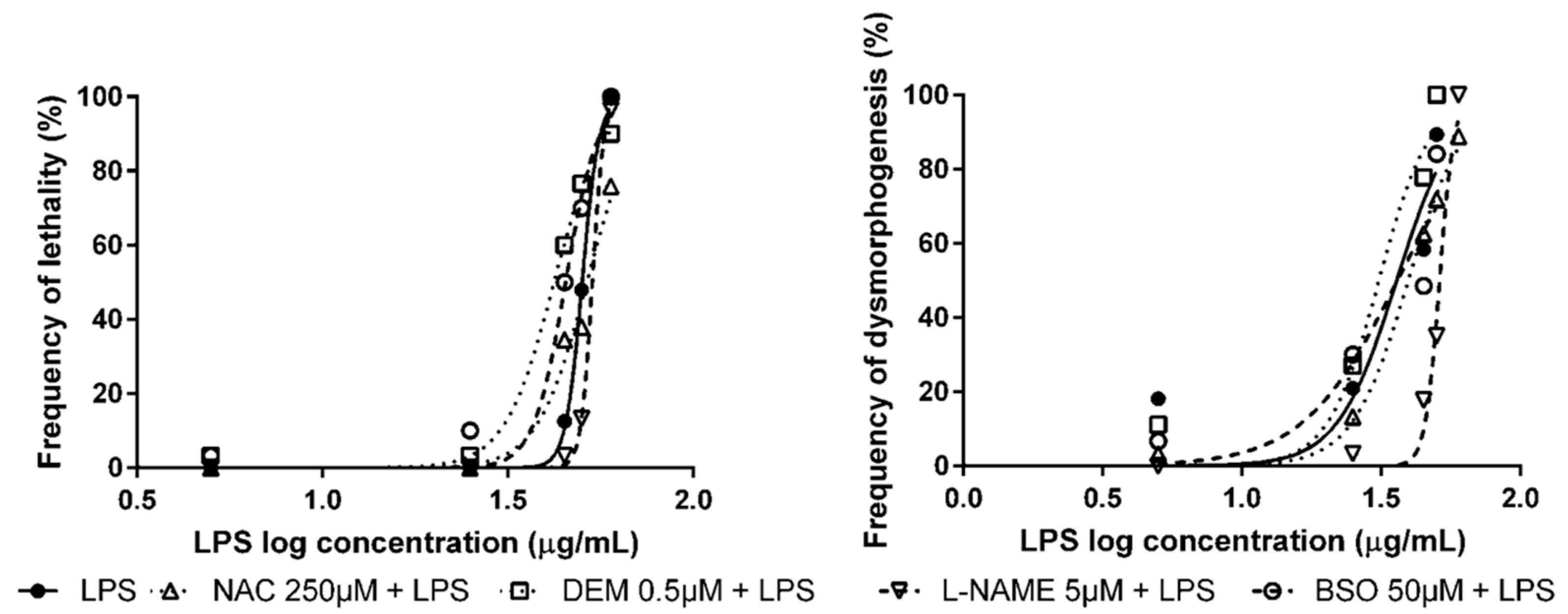
3.2. Pre-Exposure to Modulators of Antioxidant Status + Exposure to OS Inducers
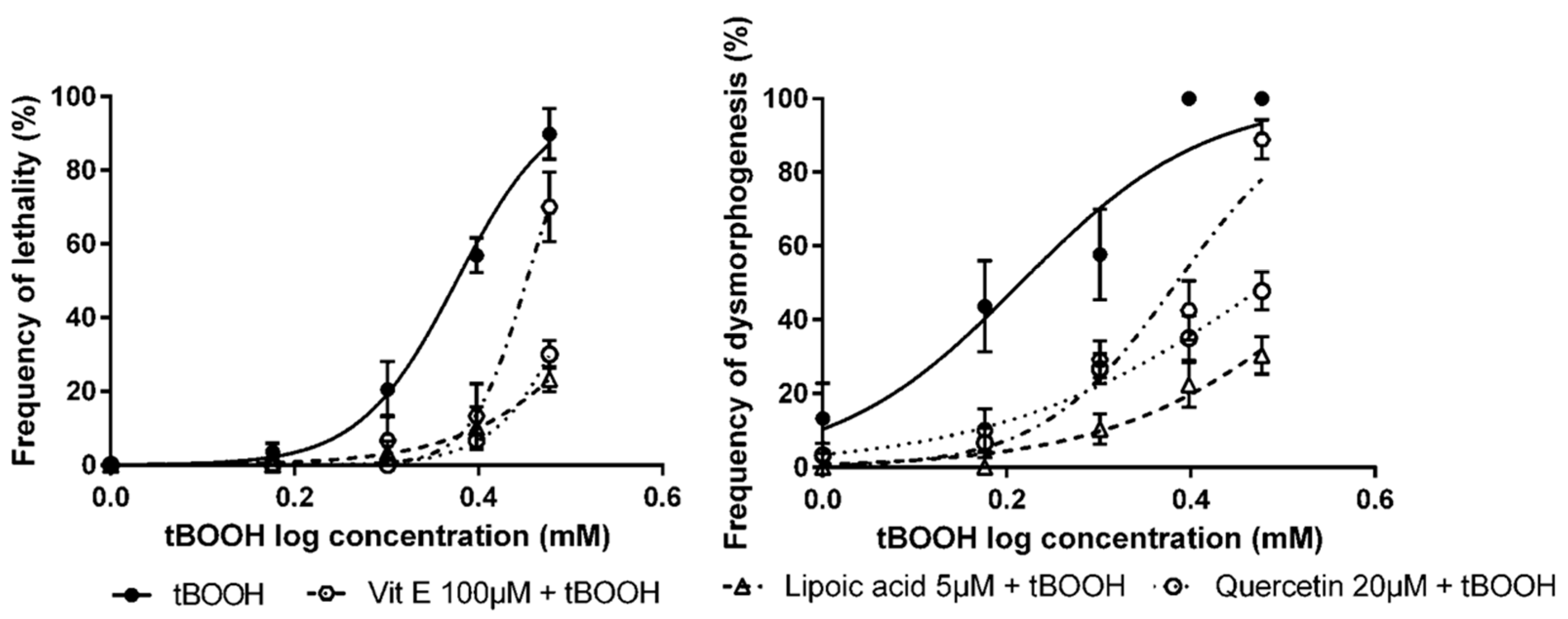
3.3. Detection of Protective Effects of Antioxidant Compounds in Zebrafish Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Überall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Finley, J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Chagas, P.M.; Weber Fulco, B.D.C.; Pesarico, A.P.; Roehrs, J.A.; Wayne, N.C. Bis(phenylimidazolselenazolyl) diselenide as an antioxidant compound: An in vitro and in vivo study. Chem. Biol. Interact. 2015, 233, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Guerra, M.C.; Minghetti, A.; Crespi-Perellino, N.; Pasini, P.; Piazza, F.; Roda, A. Oleuropein evaluated in vitro and in vivo as an antioxidant. Phyther. Res. 1998, 12, 14–24. [Google Scholar] [CrossRef]
- Phulara, S.C.; Shukla, V.; Tiwari, S.; Pandey, R. Bacopa monnieri promotes longevity in caenorhabditis elegans under stress conditions. Pharmacogn. Mag. 2015, 11, 410–416. [Google Scholar] [PubMed]
- Zhang, Z.J.; Cheang, L.C.V.; Wang, M.W.; Lee, S.M.Y. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int. J. Mol. Med. 2011, 27, 195–203. [Google Scholar] [PubMed]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high-throughput biology for 21st century. Toxicol. Curr. Environ. Heal. Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing. A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.L. Zebrafish: From disease modeling to drug discovery. Curr. Opin. Drug Discov. Devel. 2003, 6, 218–223. [Google Scholar]
- Scholz, S.; Fischer, S.; Gündel, U.; Küster, E.; Luckenbach, T.; Voelker, D. The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. 2008, 15, 394–404. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish Immunol. 2011, 31, 904–910. [Google Scholar] [CrossRef]
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 2008, 4, e1000152. [Google Scholar] [CrossRef]
- Reimers, M.J.; La Du, J.K.; Periera, C.B.; Giovanini, J.; Tanguay, R.L. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol. Teratol. 2006, 28, 497–508. [Google Scholar] [CrossRef]
- Xi, Y.; Noble, S.; Ekker, M. Modeling neurodegeneration in zebrafish. Curr. Neurol. Neurosci. Rep. 2011, 11, 274–282. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakajima-Takagi, Y.; Tsujita, T.; Akiyama, S.I.; Wakasa, T.; Mukaigasa, K.; Kaneko, H.; Tamaru, Y.; Yamamoto, M.; Kobayashi, M. Tissue-restricted expression of Nrf2 and its target genes in zebrafish with gene-specific variations in the induction profiles. PLoS ONE 2011, 6, e26884. [Google Scholar] [CrossRef] [PubMed]
