Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy and Selection of Studies
2.2. Data Extraction and Assessment of Risk of Bias
2.3. Data Analysis
3. Results
3.1. Search Results
3.2. Assessment of the Risk of Bias
3.3. Animal and Antioxidant Characteristics of the Included Studies
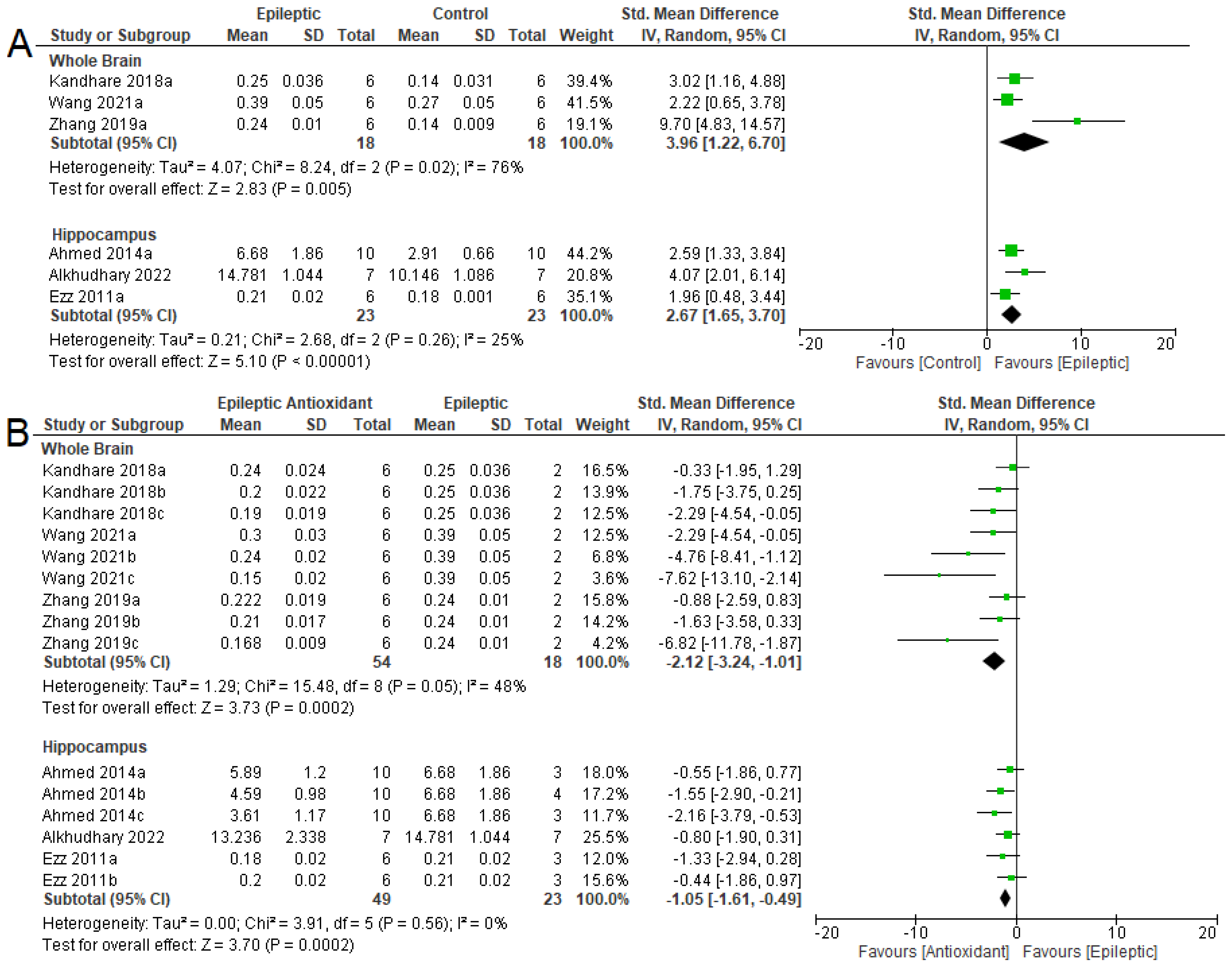
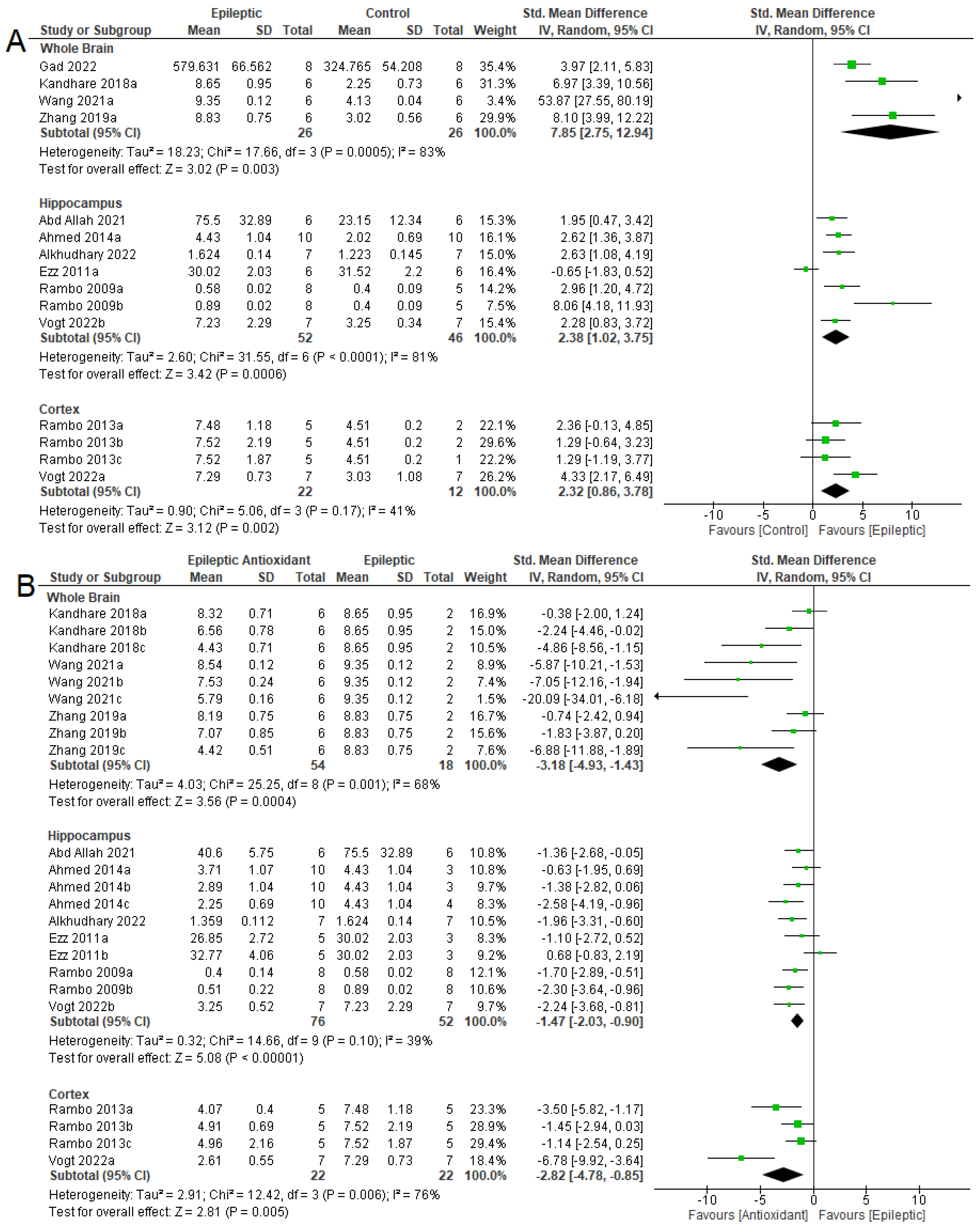
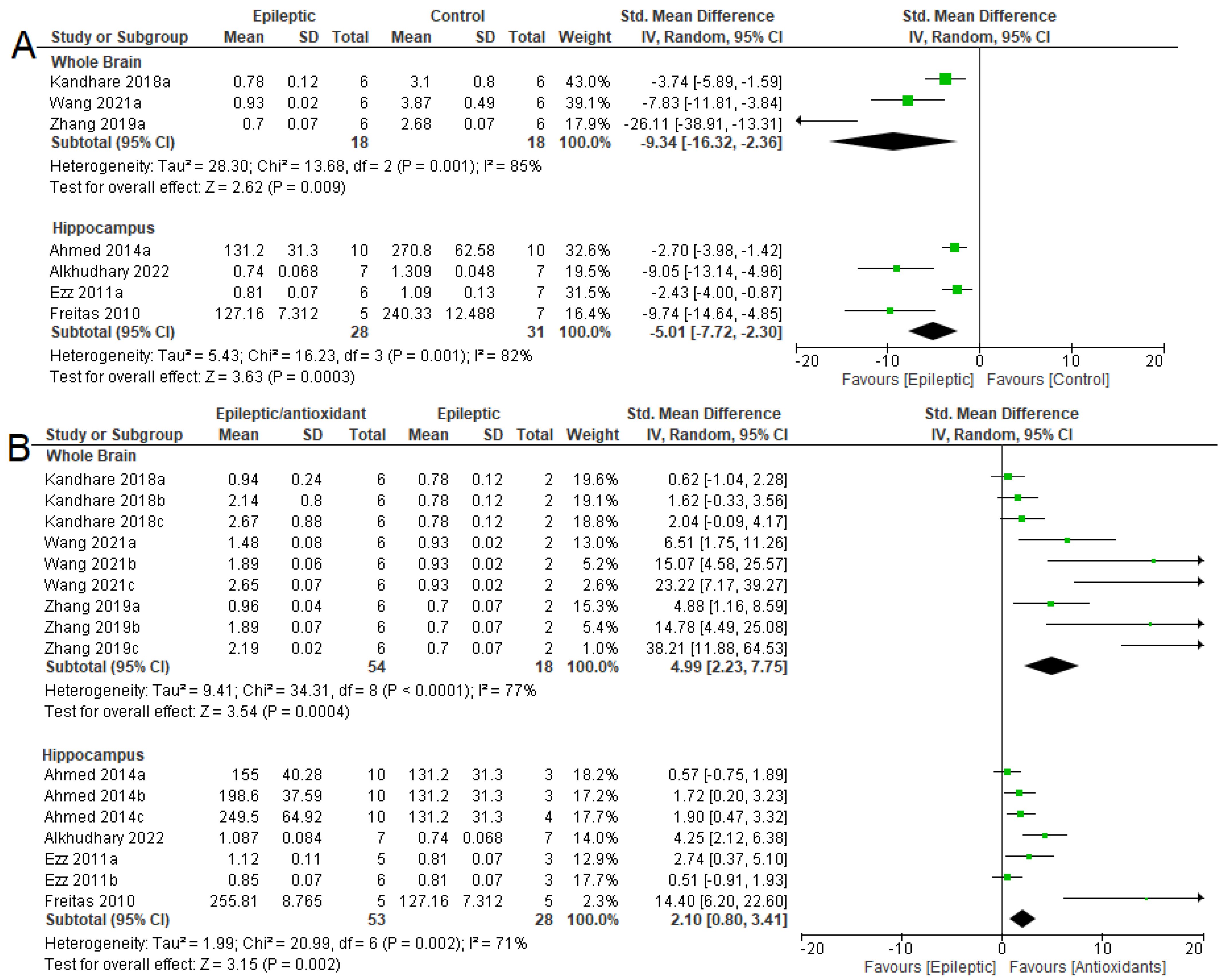
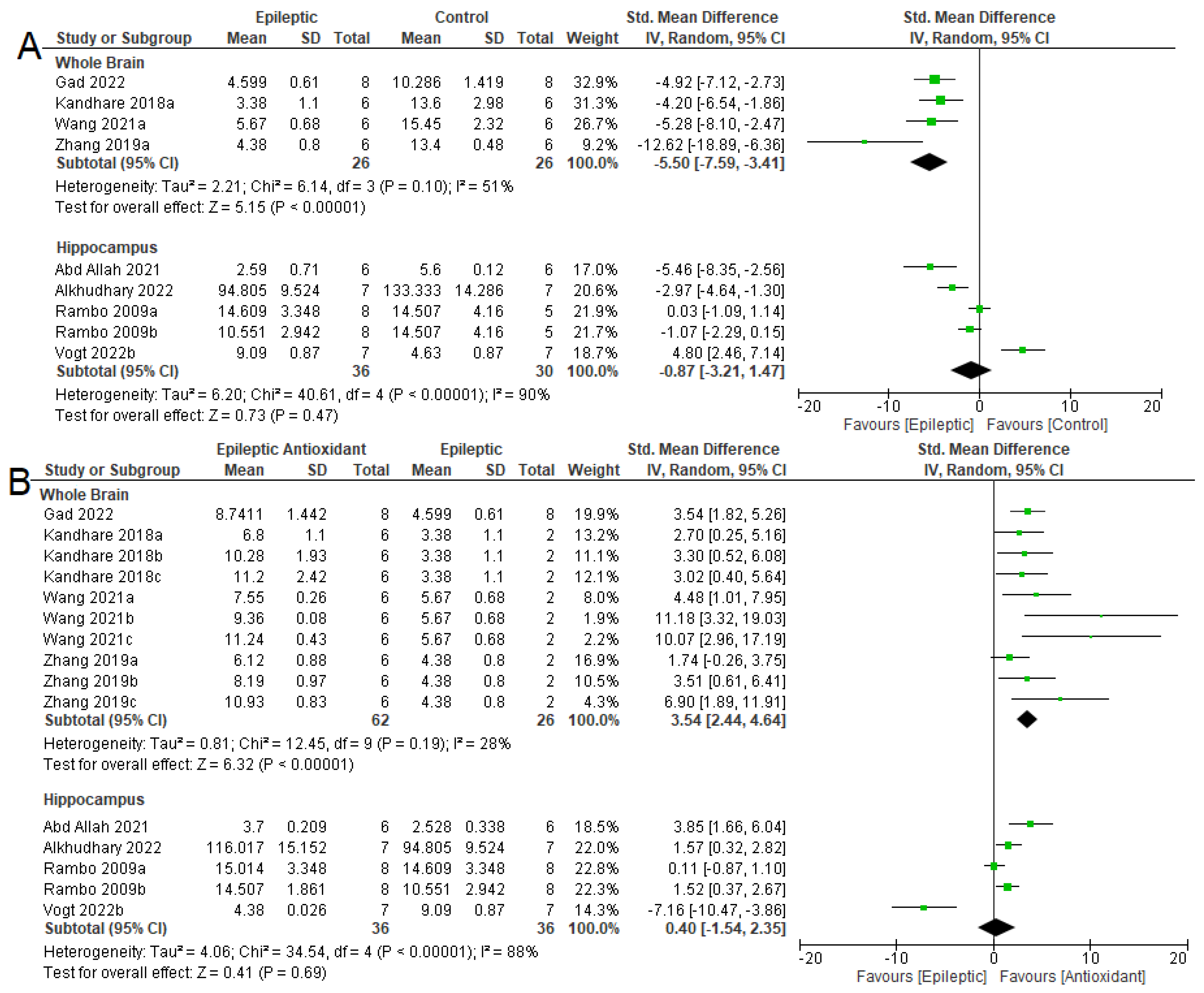
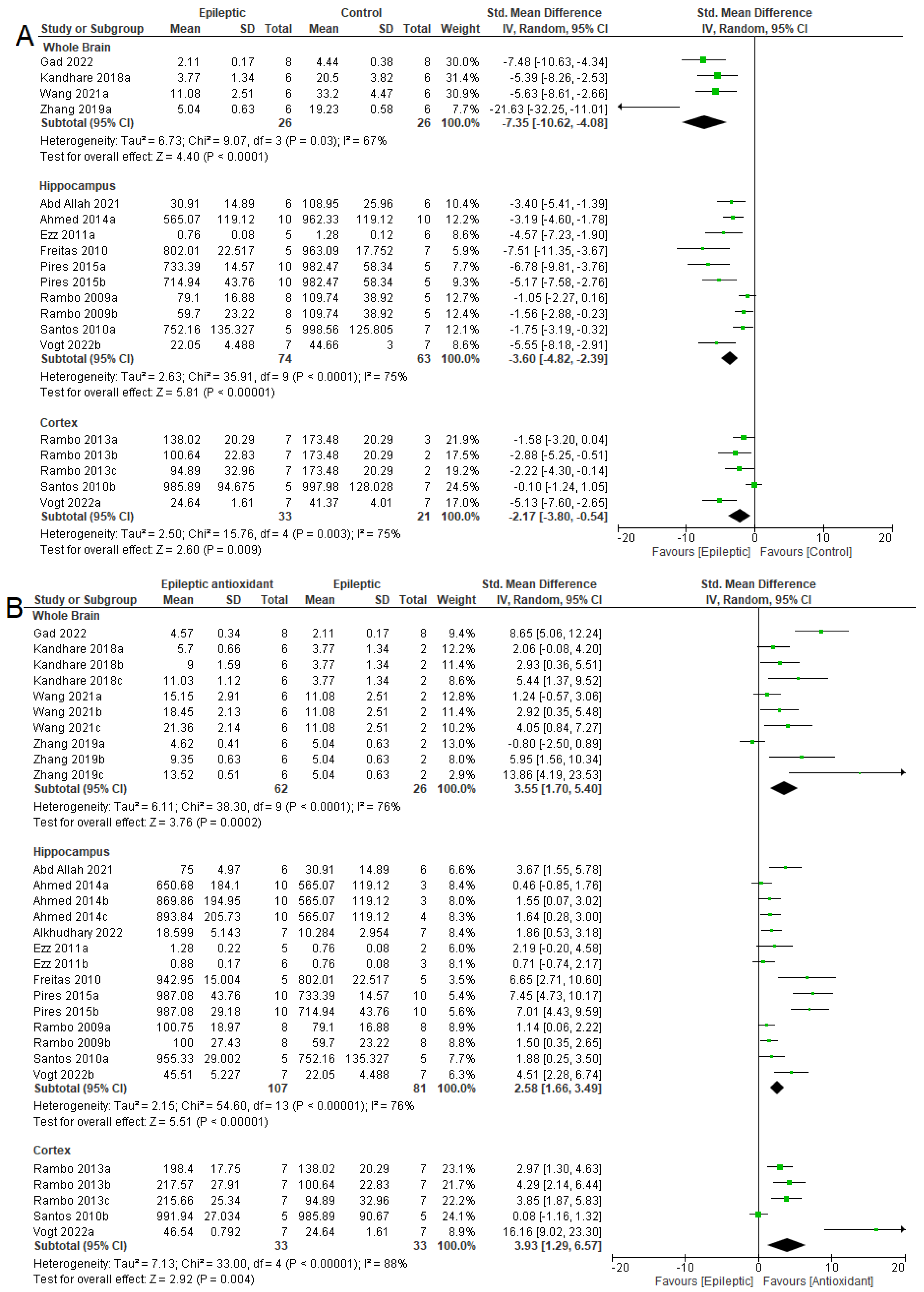
3.4. Biochemical Parameters
3.4.1. Oxidizing Agent
3.4.2. Antioxidant System
3.4.3. NKA Activity
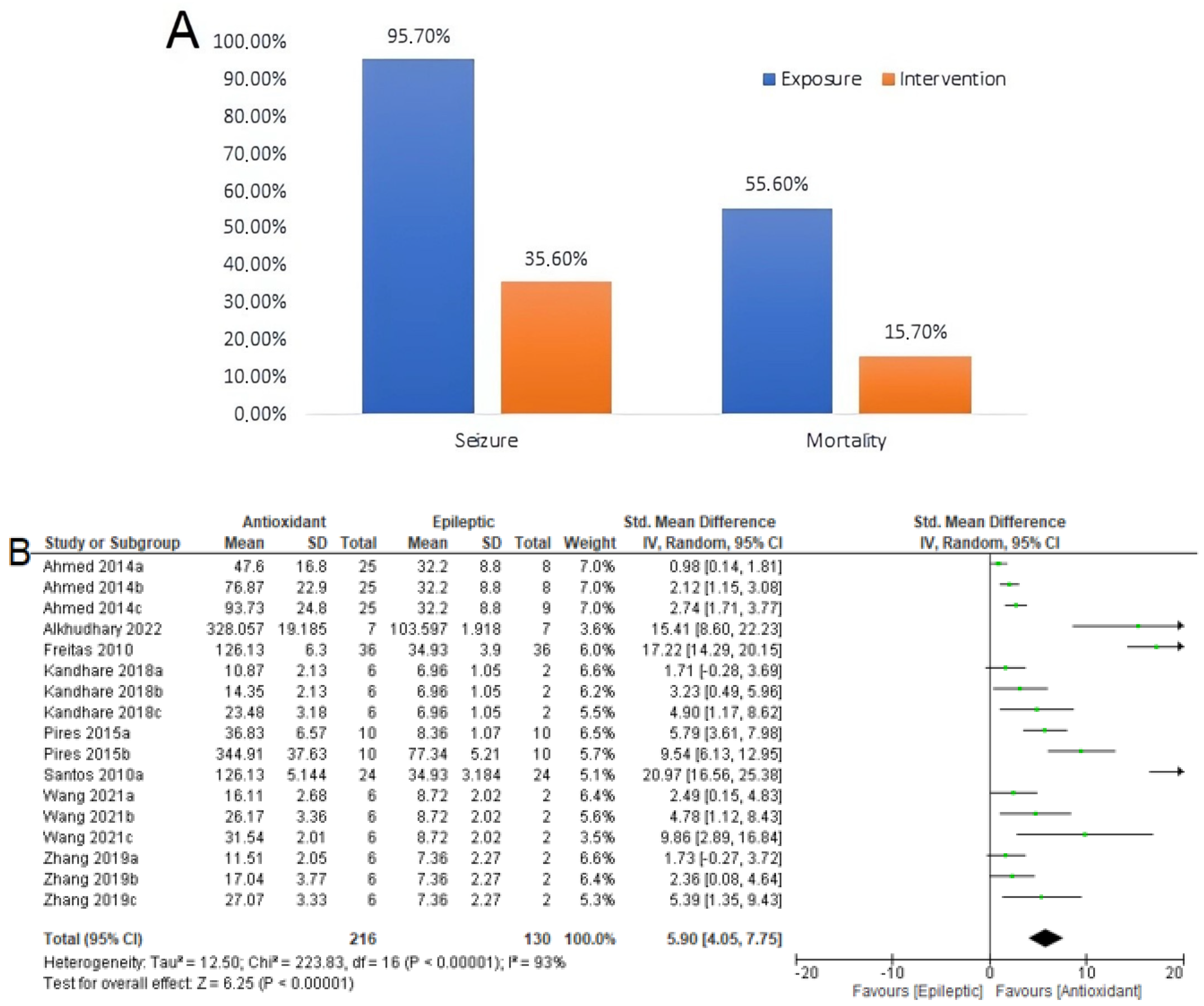
3.5. Seizure-Related Outcomes and Mortality
3.6. Summary of Meta-Analyses
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in Adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Fisher, R.S.; Van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Zhou, H.H.; Jin, W.L. Redox-Related Neuronal Death and Crosstalk as Drug Targets: Focus on Epilepsy. Front. Neurosci. 2019, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative Stress in Epilepsy. Expert Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef]
- Realmuto, S.; Zummo, L.; Cerami, C.; Agrò, L.; Dodich, A.; Canessa, N.; Zizzo, A.; Fierro, B.; Daniele, O. Social Cognition Dysfunctions in Patients with Epilepsy: Evidence from Patients with Temporal Lobe and Idiopathic Generalized Epilepsies. Epilepsy Behav. 2015, 47, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Wu, Y.; Li, T.; Wang, W. Salidroside Shows Anticonvulsant and Neuroprotective Effects by Activating the Nrf2-ARE Pathway in a Pentylenetetrazol-Kindling Epileptic Model. Brain Res. Bull. 2020, 164, 14–20. [Google Scholar] [CrossRef]
- Yang, D.Q.; Zuo, Q.N.; Wang, T.; Xu, D.; Lian, L.; Gao, L.J.; Wan, C.; Chen, L.; Wen, F.Q.; Shen, Y.C. Mitochondrial-Targeting Antioxidant SS-31 Suppresses Airway Inflammation and Oxidative Stress Induced by Cigarette Smoke. Oxid. Med. Cell. Longev. 2021, 2021, 6644238. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 2020, 1057–1073. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Fiorito, G.; Vlaanderen, J.; Polidoro, S.; Gulliver, J.; Galassi, C.; Ranzi, A.; Krogh, V.; Grioni, S.; Agnoli, C.; Sacerdote, C.; et al. Oxidative Stress and Inflammation Mediate the Effect of Air Pollution on Cardio- and Cerebrovascular Disease: A Prospective Study in Nonsmokers. Environ. Mol. Mutagen. 