Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
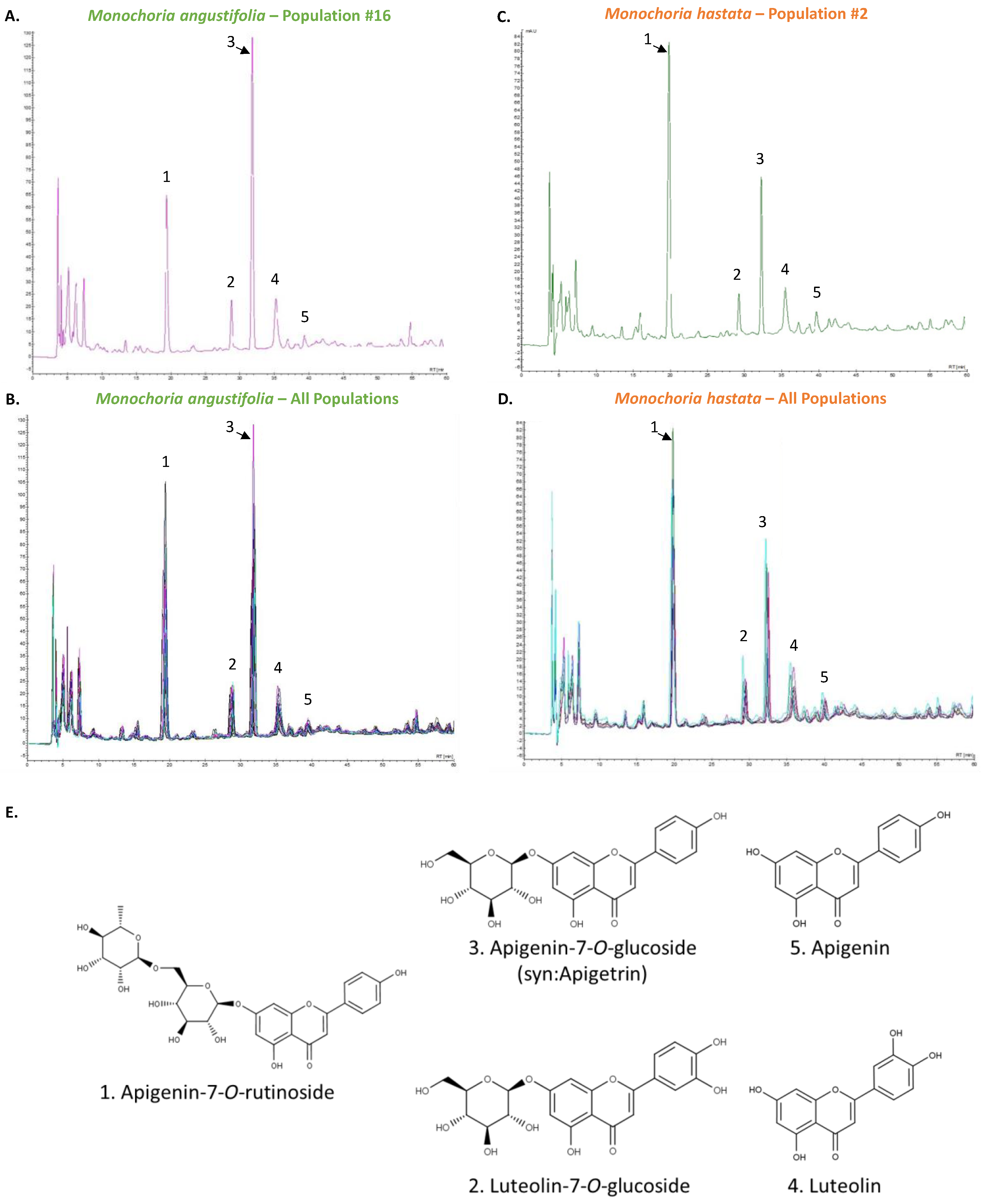
2.6. High-Performance Liquid Chromatography (HPLC) Analysis
2.7. In Vitro Cell Free Antioxidant Assays
2.8. Cellular Antioxidant Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. Plant Collection and Taxonomic Description
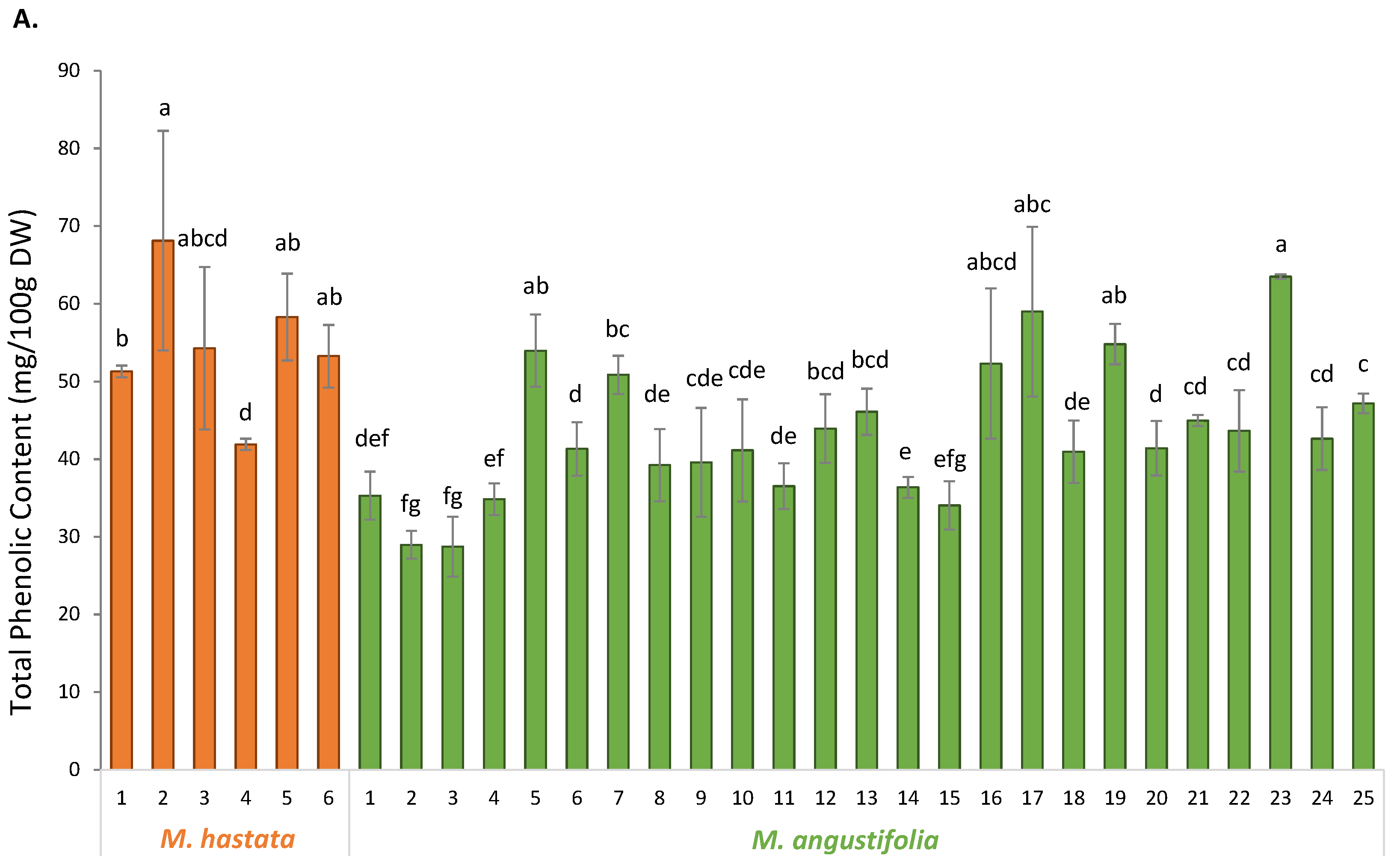
3.2. Phytochemical Characterization
3.3. Antioxidant Activity
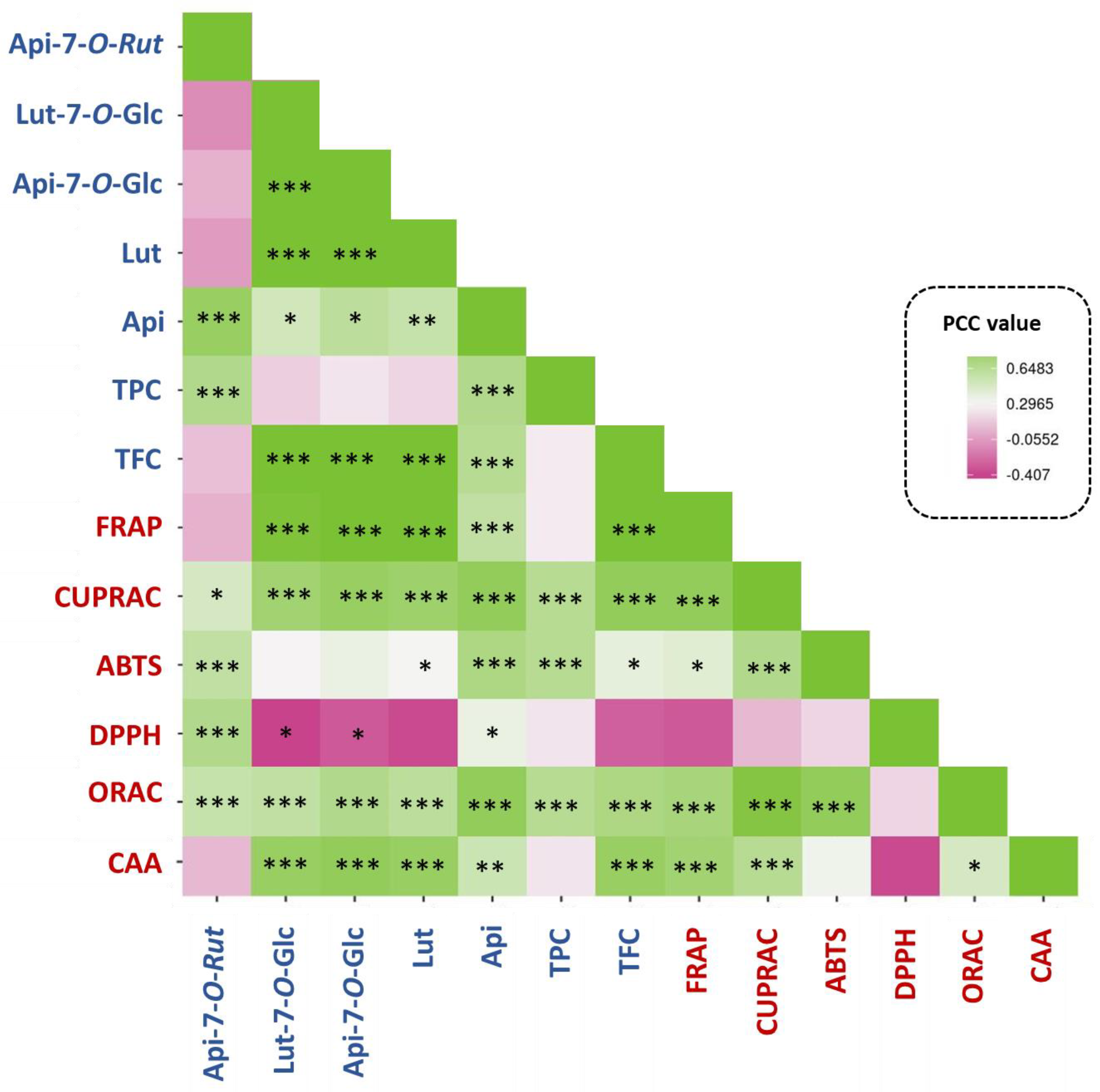
3.4. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tungmunnithum, D.; Tanaka, N.; Boonkerd, B. Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, the new species of the genus Monochoria from Thailand. Songklanakarin J. Sci. Technol. 2020, 42, 747–752. [Google Scholar]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Morphological variations among populations of Monochoria vaginalis s.l. (Pontederiaceae) in Thailand. Phytotaxa 2016, 268, 57–68. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Phenetic study of the genus Monochoria in Thailand. Songklanakarin J. Sci. Technol. 2017, 39, 49–57. [Google Scholar]
- Chayamarit, K. Pontederiaceae. In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; Parchachon: Bangkok, Thailand, 2005; pp. 51–57. [Google Scholar]
- World Checklist of Selected Plant Families. Available online: http://wcsp.science.kew.org/home.do (accessed on 2 January 2022).