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Moreno-Fernández, A.M.; Gomez-Skarmeta, J.L.; de Miguel, M.; Garrido-Maraver, J.; Oropesa-Ávila, M.; Rodríguez-Hernández, Á.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q10 and alpha-tocopherol protect against amitriptyline toxicity. Toxicol. Appl. Pharmacol. 2009, 235, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Seok, S.H.; Baek, M.W.; Lee, H.Y.; Kim, D.J.; Park, S.H.; Lee, H.K.; Park, J.H. Protective effects of vitamin E against 3,3′,4,4’,5-pentachlorobiphenyl (PCB126) induced toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2009, 72, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-Implications for the fish embryo test (FET). Aquat. Toxicol. 2013, 140–141, 229–238. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio--a general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar]
- Teixidó, E.; Piqué, E.; Gómez-Catalán, J.; Llobet, J.M. Assessment of developmental delay in the zebrafish embryo teratogenicity assay. Toxicol. Vitr. 2013, 27, 469–478. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, C.Y.; Tseng, M.C. Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Mar. Pollut. Bull. 2011, 63, 303–308. [Google Scholar] [CrossRef]
- Holmberg, A.; Olsson, C.; Holmgren, S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 2006, 209, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol. Appl. Pharmacol. 2008, 229, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free radicals and antioxidant protection: Mechanisms and significance in toxicology and disease. Hum. Toxicol. 1988, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Murado, M.A. A critical point: The problems associated with the variety of criteria to quantify the antioxidant capacity. J. Agric. Food Chem. 2014, 62, 5472–5484. [Google Scholar] [CrossRef]
- Kanupriya; Prasad, D.; Sai Ram, M.; Sawhney, R.C.; Ilavazhagan, G.; Banerjee, P.K. Mechanism of tert-butylhydroperoxide induced cytotoxicity in U-937 macrophages by alteration of mitochondrial function and generation of ROS. Toxicol. Vitr. 2007, 21, 846–854. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Van Tiem, L.A.; Linney, E.A.; Di Giulio, R.T. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol. Sci. 2009, 109, 217–227. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ho, Y.S.; Chu, S.W.; Lien, H.J.; Liu, T.H.; Lin, J.K. Induction of glutathione depletion, p53 protein accumulation and cellular transformation by tetrachlorohydroquinone, a toxic metabolite of pentachlorophenol. Chem. Biol. Interact. 1997, 105, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lee, C.C.; Chang, W.C.; Liou, H.B.; Ho, Y.S. Oxidative stress and liver toxicity in rats and human hepatoma cell line induced by pentachlorophenol and its major metabolite tetrachlorohydroquinone. Toxicol. Lett. 2001, 122, 157–169. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.H.; Wang, H.; Ji, Y.L.; Ning, H.; Wang, S.F.; Zhang, C.; Lu, J.W.; Duan, Z.H.; Xu, D.X. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol. Sci. 2008, 103, 149–157. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.K.; Jeon, N.J.; Ryu, B.I.; Hwang, J.W.; Choi, E.J.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant effect of Taurine-Rich Paroctopus dofleini extracts through inhibiting ROS production against LPS-induced oxidative stress in vitro and in vivo model. Adv. Exp. Med. Biol. 2017, 975, 1165–1177. [Google Scholar]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Nigam, A.; Bajpai, P.; Kumar, S. Diethyl maleate inhibits MCA+TPA transformed cell growth via modulation of GSH, MAPK, and cancer pathways. Chem Biol Interact. 2014, 219, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J. Biol. Chem. 1982, 257, 13704–13712. [Google Scholar] [PubMed]
- Novoa, B.; Bowman, T.V.; Zon, L.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Davis, B.M.; Richens, J.L.; Vere, K.A.; Petrov, P.G.; Winlove, C.P.; O’shea, P. α-Tocopherols modify the membrane dipole potential leading to modulation of ligand binding by P-glycoprotein 4. J. Lipid. Res. 2015, 56, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 13–14. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Invest. Ophthalmol Vis. Sci. 2011, 22, 1055–1063. [Google Scholar] [CrossRef]
- Rayamajhi, N.; Kim, S.-K.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.-G.; Ryter, S.; Chung, H. Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxidative Med. Cell. Longev. 2013, 2013, 154279. [Google Scholar] [CrossRef]
- Mourabit, S.; Fitzgerald, J.A.; Ellis, R.P.; Takesono, A.; Porteus, C.S.; Trznadel, M.; Metz, J.; Winter, M.J.; Kudoh, T.; Tyler, C.R. New insights into organ-specific oxidative stress mechanisms using a novel biosensor zebrafish. Environ. Int. 2019, 133, 105138. [Google Scholar] [CrossRef]

| Compounds | Range of Concentrations | MTC | LC50 | EC50 | Exposure Window |
|---|---|---|---|---|---|
| OS Inducers | |||||
| Tert-butyl hydroperoxide (tBOOH) | 1–4 mM | n.d.a | 2.4 mM | 1.6 mM | 26–50 hpf |
| Tetrachlorohydroquinone (TCHQ) | 2.5–20 µM | n.d.a | 16.0 µM | 3.9 µM | 26–50 hpf |
| Lipopolysaccharides from Escherichia coli 0111:B4 (LPS) | 5–60 µg/mL | 25 µg/mL | 50.1 µg/mL | 35.9 µg/mL | 26–50 hpf |
| Modulators of Antioxidant Status | |||||
| n-acetyl-l-cysteine (NAC) | 50–2500 µM | 250 µM | 1874 µM | 920.6 µM | 2–26 hpf |
| Diethyl maleate (DEM) | 0.1–100 µM | 0.5 µM | n.d.b | 1.5 µM | 2–26 hpf |
| Nω-nitro l-arginine methyl ester hydrochloride (L-NAME) | 0.1–100 µM | 5 µM | n.d.c | 44.36 µM | 2–26 hpf |
