Effects of Dietary Oleacein Treatment on Endothelial Dysfunction and Lupus Nephritis in Balb/C Pristane-Induced Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Chemical Synthesis of (−)-Oleacein
2.3. Diet Elaboration
2.4. Animals, Diets, and SLE-like Disease Induction
2.5. Kidney Histological Evaluation
2.6. Immunohistochemistry and Immunofluorescence Analysis
2.7. Western Blotting
2.8. Vascular Reactivity
2.9. RT-qPCR
2.10. miRNAs Expression
2.11. Data and Statistical Analysis
3. Results
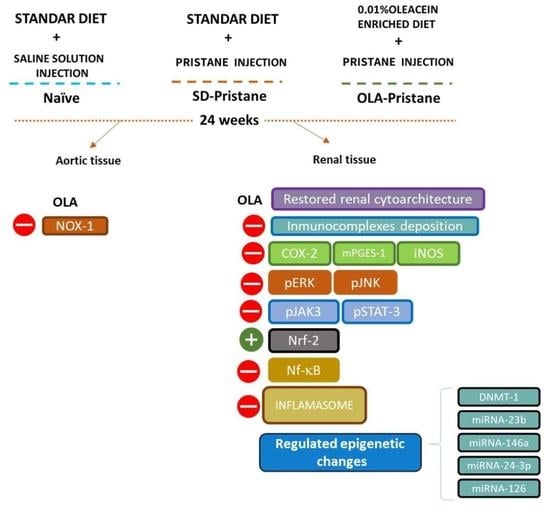
3.1. OLA-Supplemented Diet Reduced SLE Lymphoid Organs Weight in Pristane-Induced SLE BALB/c Mice
3.2. OLA Dietary Treatment Counteracts Renal Abnormalities in Pristane-Induced SLE BALB/c Mice
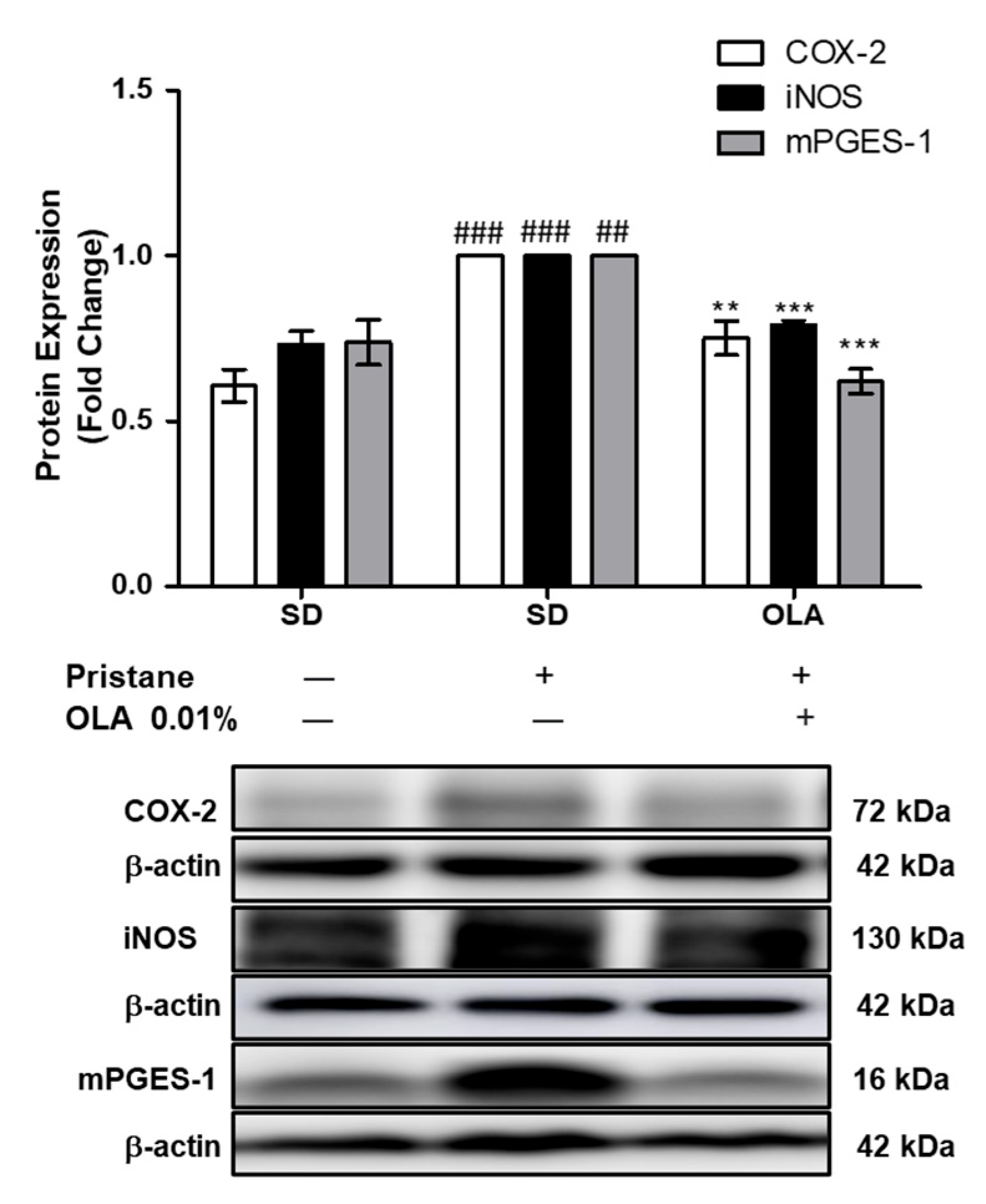
3.3. Effects of OLA Dietary Supplementation onCOX-2, iNOS, and mPGES-1 Protein Expression in Renal Tissue from Pristane-Induced SLE Mice
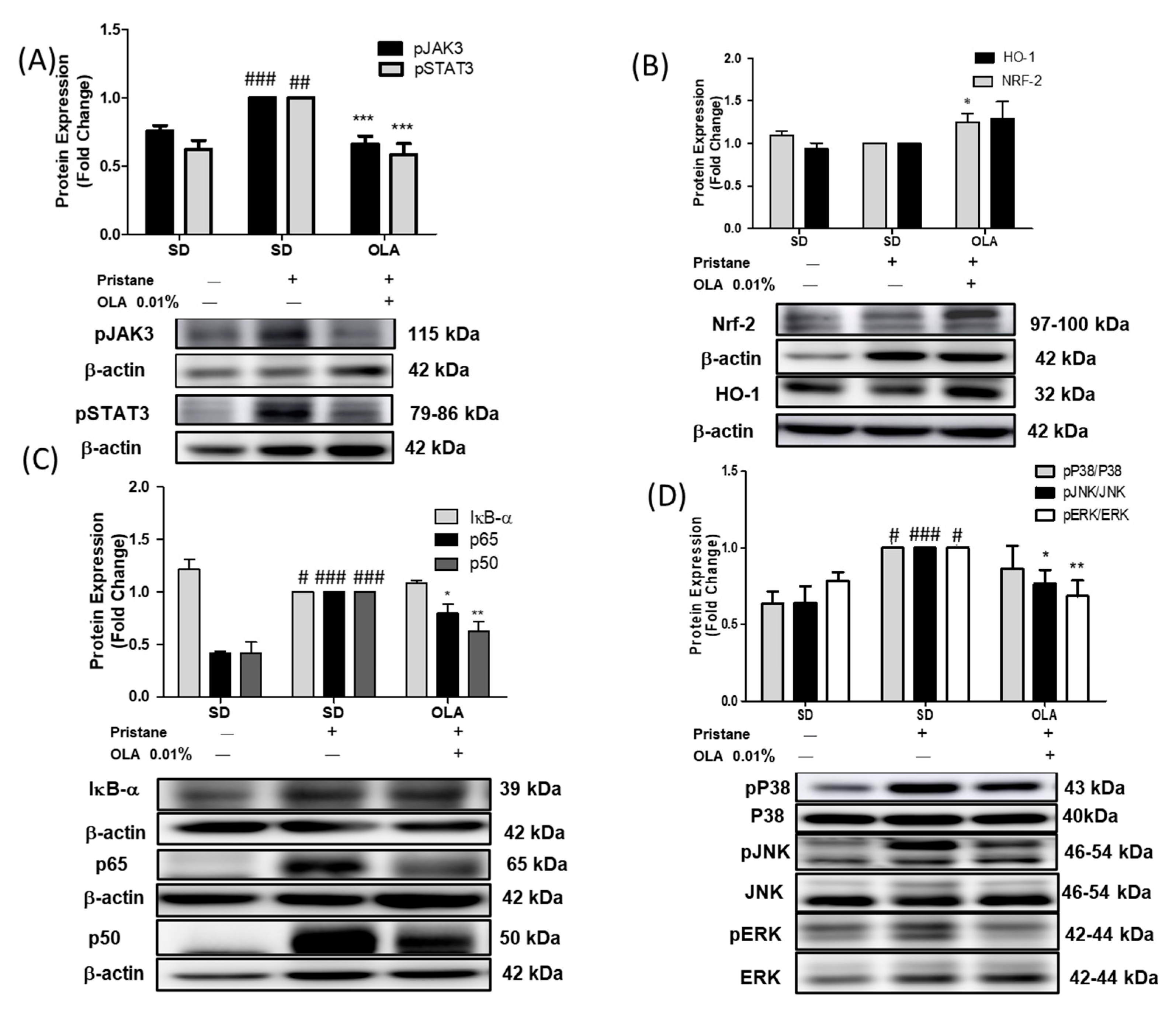
3.4. Effects of OLA Treatment on the Modulation of the MAPK, JAK/STAT, Nrf/HO-1, and Nf-kB Signaling Pathways in Pristane-Induced Mice
3.5. Nutritional OLA Supplementation Regulated Inflammasome Activation in Kidney Tissue from Pristane-Induced SLE Mice
3.6. Effects of OLA Dietary Treatment on Vascular Function in the Thoracic Aortas from Pristane-Induced Mice
3.7. An OLA-Supplemented Diet Regulated miRNAs and DNMT-1 Expression in Kidney Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lou, H.; Sheng, G.; Cao, X. Autoantibodies in Systemic Lupus Erythematosus: From Immunopathology to Therapeutic Target. J. Autoimmun. 2022, 132, 102861. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.F.; Wong, K.K.; Mohd Redzwan, N. Th1, Th2, and Th17 Cytokines in Systemic Lupus Erythematosus. Autoimmunity 2020, 53, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Padjen, I.; Fanouriakis, A.; Boumpas, D.T. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 2019, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.T.; Caster, D.J. The Potential of Nrf2 Activation as a Therapeutic Target in Systemic Lupus Erythematosus. Metabolites 2022, 12, 151. [Google Scholar] [CrossRef]
- Su, B.; Ye, H.; You, X.; Ni, H.; Chen, X.; Li, L. Icariin Alleviates Murine Lupus Nephritis via Inhibiting NF-ΚB Activation Pathway and NLRP3 Inflammasome. Life Sci. 2018, 208, 26–32. [Google Scholar] [CrossRef]