- Tungmunnithum, D.; Kidyoo, M.; Khunwasi, C. Variations of the Hoya siamica Craib (Asclepiadaceae) in Thailand. Trop. Nat. Hist. 2011, 11, 29–37. [Google Scholar]
- Guofang, W.; Horn, C.N. Pontederiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2000; pp. 40–42. [Google Scholar]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Green extraction of antioxidant flavonoids from pigeon pea (Cajanus cajan (L.) millsp.) seeds using ultrasound-assisted methodology. Molecules 2021, 26, 7557. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Characterization of bioactive phenolics and antioxidant capacity of edible bean extracts of 50 fabaceae populations grown in Thailand. Foods 2021, 10, 3118. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A quick, green and simple ultrasound-assisted extraction for the valorization of antioxidant phenolic acids from Moroccan almond cold-pressed oil residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A green ultrasound-assisted extraction optimization of the natural antioxidant and anti-aging flavonolignans from milk thistle Silybum marianum (L.) gaertn. fruits for cosmetic applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Drouet, S.; Abbasi, B.H.; Falguières, A.; Ahmad, W.; Sumaira; Ferroud, C.; Doussot, J.; Vanier, J.R.; Lainé, E.; Hano, C. Single laboratory validation of a quantitative core shell-based LC separation for the evaluation of silymarin variability and associated antioxidant activity of pakistani ecotypes of milk thistle (Silybum marianum, L.). Molecules 2018, 23, 904. [Google Scholar] [CrossRef] [Green Version]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; de Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (Linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Griaud, C.; Collins, C.M. An evaluation of the effectiveness of protected areas in Thailand. Ecol. Indic. 2021, 125, 107536. [Google Scholar] [CrossRef]