| DL-buthionine sulfoximine (BSO) | 1–5000 µM | 50 µM | n.d. c | 2722 µM | 2–26 hpf |
| Antioxidants | |||||
| Vit. E | 1–1000 µM | 100 µM | n.d.d | n.d.d | 2–26 hpf |
| Lipoic acid | 0.1–1000 µM | 5 µM | 116.4 µM | n.d.c | 2–26 hpf |
| Quercetin | 0.1–30 µM e | 20 µM | n.d.d | n.d.d | 2–26 hpf |
| Modulator of Antioxidant Status | OS Inducer | LC50 (95% CI) | EC50 (95% CI) |
|---|---|---|---|
| None 1 | Tert-butyl hydroperoxide (tBOOH) | 2.38 mM (2.28–2.48) | 1.64 mM (1.44–1.87) |
| n-acetyl-l-cysteine (NAC) | n.d. | 2.28 mM ** (2.11–2.46) | |
| Nω-nitro l-arginine methyl ester hydrochloride (L-NAME) | n.d. | 3.17 mM *** (2.85–3.52) | |
| Diethyl maleate (DEM) | 2.06 mM * (1.78–2.38) | 1.17 mM ** (1.07–1.29) | |
| DL-buthionine sulfoximine (BSO) | 1.95 mM *** (1.85–2.05) | 1.20 mM * (1.07–1.33) | |
| None | Tetrachlorohydroquinone (TCHQ) | 15.2 µM (13.8–16.7) | 8.84 µM (7.15–10.9) |
| NAC | 19.6 µM * (16.6–23.3) | 15.5 µM *** (14.8-16.3) | |
| L-NAME | 19.0 µM * (17.3–20.9) | 17.1 µM *** (16.9–17.3) | |
| DEM | 9.78 µM ** (7.31–13.1) | 4.79 µM * (3.88–5.91) | |
| BSO | 6.89 µM *** (6.13–7.75) | 4.17 µM ** (3.62–4.81) | |
| None 1 | Lipopolysaccharides from Escherichia coli 0111:B4 (LPS) | 50.1 µg/mL (48.6–51.8) | 36.0 µg/mL (28.4–45.6) |
| NAC | 51.6 µg/mL * (48.8–54.5) | 39.6 µg/mL (35.0–44.8) | |
| L-NAME | 53.4 µg/mL * (51.9–55.0) | 51.3 µg/mL ** (49.6–53.0) | |
| DEM | 42.1 µg/mL *** (37.9–46.8) | 31.1 µg/mL (26.3–36.6) | |
| BSO | 45.2 µg/mL ** (43.2–47.4) | 36.2 µg/mL (29.4–44.5) |
| Antioxidant Compounds | OS Inducer | LC50 (95% CI) | EC50 (95% CI) |
|---|---|---|---|
| None 1 | Tert-butyl hydroperoxide (tBOOH) | 2.38 mM (2.28–2.48) | 1.64 mM (1.44–1.87) |
| Vitamin E | 2.83 mM *** (2.70–2.69) | 2.42 mM *** (2.17–2.70) | |
| Lipoic acid | 3.72 mM *** (3.14–4.40) | 3.70 mM *** (3.03–4.51) | |
| Quercetin | 3.26 mM *** (2.83–3.76) | 3.05 mM *** (2.64–3.54) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boix, N.; Teixido, E.; Pique, E.; Llobet, J.M.; Gomez-Catalan, J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants 2020, 9, 721. https://doi.org/10.3390/antiox9080721
Boix N, Teixido E, Pique E, Llobet JM, Gomez-Catalan J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants. 2020; 9(8):721. https://doi.org/10.3390/antiox9080721
Chicago/Turabian StyleBoix, Nuria, Elisabet Teixido, Ester Pique, Juan Maria Llobet, and Jesus Gomez-Catalan. 2020. "Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish" Antioxidants 9, no. 8: 721. https://doi.org/10.3390/antiox9080721
APA StyleBoix, N., Teixido, E., Pique, E., Llobet, J. M., & Gomez-Catalan, J. (2020). Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants, 9(8), 721. https://doi.org/10.3390/antiox9080721