2018, 59, 234–246. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Kovac, S.; Kostova, A.T.D.; Herrmann, A.M.; Melzer, N.; Meuth, S.G.; Gorji, A. Metabolic and Homeostatic Changes in Seizures and Acquired Epilepsy—Mitochondria, Calcium Dynamics and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 1935. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, Pathophysiology and Development of Therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Moncada, S.; Bolaños, J.P. Nitric Oxide, Cell Bioenergetics and Neurodegeneration. J. Neurochem. 2006, 97, 1676–1689. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox. Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- McCoy, C.R.; Sabbagh, M.N.; Huaman, J.P.; Pickrell, A.M.; Clinton, S.M. Oxidative Metabolism Alterations in the Emotional Brain of Anxiety-Prone Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109706. [Google Scholar] [CrossRef] [PubMed]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under Oxidative Stress: Molecular Mechanisms of Injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and Pathology in Epilepsy: Clinical and Basic Perspectives. In Neurodegenerative Diseases; Advances in Neurobiology; Springer: Cham, Switzerland, 2017; Volume 15, pp. 317–334. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in Structure, Diversity in Function. Am. J. Physiol. 1998, 44, 633–650. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, R.M. Lipoic Acid Alters δ-Aminolevulinic Dehydratase, Glutathione Peroxidase and Na+,K+-ATPase Activities and Glutathione-Reduced Levels in Rat Hippocampus after Pilocarpine-Induced Seizures. Cell. Mol. Neurobiol. 2010, 30, 381–387. [Google Scholar] [CrossRef]
- Grisar, A’.B.T.; Guillaume, A’.D.; Delgado-Escueta, A.V. Contribution of Na +,K +-ATPase to Focal Epilepsy: A Brief Review. Epilepsy Res. 1992, 12, 141–149. [Google Scholar] [CrossRef]
- Barbosa, K.; Costa, N.; Alfenas, R.; DE Paula, S.; Minim, V.; Bressan, J. Revista de Nutrição Oxidative Stress: Concept, Implications and Modulating Factors. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Gaby, A.R. Natural Approaches to Epilepsy. Altern. Med. Rev. 2007, 12, 9. [Google Scholar] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Maharjan, S.; Wang, Q.; Sun, Y.; Han, X.; Wang, S.; Mao, Z.; Xin, Y.; Zhang, B. α-Lipoic Acid Promotes Neurological Recovery after Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage. Cell. Physiol. Biochem. 2017, 43, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Hegde, M.V.; Katyare, S.S. Mitochondrial Dysfunction in Psychiatric and Neurological Diseases: Cause(s), Consequence(s), and Implications of Antioxidant Therapy. BioFactors 2013, 39, 392–406. [Google Scholar] [CrossRef]