- Richter, P.; Cardoneanu, A.; Burlui, A.M.; Macovei, L.A.; Bratoiu, I.; Buliga-Finis, O.N.; Rezus, E. Why Do We Need JAK Inhibitors in Systemic Lupus Erythematosus? Int. J. Mol. Sci. 2022, 23, 11788. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Cárdeno, A.; Rosillo, M.Á.; Sánchez-Fidalgo, S.; Utrilla, J.; Martín-Lacave, I.; Alarcón-de-la-Lastra, C. Dietary Extra Virgin Olive Oil Attenuates Kidney Injury in Pristane-Induced SLE Model via Activation of HO-1/Nrf-2 Antioxidant Pathway and Suppression of JAK/STAT, NF-ΚB and MAPK Activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, Y.; Lee, N.; Wahl, E.R.; Kim, S.H.; Kang, K.S.; Lazova, R.; Kang, I. Self Double-Stranded (Ds)DNA Induces IL-1β Production from Human Monocytes by Activating NLRP3 Inflammasome in the Presence of Anti–DsDNA Antibodies. J. Immunol. 2013, 190, 1407–1415. [Google Scholar] [CrossRef]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Vazquéz-Román, M.V.; de Sotomayor, M.A.; Ortega-Vidal, J.; González, M.L.; Alarcón-de-la-Lastra, C. Oleocanthal Supplemented Diet Improves Renal Damage and Endothelial Dysfunction in Pristane-Induced Systemic Lupus Erythematosus in Mice. Food Res. Int. 2023, 163, 112140. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-De-La-Lastra, C.; González-Benjumea, A.; Vázquez-Román, M.V.; Sánchez-Hidalgo, M. Dietary Oleuropein and Its Acyl Derivative Ameliorate Inflammatory Response in Peritoneal Macrophages from Pristane-Induced SLE Mice: Via Canonical and Noncanonical NLRP3 Inflammasomes Pathway. Food Funct. 2020, 11, 6622–6631. [Google Scholar] [CrossRef]
- Pocovi-Gerardino, G.; Correa-Rodríguez, M.; Callejas-Rubio, J.L.; Ríos-Fernández, R.; Martín-Amada, M.; Cruz-Caparros, M.G.; Rueda-Medina, B.; Ortego-Centeno, N. Beneficial Effect of Mediterranean Diet on Disease Activity and Cardiovascular Risk in Systemic Lupus Erythematosus Patients: A Cross-Sectional Study. Rheumatology 2021, 60, 160–169. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; De Sancti, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-Inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug. Targets 2017, 18, 36–50. [Google Scholar] [CrossRef]
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Montoya, T.; Martín-LaCave, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary Oleuropein and Its New Acyl-Derivate Attenuate Murine Lupus Nephritis through HO-1/Nrf2 Activation and Suppressing JAK/STAT, NF-ΚB, MAPK and NLRP3 Inflammasome Signaling Pathways. J. Nutr. Biochem. 2019, 74, 108229. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Cárdeno, A.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary Hydroxytyrosol and Hydroxytyrosyl Acetate Supplementation Prevent Pristane-Induced Systemic Lupus Erythematous in Mice. J. Funct. Foods 2017, 29, 84–92. [Google Scholar] [CrossRef]