- Backer, C.A. Pontederiaceae. In Flora Malesiana; Steenis, C.G.G.J., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1951; pp. 255–259. [Google Scholar]
- Ridley, H.N. Monochoria. In The Flora of the Malay Peninsula; Ridley, H.N., Ed.; L. Reeve: London, UK, 1924; pp. 295–319. [Google Scholar]
- Yang, Y.P. Pontederiaceae. In Flora of Taiwan; Liu, T.S., Huang, T.C., Koyama, T., Devol, C.E., Eds.; Epoch Publ. Co. Ltd.: Taipei, Taiwan, 1976; pp. 138–140. [Google Scholar]
- Thiers, B.; Thiers, B.H.; Cokic, B.B.B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2009. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998; ISBN 9241545100. [Google Scholar]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in antioxidant flavonoids of stamen extracts from Nymphaea lotus L. using ultrasonic-assisted extraction and macroporous resin adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Validation of a high-performance liquid chromatography with photodiode array detection method for the separation and quantification of antioxidant and skin anti-aging flavonoids from Nelumbo nucifera Gaertn. stamen extract. Molecules 2022, 27, 1102. [Google Scholar] [CrossRef]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC Int: Gaithersburg, MD, USA, 2002; p. 1219. [Google Scholar]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a medicinal plant: Uses in traditional medicine, phytochemistry and pharmacological activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.-Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar]
- Bai, N.K.; Ilango, K. High performance thin layer chromatography-automated multiple development bioautography of phytoconstituents and quantification of stigmasterol in Monochoria vaginalis and Monochoria hastata with antioxidant potential. Pharmacogn. Mag. 2018, 14, 45. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Rosatti, T.J. The genera of Pontederiaceae in the southeastern United States. J. Arnold Arbor. 1987, 68, 35–71. [Google Scholar] [CrossRef]