- Haghnejad Azar, A.; Oryan, S.; Bohlooli, S.; Panahpour, H. Alpha-Tocopherol Reduces Brain Edema and Protects Blood-Brain Barrier Integrity Following Focal Cerebral Ischemia in Rats. Med. Princ. Pract. 2017, 26, 17–22. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, Y.; Huo, T.; Fang, Y.; Jiao, X.; Yuan, M.; Jiang, H. Effects of Realgar on GSH Synthesis in the Mouse Hippocampus: Involvement of System XAG−, System XC−, MRP-1 and Nrf2. Toxicol. Appl. Pharmacol. 2016, 308, 91–101. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Clinical and Metabolic Status in Patients with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef]
- Gutzmann, H.; Hadler, D. Sustained Efficacy and Safety of Idebenone in the Treatment of Alzheimer’s Disease: Update on a 2-Year Double-Blind Multi Centre Study. In Alzheimer’s Disease-From Basic Research to Clinical Applications; Springer: Vienna, Austria, 1998. [Google Scholar]
- Essawy, A.E.; El-Sayed, S.A.; Tousson, E.; Abd El-gawad, H.S.; Alhasani, R.H.; Abd Elkader, H.T.A.E. Anti-Kindling Effect of Ginkgo Biloba Leaf Extract and L-Carnitine in the Pentylenetetrazol Model of Epilepsy. Environ. Sci. Pollut. Res. 2022, 29, 48573–48587. [Google Scholar] [CrossRef]
- Asgharzade, S.; Rabiei, Z.; Rabiei, S.; Bijad, E.; Rafieian-Kopaei, M. Therapeutic Effects of Oleuropein in Improving Seizure, Oxidative Stress and Cognitive Disorder in Pentylenetetrazole Kindling Model of Epilepsy in Mice. Iran. J. Pharm. Res. 2020, 19, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Costello, D.J.; Delanty, N. Oxidative Injury in Epilepsy: Potential for Antioxidant Therapy? Expert Rev. Neurother. 2004, 4, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Stern, H.; Oren, H.; Peled, N.; Garty, B.Z. Effect of Melatonin on Seizure Frequency in Intractable Epilepsy: A Pilot Study. J. Child Neurol. 2012, 27, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Mehvari, J.; Motlagh, F.; Najafi, M.; Ghazvini, M.A.; Naeini, A.; Zare, M. Effects of Vitamin E on Seizure Frequency, Electroencephalogram Findings, and Oxidative Stress Status of Refractory Epileptic Patients. Adv. Biomed. Res. 