- Karnopp, T.E.; Chapacais, G.F.; Freitas, E.C.; Monticielo, O.A. Lupus Animal Models and Neuropsychiatric Implications. Clin. Rheumatol. 2021, 40, 2535–2545. [Google Scholar] [CrossRef]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein Inhibits STAT3, Activates the Apoptotic Machinery, and Exerts Anti-Metastatic Effects in the SH-SY5Y Human Neuroblastoma Cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Lepore, S.M.; Morittu, V.M.; Arcidiacono, B.; Colica, C.; Procopio, A.; Maggisano, V.; Bulotta, S.; Costa, N.; Mignogna, C.; et al. Effects of Oleacein on High-Fat Diet-Dependent Steatosis, Weight Gain, and Insulin Resistance in Mice. Front. Endocrinol. 2018, 9, 116. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef]
- Muñoz-García, R.; Sánchez-Hidalgo, M.; Montoya, T.; Alcarranza, M.; Ortega-Vidal, J.; Altarejos, J.; Alarcón-de-la-Lastra, C. Effects of Oleacein, a New Epinutraceutical Bioproduct from Extra Virgin Olive Oil, in LPS-Activated Murine Immune Cells. Pharmaceuticals 2022, 15, 1338. [Google Scholar] [CrossRef]
- Gutiérrez-Miranda, B.; Gallardo, I.; Melliou, E.; Cabero, I.; Álvarez, Y.; Magiatis, P.; Hernández, M.; Nieto, M.L. Oleacein Attenuates the Pathogenesis of Experimental Autoimmune Encephalomyelitis through Both Antioxidant and Anti-Inflammatory Effects. Antioxidants 2020, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The Emerging Role of Epigenetics in Human Autoimmune Disorders. Clin. Epigenet. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Castejón, M.L.; Muñoz-García, R.; Alarcón-De-la-Lastra, C. Epigenetic Linkage of Systemic Lupus Erythematosus and Nutrition. Nutr. Res. Rev. 2021, 36, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and Mirna Expression in Adipocytes by Attenuating Nf-Κb Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. MiRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharm. 2020, 11, 574317. [Google Scholar] [CrossRef]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Cantafio, M.E.G.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-Tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Lemus, C.; Halabalaki, M.; Pergola, C.; Werz, O.; Smith, A.B.; Michel, S.; Skaltsounis, L.; Deguin, B. One-Step Semisynthesis of Oleacein and the Determination as a 5-Lipoxygenase Inhibitor. J. Nat. Prod. 2014, 77, 441–445. [Google Scholar] [CrossRef]