- Sen, P.; Sahu, P.K.; Haldar, R.; Sahu, K.; Prasad, P.; Roy, A. Apigenin naturally occurring flavonoids: Occurrence and bioactivity. Pharm. Biosci. J. 2016, 4, 56–68. [Google Scholar] [CrossRef]
- Toki, K.; Saito, N.; Iimura, K.; Suzuki, T.; Honda, T. (Delphinidin 3-gentiobiosyl) (apigenin 7-glucosyl) malonate from the flowers of Eichhornia crassipes. Phytochemistry 1994, 36, 1181–1183. [Google Scholar] [CrossRef]
- Rao, A.N.; Brainard, D.C.; Kumar, V.; Ladha, J.K.; Johnson, D.E. Preventive weed management in direct-seeded rice: Targeting the weed seedbank. Adv. Agron. 2017, 144, 45–142. [Google Scholar]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.-P.; Hano, C. A critical cross-species comparison of pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for authentication of Thai medicinal herbal tea. Plants 2020, 9, 921. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Crawford, D.J. Flavonoids and phylogenetic reconstruction. Plant Syst. Evol. 1983, 143, 83–107. [Google Scholar] [CrossRef]
- Drouet, S.; Garros, L.; Hano, C.; Tungmunnithum, D.; Renouard, S.; Hagège, D.; Maunit, B.; Lainé, E. A critical view of different botanical, molecular, and chemical techniques used in authentication of plant materials for cosmetic applications. Cosmetics 2018, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Doménech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarc’H, N.; Hano, C. Isodon rugosus (Wall. ex Benth.) Codd in vitro cultures: Establishment, phytochemical characterization and in vitro antioxidant and anti-aging activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.V.; Nam, P.-C.; Thong, N.M.; Trung, N.T.; Phan, C.-T.D.; Mechler, A. Antioxidant motifs in flavonoids: O–H versus C–H bond dissociation. ACS Omega 2019, 4, 8935–8942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G.; Catherine, A.R.-E.; Nicholas, J.M.; George, P. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
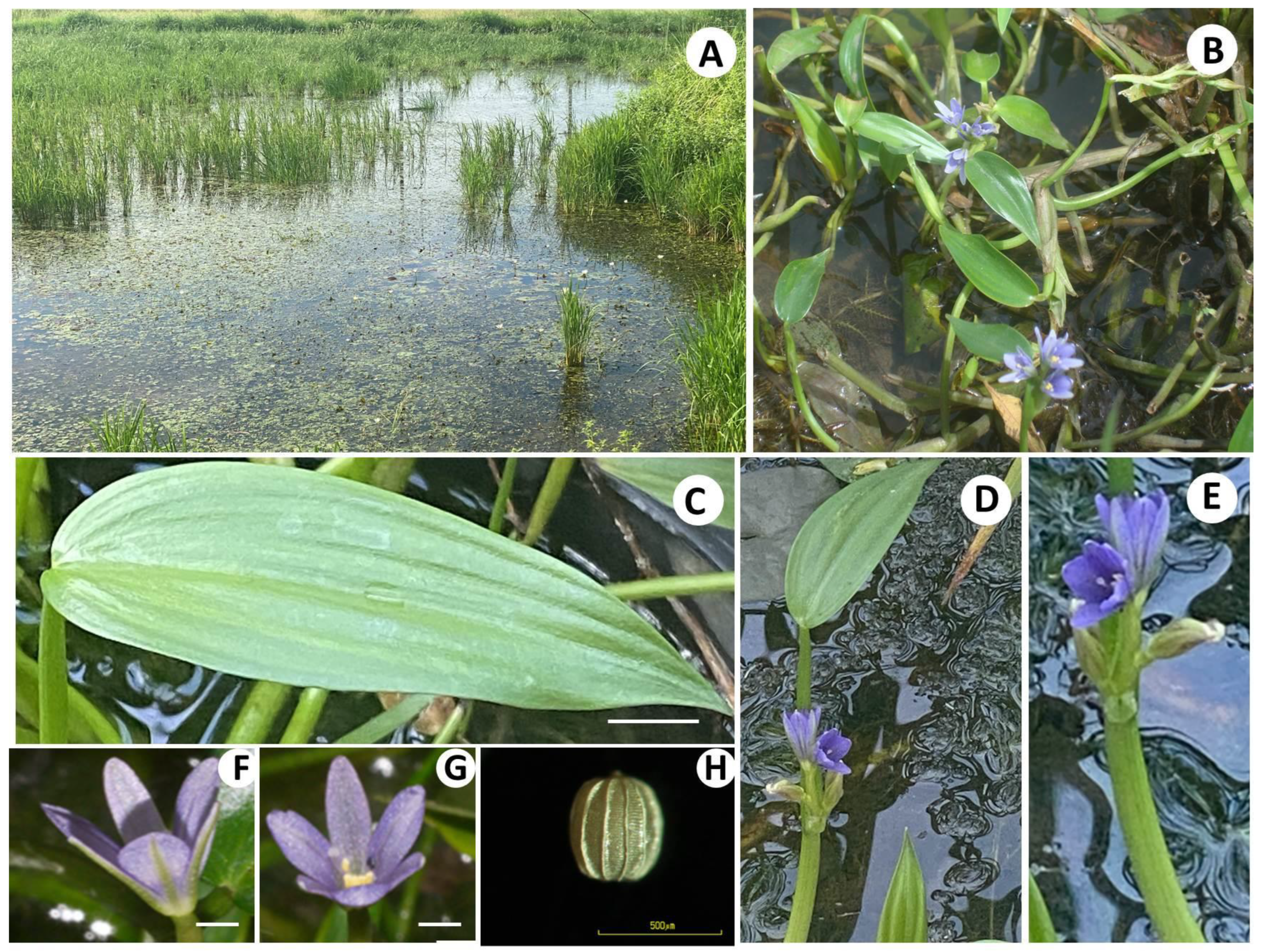
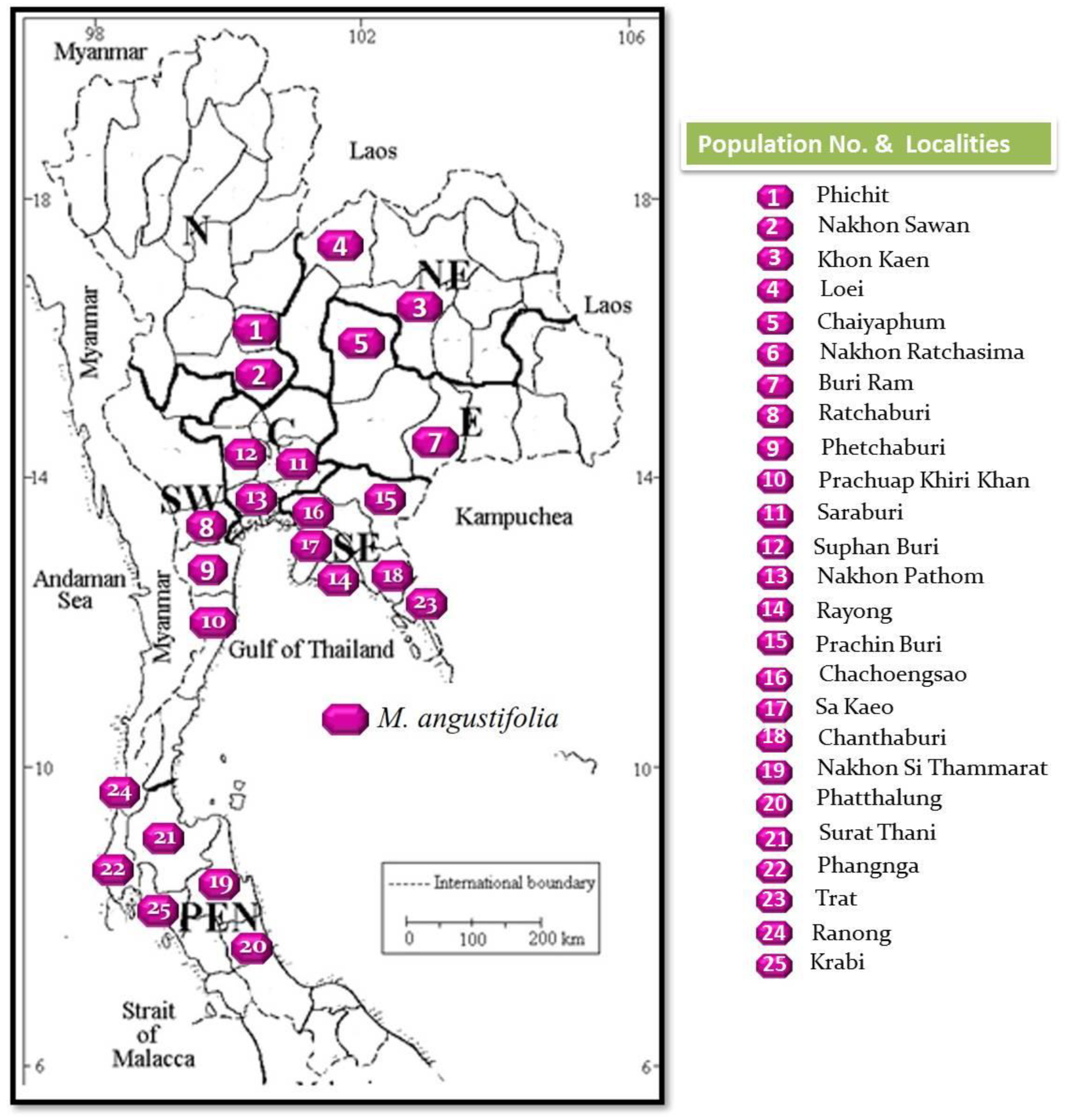