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, Y.K.; Agarwal, S.; Aneja, S.; Kohli, K. A Randomized, Double-Blind, Placebo Controlled Trial of Melatonin Add-on Therapy in Epileptic Children on Valproate Monotherapy: Effect on Glutathione Peroxidase and Glutathione Reductase Enzymes. Br. J. Clin. Pharmacol. 2004, 58, 542–547. [Google Scholar] [CrossRef]
- Raju, G.B.; Behari, M.; Prasad, K.; Ahuja, G.K. Randomized, Double-Blind, Placebo-Controlled, Clinical Trial of D-a-Tocopherol (Vitamin E) as Add-on Therapy in Uncontrolled Epilepsy. Epilepsia 1994, 35, 368–372. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, K.O. The Pilocarpine Model of Temporal Lobe Epilepsy and EEG Monitoring Using Radiotelemetry System in Mice. J. Vis. Exp. 2018, 132, e56831. [Google Scholar] [CrossRef]
- Lévesque, M.; Avoli, M. The Kainic Acid Model of Temporal Lobe Epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef]
- Raol, Y.H.; Brooks-Kayal, A.R. Experimental Models of Seizures and Epilepsies. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 105, pp. 57–82. [Google Scholar] [CrossRef]
- Turski, L.; Ikonomidou, C.; Turski, W.A.; Bortolotto, Z.A.; Cavalheiro, E.A. Review: Cholinergic Mechanisms and Epileptogenesis. The Seizures Induced by Pilocarpine: A Novel Experimental Model of Intractable Epilepsy. Synapse 1989, 3, 154–171. [Google Scholar] [CrossRef]
- Tashakori-Miyanroudi, M.; Ramazi, S.; Hashemi, P.; Nazari-Serenjeh, M.; Baluchnejadmojarad, T.; Roghani, M. Acetyl-L-Carnitine Exerts Neuroprotective and Anticonvulsant Effect in Kainate Murine Model of Temporal Lobe Epilepsy. J. Mol. Neurosci. 2022, 72, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Preclinical Assessment of Proconvulsant Drug Activity and Its Relevance for Predicting Adverse Events in Humans. Eur. J. Pharmacol. 2009, 610, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. Web Plot Digitizer, Version 4.5; EUA: California. 2021. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 5 April 2022).
- Cochrane Collaboration. Review Manager (RevMan), Version 5.4; Cochrane Collaboration: London, UK, 2020.
- Riley, R.D.; Higgins, J.P.; Deeks’, J.J. Interpretation of Random Effects Meta-Analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef]
- Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 4 July 2022).