- Satoh, M.; Reeves, W.H. Induction of Lupus-Associated Autoantibodies in BALB/c Mice by Intraperitoneal Injection of Pristane. J. Exp. Med. 1994, 180, 2341–2346. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Yabuki, A.; Sawa, M.; Kohyama, M.; Hamamoto, T.; Yamato, O. Paraffin Immunofluorescence for Detection of Immune Complexes in Renal Biopsies: An Efficient Salvage Technique for Diagnosis of Glomerulonephritis in Dogs. BMC Vet. Res. 2017, 13, 371. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Claro, C.; Ogalla, E.; Rodriguez-Rodriguez, R.; Herrera, M.D.; Alvarez de Sotomayor, M. Phenolic Content of Extra Virgin Olive Oil Is Essential to Restore Endothelial Dysfunction but Not to Prevent Vascular Inflammation in Atherosclerotic Lesions of Apo E Deficient Mice. J. Funct. Foods 2015, 15, 126–136. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Budd, E.; De Andrés, M.C.; Sanchez-Elsner, T.; Oreffo, R.O.C. MiR-146b Is down-Regulated during the Chondrogenic Differentiation of Human Bone Marrow Derived Skeletal Stem Cells and up-Regulated in Osteoarthritis. Sci. Rep. 2017, 7, 46704. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhao, Y.; Xu, N.; Lv, E.; Ci, C.; Li, X. 1,25-Dihydroxyvitamin D3 Ameliorates Lupus Nephritis through Inhibiting the NF-ΚB and MAPK Signalling Pathways in MRL/Lpr Mice. BMC Nephrol. 2022, 23, 243. [Google Scholar] [CrossRef]
- McClung, D.M.; Kalusche, W.J.; Jones, K.E.; Ryan, M.J.; Taylor, E.B. Hypertension and Endothelial Dysfunction in the Pristane Model of Systemic Lupus Erythematosus. Physiol. Rep. 2021, 9, e14734. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Z.; Chen, Y.; Hou, B.; Liu, K.; Qin, H.; Fang, L.; Du, G. Coptisine Alleviates Pristane-Induced Lupus-Like Disease and Associated Kidney and Cardiovascular Complications in Mice. Front. Pharm. 2020, 11, 929. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical Significance of MiRNAs in Autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef]
- Rao, A.P.; Raghuram, J. Systemic Lupus Erythematosus. Indian J. Pract. Pediatr. 2016, 18, 313–319. [Google Scholar] [CrossRef]