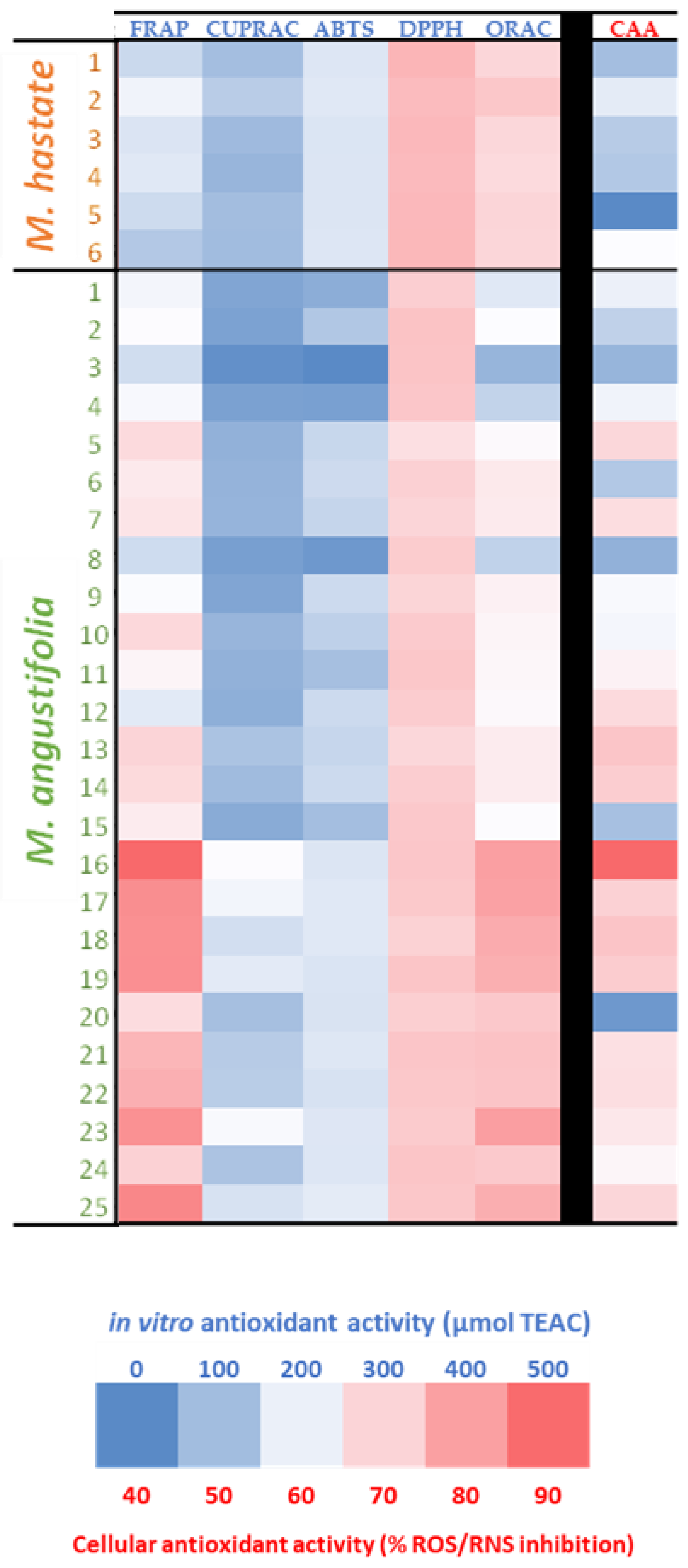

| Population No. | Population Names (The Collected Localities) | Floristic Regions | Scientific Name |
|---|---|---|---|
| 1 | Phichit | Northern (N) | M. angustifolia |
| 2 | Nakhon Sawan | Northern (N) | M. angustifolia |
| 3 | Khon Kaen | North-Eastern (NE) | M. angustifolia |
| 4 | Loei | North-Eastern (NE) | M. angustifolia |
| 5 | Chaiyaphum | Eastern (E) | M. angustifolia |
| 6 | Nakhon Ratchasima | Eastern (E) | M. angustifolia |
| 7 | Buri Ram | Eastern (E) | M. angustifolia |
| 8 | Ratchaburi | South-Western (SW) | M. angustifolia |
| 9 | Phetchaburi | South-Western (SW) | M. angustifolia |
| 10 | Prachuap Khiri Khan | South-Western (SW) | M. angustifolia |
| 11 | Saraburi | Central (C) | M. angustifolia |
| 12 | Suphan Buri | Central (C) | M. angustifolia |
| 13 | Nakhon Pathom | Central (C) | M. angustifolia |
| 14 | Rayong | South- Eastern (SE) | M. angustifolia |
| 15 | Prachin Buri | South- Eastern (SE) | M. angustifolia |
| 16 | Chachoengsao | South- Eastern (SE) | M. angustifolia |
| 17 | Sa Kaeo | South- Eastern (SE) | M. angustifolia |
| 18 | Chanthaburi | South- Eastern (SE) | M. angustifolia |
| 19 | Nakhon Si Thammarat | Peninsular (PEN) | M. angustifolia |
| 20 | Phatthalung | Peninsular (PEN) | M. angustifolia |
| 21 | Surat Thani | Peninsular (PEN) | M. angustifolia |
| 22 | Phangnga | Peninsular (PEN) | M. angustifolia |
| 23 | Trat | South- Eastern (SE) | M. angustifolia |
| 24 | Ranong | Peninsular (PEN) | M. angustifolia |
| 25 | Krabi | Peninsular (PEN) | M. angustifolia |
| Outgroup 1 | Nakhon Sawan | Northern (N) | M. hastata |
| Outgroup 2 | Loei | North-Eastern (NE) | M. hastata |
| Outgroup 3 | Nakhon Ratchasima | Eastern (E) | M. hastata |
| Outgroup 4 | Suphan Buri | Central (C) | M. hastata |
| Outgroup 5 | Chachoengsao | South- Eastern (SE) | M. hastata |
| Outgroup 6 | Ratchaburi | South-Western (SW) | M. hastata |