- Schneider Oliveira, M.; Flávia Furian, A.; Freire Royes, L.F.; Rechia Fighera, M.; De Carvalho Myskiw, J.; Gindri Fiorenza, N.; Mello, C.F. Ascorbate Modulates Pentylenetetrazol-Induced Convulsions Biphasically. Neuroscience 2004, 128, 721–728. [Google Scholar] [CrossRef]
- Fighera, M.R.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S.; Fiorenza, N.G.; Frussa-Filho, R.; Petry, J.C.; Coelho, R.C.; Mello, C.F. GM1 Ganglioside Prevents Seizures, Na+,K+-ATPase Activity Inhibition and Oxidative Stress Induced by Glutaric Acid and Pentylenetetrazole. Neurobiol. Dis. 2006, 22, 611–623. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Bortolatto, C.F.; Nogueira, C.W.; Savegnago, L. Anticonvulsant and Antioxidant Effects of 3-Alkynyl Selenophene in 21-Day-Old Rats on Pilocarpine Model of Seizures. Brain Res. Bull. 2009, 79, 281–287. [Google Scholar] [CrossRef]
- Rambo, L.M.; Ribeiro, L.R.; Oliveira, M.S.; Furian, A.F.; Lima, F.D.; Souza, M.A.; Silva, L.F.A.; Retamoso, L.T.; Corte, C.L.D.; Puntel, G.O.; et al. Additive Anticonvulsant Effects of Creatine Supplementation and Physical Exercise against Pentylenetetrazol-Induced Seizures. Neurochem. Int. 2009, 55, 333–340. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Roman, S.S.; Bortolatto, C.F.; Nogueira, C.W. Anticonvulsant Effect of (E)-2-Benzylidene-4-Phenyl-1,3-Diselenole in a Pilocarpine Model in Mice. Life Sci. 2010, 87, 620–627. [Google Scholar] [CrossRef]
- de Sales Santos, Í.M.; da Rocha Tomé, A.; Feitosa, C.M.; de Souza, G.F.; Feng, D.; de Freitas, R.M.; Jordán, J. Lipoic Acid Blocks Seizures Induced by Pilocarpine via Increases in δ-Aminolevulinic Dehydratase and Na+, K+-ATPase Activity in Rat Brain. Pharmacol. Biochem. Behav. 2010, 95, 88–91. [Google Scholar] [CrossRef]
- Aboul Ezz, H.S.; Khadrawy, Y.A.; Noor, N.A. The Neuroprotective Effect of Curcumin and Nigella Sativa Oil against Oxidative Stress in the Pilocarpine Model of Epilepsy: A Comparison with Valproate. Neurochem. Res. 2011, 36, 2195–2204. [Google Scholar] [CrossRef]
- Bortolatto, C.F.; Jesse, C.R.; Wilhelm, E.A.; Ribeiro, L.R.; Rambo, L.M.; Royes, L.F.F.; Roman, S.S.; Nogueira, C.W. Protective Effect of 2,2′-Dithienyl Diselenide on Kainic Acid-Induced Neurotoxicity in Rat Hippocampus. Neuroscience 2011, 193, 300–309. [Google Scholar] [CrossRef]
- Souza, M.A.; Mota, B.C.; Gerbatin, R.R.; Rodrigues, F.S.; Castro, M.; Fighera, M.R.; Royes, L.F.F. Antioxidant Activity Elicited by Low Dose of Caffeine Attenuates Pentylenetetrazol-Induced Seizures and Oxidative Damage in Rats. Neurochem. Int. 2013, 62, 821–830. [Google Scholar] [CrossRef]
- Della-Pace, I.D.; Rambo, L.M.; Ribeiro, L.R.; Saraiva, A.L.L.; De Oliveira, S.M.; Silva, C.R.; Villarinho, J.G.; Rossato, M.F.; Ferreira, J.; De Carvalho, L.M.; et al. Triterpene 3β, 6β, 16β Trihidroxilup-20(29)-Ene Protects against Excitability and Oxidative Damage Induced by Pentylenetetrazol: The Role of Na+, K+-ATPase Activity. Neuropharmacology 2013, 67, 455–464. [Google Scholar] [CrossRef]
- Rambo, L.M.; Ribeiro, L.R.; Della-Pace, I.D.; Stamm, D.N.; Da Rosa Gerbatin, R.; Prigol, M.; Pinton, S.; Nogueira, C.W.; Furian, A.F.; Oliveira, M.S.; et al. Acute Creatine Administration Improves Mitochondrial Membrane Potential and Protects against Pentylenetetrazol-Induced Seizures. Amino Acids 2013, 44, 857–868. [Google Scholar] [CrossRef]
- Ahmed, M.A.E. Neuroprotective Effects of Idebenone against Pilocarpine-Induced Seizures: Modulation of Antioxidant Status, DNA Damage and Na+, K+-ATPase Activity in Rat Hippocampus. Neurochem. Res. 2014, 39, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; de Almeida, A.A.C.; Silva, O.A.; Cerqueira, G.S.; de Sousa, D.P.; Pires, R.M.C.; Satyal, P.; de Freitas, R.M. Neuropharmacological Effects of Carvacryl Acetate on δ-Aminolevulinic Dehydratase, Na+, K+-ATPase Activities and Amino Acids Levels in Mice Hippocampus after Seizures. Chem. Biol. Interact. 2015, 226, 49–57. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Anti-Epileptic Effect of Morin against Experimental Pentylenetetrazol-Induced Seizures via Modulating Brain Monoamines and Oxidative Stress. Asian Pac. J. Trop. Biomed. 2018, 8, 352–359. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Kandhare, A.; Mukherjee, A.; Guo, G.; Bodhankar, S. Elucidation of the Molecular Mechanism of Tempol in Pentylenetetrazol-Induced Epilepsy in Mice: Role of Gamma-Aminobutyric Acid, Tumor Necrosis Factor-Alpha, Interleukin-1β and c-Fos. Pharmacogn Mag. 2018, 14, S520–S527. [Google Scholar] [CrossRef]
- Tao, Z.; Chun-Yan, H.; Hua, P.; Bin-Bin, Y.; Xiaoping, T. Phyllathin From Phyllanthus Amarus Ameliorates Epileptic Convulsion and Kindling Associated Post-Ictal Depression in Mice via Inhibition of NF-ΚB/TLR-4 Pathway. Dose-Response 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, H.N.; Abdul-Hamid, M.; Mahmoud, A.M.; Abdel-Reheim, E.S. Melissa Officinalis L. Ameliorates Oxidative Stress and Inflammation and Upregulates Nrf2/HO-1 Signaling in the Hippocampus of Pilocarpine-Induced Rats. Environ. Sci. Pollut. Res. 2022, 29, 2214–2226. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Shi, Y.; Yan, M.; Rengarajan, T.; Feng, X. Amomum Tsaoko Fruit Extract Exerts Anticonvulsant Effects through Suppression of Oxidative Stress and Neuroinflammation in a Pentylenetetrazol Kindling Model of Epilepsy in Mice. Saudi J. Biol. Sci. 2021, 28, 4247–4254. [Google Scholar] [CrossRef]
- Vogt, A.G.; de Oliveira, R.L.; Voss, G.T.; Blödorn, G.B.; Alves, D.; Wilhelm, E.A.; Luchese, C. QCTA-1, a Quinoline Derivative, Ameliorates Pentylenetetrazole-Induced Kindling and Memory Comorbidity in Mice: Involvement of Antioxidant System of Brain. Pharmacol. Biochem. Behav. 2022, 215, 173357. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhayri, A.; Abdel Moneim, A.E.; Rizk, S.; Bauomy, A.A.; Dkhil, M.A. The Neuroprotective Effect Associated with Echinops Spinosus in an Acute Seizure Model Induced by Pentylenetetrazole. Neurochem. Res. 2023, 48, 273–283. [Google Scholar] [CrossRef]
- Gad, R.A.; Abdel-Reheim, E.S.; Ebaid, H.; Alhazza, I.M.; Abuelsaad, A.S.A. Mitigating Effects of Passiflora incarnata on Oxidative Stress and Neuroinflammation in Case of Pilocarpine-Induced Status Epilepticus Model. J. King Saud Univ. Sci. 2022, 34, 101886. [Google Scholar] [CrossRef]