- Zucchi, D.; Elefante, E.; Calabresi, E.; Signorini, V.; Bortoluzzi, A.; Tani, C. One Year in Review 2019: Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2019, 37, 715–722. [Google Scholar] [PubMed]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Alarcón-De-La-Lastra, M. An Update on Diet and Nutritional Factors in Systemic Lupus Erythematosus Management. Nutr. Res. Rev. 2017, 30, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, L.; Zhang, X.; Chen, X. An Update on Genetic Susceptibility in Lupus Nephritis. Clin. Immunol. 2020, 215, 108389, Erratum in: Clin. Immunol. 2020, 10, S1521661619303365. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Oxidative Stress and Immune Complexes: Pathogenic Mechanisms in Pristane Induced Murine Model of Lupus. Immunobiology 2020, 225, 151871. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular Mechanisms Underlying Prostaglandin E2-Exacerbated Inflammation and Immune Diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, L.; Zou, W.; Shen, W.; Zhang, H.; He, Q. Activation of Cyclooxygenase-2 by ATF4 during Endoplasmic Reticulum Stress Regulates Kidney Podocyte Autophagy Induced by Lupus Nephritis. Cell Physiol. Biochem. 2018, 48, 753–764. [Google Scholar] [CrossRef]
- Pan, L.; Yang, S.; Wang, J.; Xu, M.; Wang, S.; Yi, H. Inducible Nitric Oxide Synthase and Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. BMC Immunol. 2020, 21, 6. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 Induces Nrf2 Translocation and Glutathione S-Transferase P1 Expression via ERKs and P38-MAPK Pathways and Protect Human Colonic Cells against Oxidative Stress. Eur. J. Nutr. 2012, 51, 881–892. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 Suppresses Lupus Nephritis through Inhibition of Oxidative Injury and the NF-ΚB-Mediated Inflammatory Response. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Yoh, K.; Itoh, K.; Enomoto, A.; Hirayama, A.; Yamaguchi, N.; Kobayashi, M.; Morito, N.; Koyama, A.; Yamamoto, M.; Takahashi, S. Nrf2-Deficient Female Mice Develop Lupus-like Autoimmune Nephritis. Kidney Int. 2001, 60, 1343–1353. [Google Scholar] [CrossRef]
- Parzonko, A.; Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and Oleacein May Restore Biological Functions of Endothelial Progenitor Cells Impaired by Angiotensin II via Activation of Nrf2/Heme Oxygenase-1 Pathway. Phytomedicine 2013, 20, 1088–1094. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-ΚB/NLRP3 and Inhibition of Efferocytosis in Osteoclast-Mediated Diabetic Osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef]