| Flavonoid | Retention Time (tR) | Calibration Curve | LOD (µg/mL) | LOQ (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | RSD (%) | Rs | Sym Fact | Slope | Intercept | R2 | |||
| Api-7-Rut | 19.90 | 0.76 | 3.33 | 1.07 | 2291.9 | 569.6 | 0.9996 | 0.08 | 0.25 |
| Lut-7-Glc | 29.19 | 0.20 | 3.63 | 1.01 | 2553.3 | 157.1 | 0.9994 | 0.02 | 0.06 |
| Api-7-Glc | 32.58 | 0.51 | 3.04 | 1.11 | 2468.8 | 642.5 | 0.9999 | 0.09 | 0.26 |
| Lut | 36.28 | 0.41 | 1.87 | 1.03 | 3670.2 | 285.1 | 0.9997 | 0.03 | 0.08 |
| Api | 40.56 | 0.24 | 1.67 | 0.98 | 3742.3 | 250.5 | 0.9992 | 0.02 | 0.07 |
| Flavonoid | Concentration | RSD (%) | HortRat | Accuracy | Intra-Day Precision | Inter-Day Precision | |||
|---|---|---|---|---|---|---|---|---|---|
| (mg/g DW) | Recovery (%) | RSD | % | RSD | % | RSD | |||
| Api-7-Rut | 22.04 ± 0.02 | 0.09 | 0.03 | 100.32 | 1.34 | 99.15 | 3.88 | 99.85 | 0.67 |
| Lut-7-Glc | 18.91 ± 0.13 | 0.67 | 0.22 | 99.90 | 0.46 | 99.37 | 3.35 | 95.72 | 3.67 |
| Api-7-Glc | 36.84 ± 0.49 | 1.33 | 0.39 | 99.80 | 0.53 | 98.80 | 3.31 | 97.91 | 4.81 |
| Lut | 25.09 ± 0.15 | 0.58 | 0.18 | 100.11 | 0.41 | 99.64 | 1.43 | 98.77 | 4.97 |
| Api | 3.41 ± 0.20 | 5.93 | 2.47 | 98.40 | 3.10 | 97.99 | 4.31 | 96.98 | 4.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Drouet, S.; Garros, L.; Lorenzo, J.M.; Hano, C. Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants 2022, 11, 952. https://doi.org/10.3390/antiox11050952
Tungmunnithum D, Drouet S, Garros L, Lorenzo JM, Hano C. Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants. 2022; 11(5):952. https://doi.org/10.3390/antiox11050952
Chicago/Turabian StyleTungmunnithum, Duangjai, Samantha Drouet, Laurine Garros, Jose Manuel Lorenzo, and Christophe Hano. 2022. "Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl" Antioxidants 11, no. 5: 952. https://doi.org/10.3390/antiox11050952
APA StyleTungmunnithum, D., Drouet, S., Garros, L., Lorenzo, J. M., & Hano, C. (2022). Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants, 11(5), 952. https://doi.org/10.3390/antiox11050952