- Garthwaite, J. Concepts of Neural Nitric Oxide-Mediated Transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Wang, G.; Gallo, E.F.; Anrather, J.; Zhou, P.; Pickel, V.M.; Iadecola, C. NMDA Receptor Activation Increases Free Radical Production through Nitric Oxide and NOX2. J. Neurosci. 2009, 29, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, E.K. Molecular Biology of Glutamate Receptors in the Central Nervous System and Their Role in Excitotoxicity, Oxidative Stress and Aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G.; Chinopoulos, C.; Lorenzo, B.J.; Browne, S.E.; Patel, M.S.; Beal, M.F. Mitochondrial α-Ketoglutarate Dehydrogenase Complex Generates Reactive Oxygen Species. J. Neurosci. 2004, 24, 7779–7788. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Cararo-Lopes, M.M.; Kinoshita, P.F.; Quintas, L.E.M.; Lima, L.S.; Andreotti, D.Z.; Scavone, C. Influence of Nitric Oxide–Cyclic GMP and Oxidative STRESS on Amyloid-β Peptide Induced Decrease of Na,K-ATPase Activity in Rat Hippocampal Slices. J. Membr. Biol. 2021, 254, 463–473. [Google Scholar] [CrossRef]
- Petrillo, S.; Pietrafusa, N.; Trivisano, M.; Calabrese, C.; Saura, F.; Gallo, M.G.; Bertini, E.S.; Vigevano, F.; Specchio, N.; Piemonte, F. Imbalance of Systemic Redox Biomarkers in Children with Epilepsy: Role of Ferroptosis. Antioxidants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Low Plasma Antioxidant Status in Patients with Epilepsy and the Role of Antiepileptic Drugs on Oxidative Stress. Ann. Indian Acad. Neurol. 2014, 17, 398–404. [Google Scholar] [CrossRef]
- Hussein, S.A.; Abdel-Mageid, A.D.; Abd-Elhamed, O.M.; Al-Harthy, H.S. Biochemical Role of Curcumin on Kainic Acid-Induced Epilepsy in Male Swiss Albino Mice. Benha. Vet. Med. J. 2014, 27, 225–240. [Google Scholar]
- Reddy, A.; Dubey, A.K.; Handu, S.; Sharma, P.; Mediratta, P.; Ahmed, Q.; Jain, S. Anticonvulsant and Antioxidant Effects of Musa Sapientum Stem Extract on Acute and Chronic Experimental Models of Epilepsy. Pharmacogn. Res. 2018, 10, 49–54. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Wang, N.; Mishra, A.; Li, H.; Liu, H.; Fan, Y.; Liu, N.; Wu, Z. Wogonin Preventive Impact on Hippocampal Neurodegeneration, Inflammation and Cognitive Defects in Temporal Lobe Epilepsy. Saudi J. Biol. Sci. 2020, 27, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Tashakori-Miyanroudi, M.; Nourabadi, D.; Nazari-Serenjeh, M.; Roghani, M.; Baluchnejadmojarad, T. Neuroprotective and Anticonvulsant Effects of Sinomenine in Kainate Rat Model of Temporal Lobe Epilepsy: Involvement of Oxidative Stress, Inflammation and Pyroptosis. J. Chem. Neuroanat. 2020, 108, 101800. [Google Scholar] [CrossRef]
- Moto, F.C.O.; Arsa’a, A.; Ngoupaye, G.T.; Taiwe, G.S.; Njapdounke, J.S.K.; Kandeda, A.K.; Nkantchoua, G.C.N.; Omam Omam, J.P.; Pale, S.; Kouemou, N.E.; et al. Anxiolytic and Antiepileptic Properties of the Aqueous Extract of Cissus Quadrangularis (Vitaceae) in Mice Pilocarpine Model of Epilepsy. Front. Pharmacol. 2018, 9, 751. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Neuroprotective Properties of the Excitatory Amino Acid Carrier 1 (EAAC1). Amino Acids 2013, 45, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Sang, W.S.; Hamby, A.M.; Liu, J.; Wai, Y.C.; Chen, Y.; Swanson, R.A. Neuronal Glutathione Deficiency and Age-Dependent Neurodegeneration in the EAAC1 Deficient Mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Rodríguez-Campuzano, A.G.; Ortega, A. Glutamate Transporters: Critical Components of Glutamatergic Transmission. Neuropharmacology 2021, 192, 108602. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Tokumaru, J.; Yokoyama, H.; Nakajima, A.; Mitsuyama, Y.; Ohya-Nishiguchi, H.; Kamada2, H.; Willmore, L.J. Collapse of Extracellular Glutamate Regulation during Epileptogenesis: Down-Regulation and Functional Failure of Glutamate Transporter Function in Rats with Chronic Seizures Induced by Kainic Acid. J. Neurochem. 2001, 76, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Ingram, E.M.; Wiseman, J.W.; Tessler, S.; Emson, P.C. Reduction of Glial Glutamate Transporters in the Parietal Cortex and Hippocampus of the EL Mouse. J. Neurochem. 2001, 79, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Petr, G.T.; Sun, Y.; Frederick, N.M.; Zhou, Y.; Dhamne, S.C.; Hameed, M.Q.; Miranda, C.; Bedoya, E.A.; Fischer, K.D.; Armsen, W.; et al. Conditional Deletion of the Glutamate Transporter GLT-1 Reveals That Astrocytic GLT-1 Protects against Fatal Epilepsy While Neuronal GLT-1 Contributes Significantly to Glutamate Uptake into Synaptosomes. J. Neurosci. 2015, 35, 5187–5201. [Google Scholar] [CrossRef]
- Tanaka, K.; Watase, K.; Manabe, T.; Yamada, K.; Watanabe, M.; Takahashi, K.; Iwama, H.; Nishikawa, T.; Ichihara, N.; Kikuchi, T.; et al. Epilepsy and Exacerbation of Brain Injury in Mice Lacking the Glutamate Transporter GLT-1. Science 1997, 276, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Oja, S.S.; Janaky, R.; Varga, V.; Saransaari, P. Modulation of Glutamate Receptor Functions by Glutathione. Neurochem. Int. 2000, 37, 299–306. [Google Scholar] [CrossRef]
- Szaroma, W.; Dziubek, K.; Kapusta, E. Effect of N-Methyl-D-Aspartic Acid on Activity of Superoxide Dismutase, Catalase, Glutathione Peroxidase and Reduced Glutathione Level in Selected Organs of the Mouse. Acta Physiol. Hung. 2014, 101, 377–387. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. Inhibition of Copper-Zinc Superoxide Dismutase Activity by Selected Environmental Xenobiotics. Environ. Toxicol. Pharmacol. 2018, 58, 105–113. [Google Scholar] [CrossRef]
- Taiwe, G.S.; Ndieudieu Kouamou, A.L.; Dabole, B.; Ambassa, A.R.M.; Mambou, H.M.A.Y.; Bila, R.B.; Tchoya, T.B.; Menanga, J.R.; Djomeni Dzeufiet, P.D.; Ngo Bum, E. Protective Effects of Anthocleista Djalonensis Extracts against Pentylenetetrazole-Induced Epileptic Seizures and Neuronal Cell Loss: Role of Antioxidant Defense System. Evid. Based Complement. Altern. Med. 2021, 2021, 5523705. [Google Scholar] [CrossRef]
- Tambe, R.; Jain, P.; Patil, S.; Ghumatkar, P.; Sathaye, S. Antiepileptogenic Effects of Borneol in Pentylenetetrazole-Induced Kindling in Mice. Naunyn-Schmiedeb. Arch. Pharmacol. 2016, 389, 467–475. [Google Scholar] [CrossRef]
- Gao, F.; Gao, Y.; Liu, Y.F.; Wang, L.; Li, Y.J. Berberine Exerts an Anticonvulsant Effect and Ameliorates Memory Impairment and Oxidative Stress in a Pilocarpine-Induced Epilepsy Model in the Rat. Neuropsychiatr. Dis. Treat 2014, 10, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Abu-Taweel, G.M.; Aboshaiqah, A.E.; Ajarem, J.S. The Effects of Quinacrine, Proglumide, and Pentoxifylline on Seizure Activity, Cognitive Deficit, and Oxidative Stress in Rat Lithium-Pilocarpine Model of Status Epilepticus. Oxid. Med. Cell. Longev. 2014, 2014, 630509. [Google Scholar] [CrossRef] [PubMed]
- Alvi, A.M.; Al Kury, L.T.; Alattar, A.; Ullah, I.; Muhammad, A.J.; Alshaman, R.; Shah, F.A.; Khan, A.U.; Feng, J.; Li, S. Carveol Attenuates Seizure Severity and Neuroinflammation in Pentylenetetrazole-Kindled Epileptic Rats by Regulating the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 9966663. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.H.; Agbo, E.; Zhang, S.H.; Zhu, J.L. Anticonvulsant and Neuroprotective Effects of Paeonol in Epileptic Rats. Neurochem. Res. 2019, 44, 2556–2565. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Taiwe, G.S.; Moto, F.C.O.; Ngoupaye, G.T.; Nkantchoua, G.C.N.; Njapdounke, J.S.K.; Omam, J.P.O.; Pale, S.; Kouemou, N.; Bum, E.N. Antiepileptogenic and Neuroprotective Effects of Pergularia daemia on Pilocarpine Model of Epilepsy. Front. Pharmacol. 2017, 8, 440. [Google Scholar] [CrossRef]