- Banerjee, N.; Wang, H.; Wang, G.; Boor, P.J.; Khan, M.F. Redox-Sensitive Nrf2 and MAPK Signaling Pathways Contribute to Trichloroethene-Mediated Autoimmune Disease Progression. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Kim, M.; Morales, L.D.; Jang, I.S.; Cho, Y.Y.; Kim, D.J. Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling. Int. J. Mol. Sci. 2018, 19, 2708. [Google Scholar] [CrossRef]
- Jamilloux, Y.; El Jammal, T.; Vuitton, L.; Gerfaud-Valentin, M.; Kerever, S.; Sève, P. JAK Inhibitors for the Treatment of Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2019, 18, 102390. [Google Scholar] [CrossRef]
- Benucci, M.; Bernardini, P.; Coccia, C.; De Luca, R.; Levani, J.; Economou, A.; Damiani, A.; Russo, E.; Amedei, A.; Guiducci, S.; et al. JAK Inhibitors and Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2023, 22, 103276. [Google Scholar] [CrossRef]
- Furumoto, Y.; Smith, C.K.; Blanco, L.; Zhao, W.; Brooks, S.R.; Thacker, S.G.; Zarzour, A.; Sciumè, G.; Tsai, W.L.; Trier, A.M.; et al. Tofacitinib Ameliorates Murine Lupus and Its Associated Vascular Dysfunction. Arthritis Rheumatol. 2017, 69, 148–160. [Google Scholar]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. 2020, 5, 209. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in Cancer Inflammation and Immunity: A Leading Role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Huang, Y.; Wang, H.; Wang, S.; Zhao, C.; Liang, Y.; Yang, N. Bay11-7082 Attenuates Murine Lupus Nephritis via Inhibiting NLRP3 Inflammasome and NF-ΚB Activation. Int. Immunopharmacol. 2013, 17, 116–122. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Li, L. Lentivirus-Mediated Knockdown of FcγRI (CD64) Attenuated Lupus Nephritis via Inhibition of NF-ΚB Regulating NLRP3 Inflammasome Activation in MRL/Lpr Mice. J. Pharm. Sci. 2018, 137, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ai, L.; Sun, Y.; Yang, B.; Chen, S.; Wang, Q.; Kuang, H. Role of NLRP3 Inflammasome in Lupus Nephritis and Therapeutic Targeting by Phytochemicals. Front. Pharm. 2021, 12, 621300. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, A.L.; Mann, N.H.; Larkum, A.W.D. This Article Is Protected by Copyright. All Rights 1 Reserved. Ultrasound Obs. Gynecol. 2006, 50, 776–780. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell. Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Kostopoulou, M.; Nikolopoulos, D.; Parodis, I.; Bertsias, G. Cardiovascular Disease in Systemic Lupus Erythematosus: Recent Data on Epidemiology, Risk Factors and Prevention. Curr. Vasc. Pharm. 2019, 18, 549–565. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 Inflammasome in Autoimmune Diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Kelm, M. Central Role of ENOS in the Maintenance of Endothelial Homeostasis. Antioxid. Redox Signal. 2015, 22, 1230–1242. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Zarzuelo, M.J.; Gómez-Morales, M.; O’Valle, F.; López-Farré, A.J.; Algieri, F.; Gálvez, J.; et al. Chronic Hydroxychloroquine Improves Endothelial Dysfunction and Protects Kidney in a Mouse Model of Systemic Lupus Erythematosus. Hypertension 2014, 64, 330–337. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Robles-Vera, I.; Sánchez, M.; Jiménez, R.; O’Valle, F.; Rodriguez-Nogales, A.; Pérez-Vizcaino, F.; Gálvez, J.; Duarte, J. Activation of Peroxisome Proliferator Activator Receptor β/δ Improves Endothelial Dysfunction and Protects Kidney in Murine Lupus. Hypertension 2017, 69, 641–650. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Habibi, F.; Soufi, F.G.; Ghiasi, R.; Khamaneh, A.M.; Alipour, M.R. Alteration in Inflammation-Related MiR-146a Expression in NF-KB Signaling Pathway in Diabetic Rat Hippocampus. Adv. Pharm. Bull. 2016, 6, 99–103. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, Z.; Di, L. Regulation of MiR-146a and TRAF6 in the Diagnose of Lupus Nephritis. Med. Sci. Monit. 2017, 23, 2550–2557. [Google Scholar] [CrossRef]
- Fu, H.; Fan, X.P.; Li, M.; Liu, M.; Sun, Q. MiR-146a Relieves Kidney Injury in Mice with Systemic Lupus Erythematosus through Regulating NF-ΚB Pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 7024–7032. [Google Scholar]
- Zhu, S.; Pan, W.; Song, X.; Liu, Y.; Shao, X.; Tang, Y.; Liang, D.; He, D.; Wang, H.; Liu, W.; et al. The MicroRNA MiR-23b Suppresses IL-17-Associated Autoimmune Inflammation by Targeting TAB2, TAB3 and IKK-α. Nat. Med. 2012, 18, 1077–1086. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Li, Y.; Imam, B.H.; Ma, X.; Shen, W.; Wu, X.; Jiang, W.; Yang, J.; Lv, Y.; Ding, X.; et al. MicroRNA MiR-24-3p Mediates the Negative Regulation of Lipopolysaccharide-Induced Endometrial Inflammatory Response by Targeting TNF Receptor-Associated Factor 6 (TRAF6). J. Inflamm. Res. 2022, 15, 807–825. [Google Scholar] [CrossRef]
- Tan, H.; Qi, J.; Fan, B.Y.; Zhang, J.; Su, F.F.; Wang, H.T. MicroRNA-24-3p Attenuates Myocardial Ischemia/Reperfusion Injury by Suppressing RIPK1 Expression in Mice. Cell. Physiol. Biochem. 2018, 51, 46–62. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Bai, J. Micro RNA-126 Expression and i Ts Me Ch Anism of Action in Pati En Ts Withsy Ste Mic Lu Pus Erythematosu. Eur. Rev. Med. Pharm. Sci. 2015, 19, 3838–3842. [Google Scholar]
- Hedrich, C.M. Mechanistic Aspects of Epigenetic Dysregulation in SLE. Clin. Immunol. 2018, 196, 3–11. [Google Scholar] [CrossRef]






| MiRNA | TaqMan miRNA Assay Name | Assay ID |
|---|---|---|
| miR126 | hsa-miR-126 | 002228 |
| miR146a | hsa-miR-146a | 000468 |
| miR24-3p | hsa-miR24-3p | 000402 |
| miR23b | hsa-miR23b | 000400 |
| MammU6 | U6 snRNA | 001973 |
| Hemorrhage | Inflammatory Cells | Glomeruli Alterations | Interstitial Fibrosis | |
|---|---|---|---|---|
| Pristane | 75% | 80% | 50% | 80% |
| OLA | 5% | 30% | 25% | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-García, R.; Sánchez-Hidalgo, M.; Alcarranza, M.; Vazquéz-Román, M.V.; de Sotomayor, M.A.; González-Rodríguez, M.L.; de Andrés, M.C.; Alarcón-de-la-Lastra, C. Effects of Dietary Oleacein Treatment on Endothelial Dysfunction and Lupus Nephritis in Balb/C Pristane-Induced Mice. Antioxidants 2023, 12, 1303. https://doi.org/10.3390/antiox12061303
Muñoz-García R, Sánchez-Hidalgo M, Alcarranza M, Vazquéz-Román MV, de Sotomayor MA, González-Rodríguez ML, de Andrés MC, Alarcón-de-la-Lastra C. Effects of Dietary Oleacein Treatment on Endothelial Dysfunction and Lupus Nephritis in Balb/C Pristane-Induced Mice. Antioxidants. 2023; 12(6):1303. https://doi.org/10.3390/antiox12061303
Chicago/Turabian StyleMuñoz-García, Rocío, Marina Sánchez-Hidalgo, Manuel Alcarranza, María Victoria Vazquéz-Román, María Alvarez de Sotomayor, María Luisa González-Rodríguez, María C. de Andrés, and Catalina Alarcón-de-la-Lastra. 2023. "Effects of Dietary Oleacein Treatment on Endothelial Dysfunction and Lupus Nephritis in Balb/C Pristane-Induced Mice" Antioxidants 12, no. 6: 1303. https://doi.org/10.3390/antiox12061303
APA StyleMuñoz-García, R., Sánchez-Hidalgo, M., Alcarranza, M., Vazquéz-Román, M. V., de Sotomayor, M. A., González-Rodríguez, M. L., de Andrés, M. C., & Alarcón-de-la-Lastra, C. (2023). Effects of Dietary Oleacein Treatment on Endothelial Dysfunction and Lupus Nephritis in Balb/C Pristane-Induced Mice. Antioxidants, 12(6), 1303. https://doi.org/10.3390/antiox12061303