- Sani, M.; Sebaï, H.; Gadacha, W.; Boughattas, N.A.; Reinberg, A.; Mossadok, B.A. Catalase Activity and Rhythmic Patterns in Mouse Brain, Kidney and Liver. Comp. Biochem. Physiol. B Biochem. 2006, 145, 331–337. [Google Scholar] [CrossRef]
- Holm, T.H.; Lykke-Hartmann, K. Insights into the Pathology of the A3 Na+/K+-ATPase Ion Pump in Neurological Disorders; Lessons from Animal Models. Front. Physiol. 2016, 7, 209. [Google Scholar] [CrossRef]
- Das, J.; Singh, R.; Sharma, D. Antiepileptic Effect of Fisetin in Iron-Induced Experimental Model of Traumatic Epilepsy in Rats in the Light of Electrophysiological, Biochemical, and Behavioral Observations. Nutr Neurosci. 2017, 20, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Funck, V.R.; Ribeiro, L.R.; Pereira, L.M.; de Oliveira, C.V.; Grigoletto, J.; Fighera, M.R.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S. Long-Term Decrease in Na+,K+-ATPase Activity after Pilocarpine-Induced Status Epilepticus Is Associated with Nitration of Its Alpha Subunit. Epilepsy Res. 2014, 108, 1705–1710. [Google Scholar] [CrossRef]
- Marquezan, B.P.; Funck, V.R.; Oliveira, C.V.; Pereira, L.M.; Araújo, S.M.; Zarzecki, M.S.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S. Pentylenetetrazol-Induced Seizures Are Associated with Na+, K+-ATPase Activity Decrease and Alpha Subunit Phosphorylation State in the Mice Cerebral Cortex. Epilepsy Res. 2013, 105, 396–400. [Google Scholar] [CrossRef]
- Bogdanova, A.; Petrushanko, I.Y.; Hernansanz-Agustín, P.; Martínez-Ruiz, A. “Oxygen Sensing” by Na,K-ATPase: These Miraculous Thiols. Front. Physiol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Mitkevich, V.A.; Lakunina, V.A.; Anashkina, A.A.; Spirin, P.V.; Rubtsov, P.M.; Prassolov, V.S.; Bogdanov, N.B.; Hänggi, P.; Fuller, W.; et al. Cysteine Residues 244 and 458–459 within the Catalytic Subunit of Na,K-ATPase Control the Enzyme’s Hydrolytic and Signaling Function under Hypoxic Conditions. Redox Biol. 2017, 13, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Poluektov, Y.M.; Dergousova, E.A.; Lopina, O.D.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Na,K-ATPase α-Subunit Conformation Determines Glutathionylation Efficiency. Biochem. Biophys. Res. Commun. 2019, 510, 86–90. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y.V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-Glutathionylation of the Na, K-ATPase Catalytic α Subunit Is a Determinant of the Enzyme Redox Sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef]
- Genda, E.N.; Jackson, J.G.; Sheldon, A.L.; Locke, S.F.; Greco, T.M.; O’Donnell, J.C.; Spruce, L.A.; Xiao, R.; Guo, W.; Putt, M.; et al. Co-Compartmentalization of the Astroglial Glutamate Transporter, GLT-1, with Glycolytic Enzymes and Mitochondria. J. Neurosci. 2011, 31, 18275–18288. [Google Scholar] [CrossRef]
- Rose, E.M.; Koo, J.C.P.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate Transporter Coupling to Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Illarionava, N.B.; Brismar, H.; Aperia, A.; Gunnarson, E. Role of Na,K-ATPase A1 and A2 Isoforms in the Support of Astrocyte Glutamate Uptake. PLoS ONE 2014, 9, e110095. [Google Scholar] [CrossRef] [PubMed]
- Sumbul, O.; Aygun, H. Chronic Effects of Different Quercetin Doses in Penicillin-Induced Focal Seizure Model. Neurosci. Lett. 2021, 753, 135848. [Google Scholar] [CrossRef]
- Shawki, M.; El Wakeel, L.M.; Shatla, R.; El-Saeed, G.; Ibrahim, S.; Badary, O. The Clinical Outcome of Adjuvant Therapy with Black Seed Oil on Intractable Paediatric Seizures: A Pilot Study. Epileptic Disord. 2013, 15, 295–301. [Google Scholar] [CrossRef]
- Colomeu, T.C.; de Figueiredo, D.; de Zollner, R.L.; Meletti, L.M.M. Comparison of Antioxidant and Ant Proliferative Effect among Four Passiflora Spp. J. Agric. Life Sci. 2017, 4, 2375–4222. [Google Scholar]
- Appel, K.; Rose, T.; Fiebich, B.; Kammler, T.; Hoffmann, C.; Weiss, G. Modulation of the γ-Aminobutyric Acid (GABA) System by Passiflora incarnata L. Phytother Res. 2011, 25, 838–843. [Google Scholar] [CrossRef]


| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Oliveira 2004 [51] |  |  |  |  |  |  |  |  |  |  |
| Fighera 2006 [52] |  |  |  |  |  |  |  |  |  |  |
| Wilhelm 2009 [53] |  |  |  |  |  |  |  |  |  |  |
| Rambo 2009 [54] |  |  |  |  |  |  |  |  |  |  |
| Wilhelm 2010 [55] |  |  |  |  |  |  |  |  |  |  |
| Freitas 2010 [20] |  |  |  |  |  |  |  |  |  |  |
| Santos 2010 [56] |  |  |  |  |  |  |  |  |  |  |
| Ezz 2011 [57] |  |  |  |  |  |  |  |  |  |  |
| Bortolatto 2011 [58] |  |  |  |  |  |  |  |  |  |  |
| Souza 2013 [59] |  |  |  |  |  |  |  |  |  |  |
| Della-Pace 2013 [60] |  |  |  |  |  |  |  |  |  |  |
| Rambo 2013 [61] |  |  |  |  |  |  |  |  |  |  |
| Ahmed 2014 [62] |  |  |  |  |  |  |  |  |  |  |
| Pires 2015 [63] |  |  |  |  |  |  |  |  |  |  |
| Kandhare 2018 [64] |  |  |  |  |  |  |  |  |  |  |
| Zhang 2018 [65] |  |  |  |  |  |  |  |  |  |  |
| Tao 2020 [66] |  |  |  |  |  |  |  |  |  |  |
| Abd Allah 2021 [67] |  |  |  |  |  |  |  |  |  |  |
| Wang 2021 [68] |  |  |  |  |  |  |  |  |  |  |
| Vogt 2022 [69] |  |  |  |  |  |  |  |  |  |  |
| Alkhudhary 2022 [70] |  |  |  |  |  |  |  |  |  |  |
| Gad 2022 [71] |  |  |  |  |  |  |  |  |  |  |
 ); high risk of bias (
); high risk of bias ( ); unclear risk of bias (
); unclear risk of bias ( ). Questions Q1, Q2, and Q3 relate to selection bias; Q4 and Q5 relate to performance bias; Q6 and Q7 relate to detection bias; and questions Q8, Q9, and Q10 relate to attrition, information, and other biases, respectively. Details are provided in Supplementary Data N° 1.
). Questions Q1, Q2, and Q3 relate to selection bias; Q4 and Q5 relate to performance bias; Q6 and Q7 relate to detection bias; and questions Q8, Q9, and Q10 relate to attrition, information, and other biases, respectively. Details are provided in Supplementary Data N° 1.| Reference | Brain Areas Sample Size | Animal Model Characteristics | Antioxidant | Results |
|---|---|---|---|---|
| * Oliveira et al., 2004 [51] | Striatum 10–14 | Wistar Rats/270–300 g PTZ 1.8 μmol/2 μL Intraestrial | Ascorbic acid (30, 100 or 300 mg/Kg)/i.p. 30 min before PTZ | Epilepsy: NKA ↓; PCA ↑ Epilepsy + antioxidants: NKA ↑ (1); n.s (2); PCA ↓ (1); n.s (2) |
| * Fighera et al., 2006 [52] | Striatum 6–8 | Wistar Rats/270–300 g PTZ 1.8 µmol/2 µL Intraestrial | GM1 ganglioside 50 mg/kg/i.p. 30 min before the injection of PTZ | Epilepsy: NKA ↓;PCA ↑; MDA ↑ Epilepsy + antioxidants: NKA ↑; PCA ↓; MDA ↓ |
| * Wilhelm et al., 2009 [53] | Whole Brain 8–12 | Wistar Rats/40–50 g PILO 400 mg/Kg/i.p. | 3-ASP (10, 25 or 50 mg/Kg)/Oral 30 min before of PILO | Epilepsy: NKA ↓; GPx ↓; GST ↑; SOD ↓; CAT ↑; RS ↑ Epilepsy + antioxidants: NKA ↑; GPx n.s; GST n.s; SOD ↑ (1)/n.s (2); CAT ↓ (2)/n.s (1); RS ↓ |
| Rambo et al., 2009 [54] | Hippocampus 8–10 | Wistar Rats/250–300 g PTZ (30 or 60 mg/Kg)/i.p. | Creatine 300 mg/Kg Oral gavage 6 weeks | Epilepsy: NKA ↓; SOD n.s; CAT ↓ (1)/n.s (1); MDA ↑; PCA ↑ Epilepsy + antioxidants: NKA ↑; SOD ↑ (1)/n.s (1); CAT ↑ (1)/n.s (1); MDA ↓; PCA ↓ (1)/n.s |
| * Wilhelm et al., 2010 [55] | Whole Brain 5–8 | Swiss Mice/25–35 g PILO 400 mg/Kg/i.p. | BPD (1, 5, 10, 25, 50 or 100 mg/Kg) Oral gavage 30 min before PILO | Epilepsy: NKA ↓; GPx ↓; GST ↑; CAT ↑; MDA↑; RS ↑ Epilepsy + antioxidants: NKA ↑; GPx n.s; GST ↑(1)/n.s (5); CAT n.s; MDA ↓ (4)/n.s (2); RS ↓ (5)/n.s (1) |
| Freitas, 2010 [20] | Hippocampus 5–7 | Wistar Rats/250–280 g PILO 400 mg/Kg/i.p. | LA 10 mg/Kg/i.p./30 min before PILO | Epilepsy: NKA ↓; GPx ↑; GSH ↓; GR n.s Epilepsy + antioxidants: NKA ↑; GPx ↑; GSH ↑; GR n.s |
| Santos et al., 2010 [56] | Hippocampus 5–7 | Wistar Rats/250–280 g PILO 400 mg/Kg/i.p. | LA 10 mg/Kg/i.p./30 min before PILO | Epilepsy: NKA ↓ Epilepsy + antioxidants: NKA ↑ |
| Striatum 5–7 | Epilepsy: NKA ↓ Epilepsy + antioxidants: NKA ↑ | |||
| Cortex 5–7 | Epilepsy: NKA n.s Epilepsy + antioxidants: NKA n.s | |||
| Cerebellum 5–7 | Epilepsy: NKA n.s Epilepsy + antioxidants: NKA n.s | |||
| Ezz et al., 2011 [57] | Hippocampus 5–7 | Wistar Rats/200–250 g PILO 380 mg/Kg/i.p. | Curcumin 80 mg/Kg or Nigella sativa oil (NSO) 4 mL/Kg/Orally/21 days | Epilepsy: NKA ↓; GSH ↓; CAT ↓; NO ↑; MDA n.s Epilepsy + antioxidants: NKA and GSH: ↑ (1)/n.s (1); CAT and NO: ↓ (1)/n.s (1); MDA n.s |
| * Bortolatto et al., 2011 [58] | Hippocampus 8–10 | Wistar Rats/200–300 g KA 10 mg/Kg/i.p. | DTDS (50 or 100 mg/Kg)/Oral by gavage 1 h after the animals received KA | Epilepsy: NKA ↑; GPx n.s; PCA ↑; RS ↑ Epilepsy + antioxidants: NKA ↓; GPx n.s; PCA ↓; RS ↓ |
| Cortex 8–10 | Epilepsy: NKA ↑; GPx, PCA and RS: n.s Epilepsy + antioxidants: NKA ↓; GPx, PCA and RS: n.s | |||
| * Souza et al., 2013 [59] | Cortex 7–8 | Wistar Rats/270–300 g PTZ 60 mg/Kg/i.p. | Caffeine 6 mg/Kg/Oral by gavage 60 min before PTZ | Epilepsy: NKA ↓; GSH ↓; MDA↑ Epilepsy + antioxidants: NKA ↑; GSH ↑; MDA ↓ |
| * Della-Pace et al., 2013 [60] | Cortex 8–9 | Swiss Mice/25–35 g PTZ 80 mg/Kg/i.p. | TTHL 30 mg/Kg/Orally by gavage/60 min before PTZ | Epilepsy: NKA ↓; MDA↑; PCA ↑ Epilepsy + antioxidants: NKA ↑; MDA ↓; PCA ↓ |
| Rambo et al., 2013 [61] | Cortex 5–7 | Wistar Rats/270–300 g PTZ (30, 45 or 60 mg/Kg)/i.p. | Creatine 300 mg/Kg/Orally/ 45 min before PTZ | Epilepsy: NKA ↓; MDA↑; PCA ↑ Epilepsy + antioxidants: NKA ↑; MDA ↓; PCA ↓ |
| Ahmed, 2014 [62] | Hippocampus 10 | Sprague-Dawley Rats/250–280 g PILO 400 mg/Kg/i.p. | Idebenone (50, 100 or 200 mg/kg) i.p./ 3 successive days | Epilepsy: NKA ↓; GSH ↓; CAT ↑; NO ↑; MDA↑ Epilepsy + antioxidants: NKA ↑ (2)/n.s (1); GSH ↑ (1)/n.s (2); CAT, NO and MDA: ↓ (2)/n.s (1) |
| Pires et al., 2015 [63] | Hippocampus 10 | Swiss Mice/25–30 g/PTZ 60 mg/Kg or PILO400 mg/Kg/i.p | CA 100 mg/Kg/i.p./30 min before PILO or PTZ | Epilepsy: NKA ↓ Epilepsy + antioxidants: NKA ↑ |
| Kandhare et al., 2018 [64] | Whole Brain 6 | Swiss Mice/18–22 g/PTZ 90 mg/Kg/i.p. | Morin (10, 20 or 40 mg/Kg)/i.p./45 min before PTZ | Epilepsy: NKA ↓; GSH ↓; SOD ↓; NO ↑; MDA↑ Epilepsy + antioxidants: NKA and GSH: ↑ (2)/n.s (1); SOD ↑; NO and MDA: ↓ (2)/n.s (1) |
| Zhang et al., 2018 [65] | Whole Brain 6 | Swiss Mice/18–22 g/PTZ 90 mg/Kg/i.p. | TEMPOL (50, 100 or 200 mg/Kg)/Oral 45 min before PTZ | Epilepsy: NKA ↓; GSH ↓; SOD ↓; NO ↑; MDA ↑ Epilepsy + antioxidants: NKA ↑ (2)/n.s (1); GSH ↑; SOD ↑; NO and MDA: ↓ (2)/n.s (1) |
| * Tao et al., 2020 [66] | Whole Brain 5–6 | Swiss Mice/18–22 g PTZ 90 mg/Kg/i.p. | PA (50, 100 or 200 mg/Kg)/i.p. 45 min before PTZ | Epilepsy: NKA ↓; GSH ↓; SOD ↓; NO ↑; MDA↑ Epilepsy + antioxidants: NO and MDA: ↓ (2)/n.s (1); NKA, GSH and SOD: ↑ (2)/n.s (1) |
| Adb Allah et al., 2021 [67] | Hippocampus 6 | Wistar Rats/150–170 g PILO 300 mg/kg/i.p. | M. officinalis extract (MOE)/250 mg/Kg Oral for 2 weeks | Epilepsy: NKA ↓; SOD ↓; CAT ↓; MDA↑ Epilepsy + antioxidants: NKA ↑; SOD ↑; CAT ↑; MDA ↓ |
| Wang et al., 2021 [68] | Whole Brain 6 | Swiss Mice/20–30 g PTZ 70 mg/Kg/i.p. | EE-ATF (50, 75 or 100 mg/Kg)/Oral 30 min before PTZ | Epilepsy: NKA ↓; GSH ↓; SOD ↓; NO ↑; MDA ↑ Epilepsy + antioxidants: NKA ↑; SOD ↑; NO ↓; MDA ↓ |
| Vogt et al., 2022 [69] | Hippocampus 7 | Swiss Mice/25–35 g PTZ 35 mg/Kg/i.p. | QTCA-1 10 mg/kg by gavage 30 min before PTZ | Epilepsy: NKA ↓; SOD ↑; MDA ↑; RS ↑ Epilepsy + antioxidants: NKA ↑; SOD ↓; MDA ↓; RS ↓ |
| Cortex 7 | Epilepsy: NKA ↓; SOD ↑; MDA↑; RS ↑ Epilepsy + antioxidants: NKA ↑; SOD ↓; MDA ↓; RS ↓ | |||
| Alkhudhary et al., 2022 [70] | Hippocampus 7 | Wistar Rats/180–200 g PTZ 60 mg/Kg/i.p. | ESE (250 mg/Kg)/Oral/for 7 days | Epilepsy: NKA ↓; GSH ↓; SOD ↓; CAT ↓; GPx ↓; GR↓; NO ↑; MDA ↑ Epilepsy + antioxidants: NKA ↑; GSH ↑; SOD ↑; CAT ↑; GPx ↑; GR ↑; NO ↓; MDA ↓ |
| Gad et al., 2022 [71] | Whole Brain 8 | Sprague-Dawley Rats/150–180 g/PILO 300 mg/Kg/i.p. | Passiflora extract 200 mg/Kg/intragastric intubation/4 weeks | Epilepsy: NKA ↓; SOD ↓; CAT ↓; GR ↓; MDA↑ Epilepsy + antioxidants: NKA ↑; SOD ↑; CAT ↑; GR ↑; MDA n.s |
| Outcomes | Brain Part | Epileptics | Epileptics/Antioxidant |
|---|---|---|---|
| GSH | WB, HIP | ↓ | ↑ |
| SOD | WB, HIP | ↓, n.s | ↑, n.s |
| CAT | HIP | n.s | n.s |
| NO | WB, HIP | ↑ | ↓ |
| MDA | WB, HIP, COR | ↑ | ↓ |
| NKA | WB, HIP, COR | ↓ | ↑ |
| Latency | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo, A.D.; Freire, V.A.F.; Diogo, Í.L.; Santos, H.d.L.; Barbosa, L.A.; de Carvalho, L.E.D. Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1397. https://doi.org/10.3390/antiox12071397
de Melo AD, Freire VAF, Diogo ÍL, Santos HdL, Barbosa LA, de Carvalho LED. Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis. Antioxidants. 2023; 12(7):1397. https://doi.org/10.3390/antiox12071397
Chicago/Turabian Stylede Melo, Anderson Dutra, Victor Antonio Ferreira Freire, Ítalo Leonardo Diogo, Hérica de Lima Santos, Leandro Augusto Barbosa, and Luciana Estefani Drumond de Carvalho. 2023. "Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis" Antioxidants 12, no. 7: 1397. https://doi.org/10.3390/antiox12071397
APA Stylede Melo, A. D., Freire, V. A. F., Diogo, Í. L., Santos, H. d. L., Barbosa, L. A., & de Carvalho, L. E. D. (2023). Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis. Antioxidants, 12(7), 1397. https://doi.org/10.3390/antiox12071397








