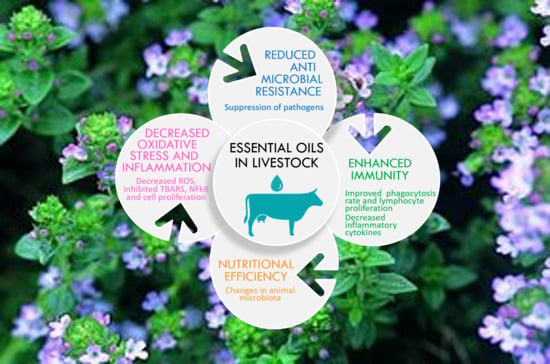
Essential Oils in Livestock: From Health to Food Quality
Abstract
1. Introduction
2. Methods
3. Importance of EOs
3.1. Definition of EOs
3.2. Historical Use of EOs
3.3. Current Use of EOs
4. Extraction Methods for EOs
4.1. Conventional Extraction Methods
4.2. Innovative Extraction Methods
5. Chemical Composition of EOs
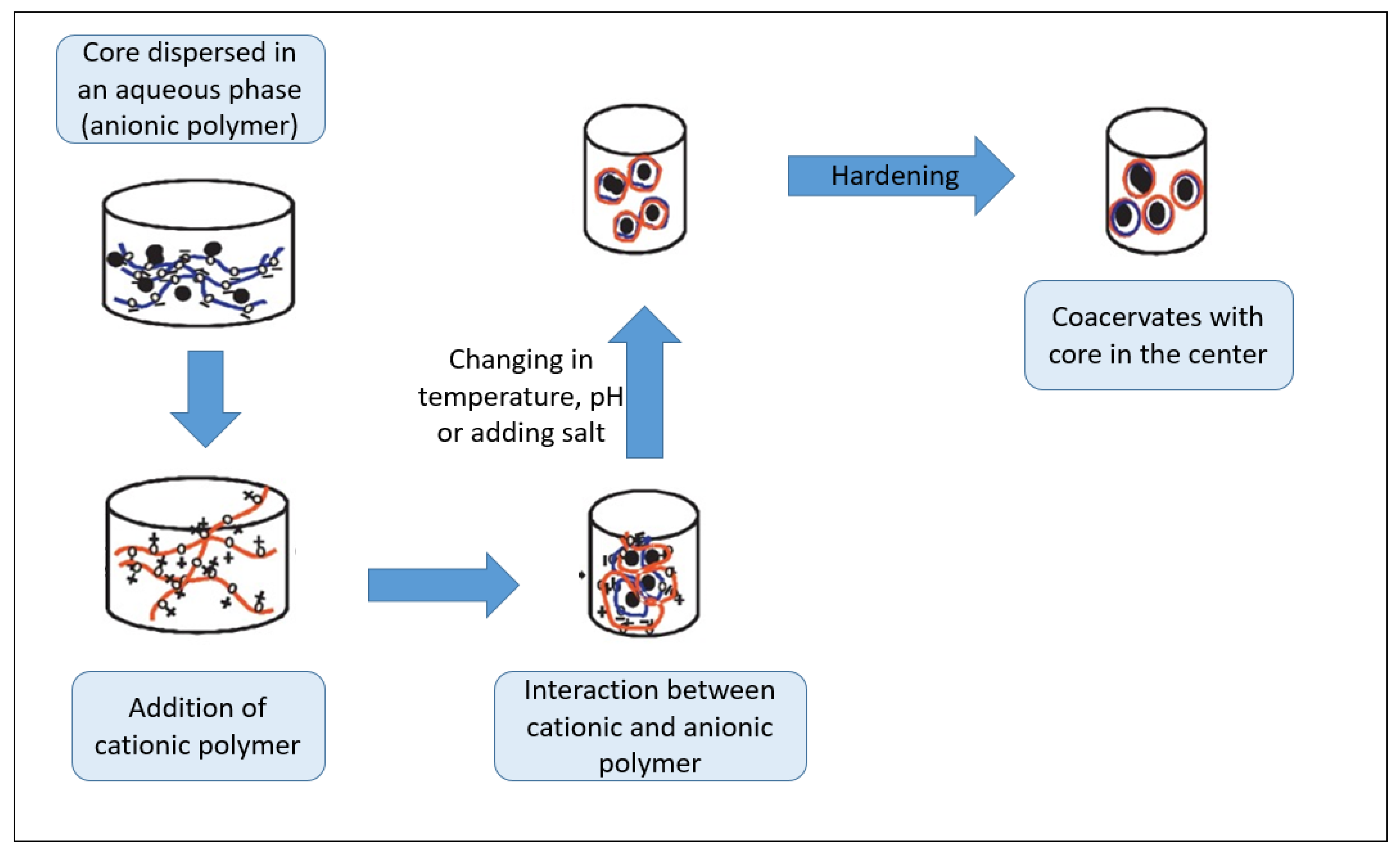
6. Stability and Encapsulation of EOs
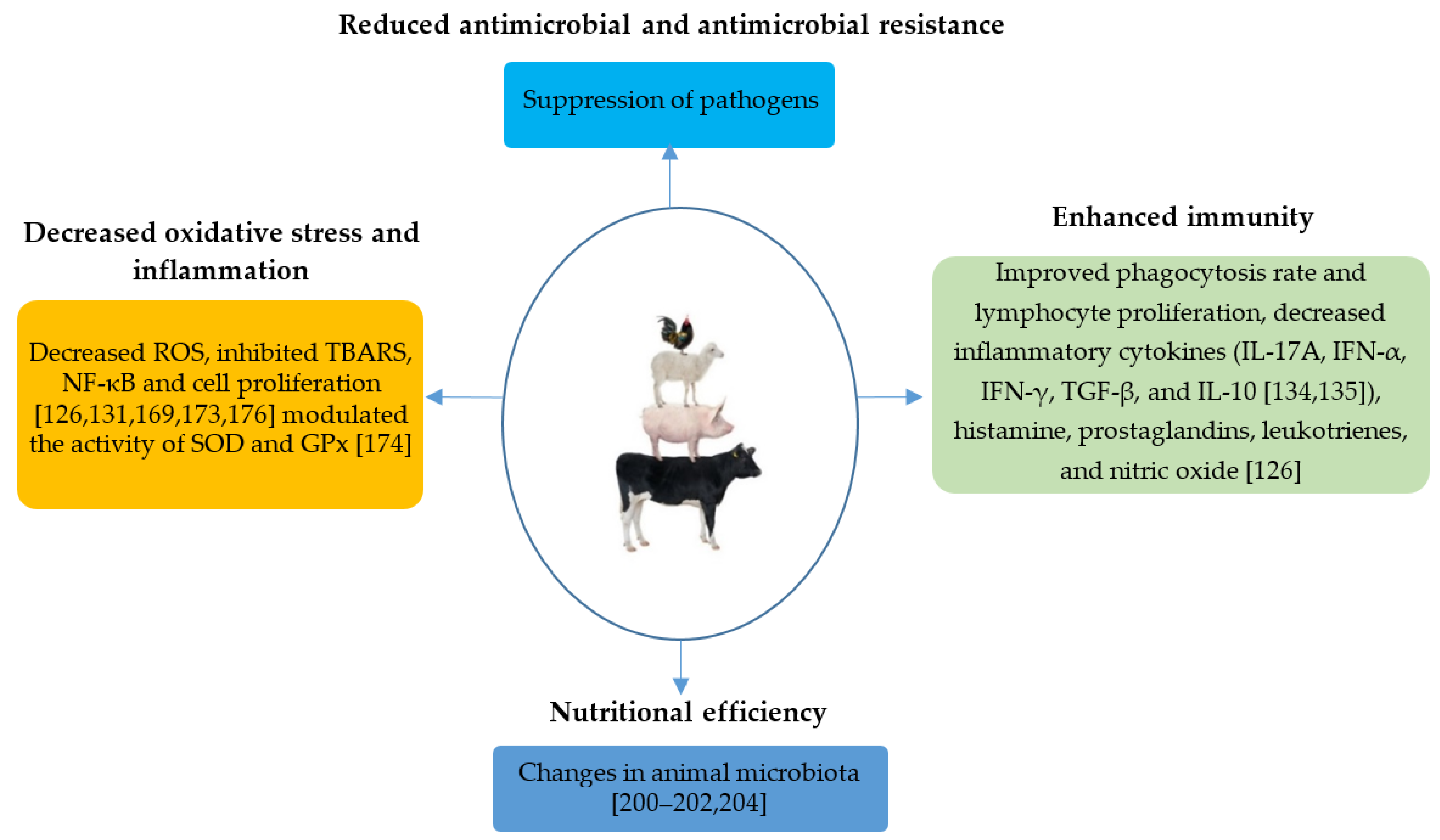
7. Main Properties of EOs
7.1. Antimicrobial Activity
7.2. AntiInflammatory Properties
8. EO and Inflammation: The Bovine Mastitis as a Paradigm Case
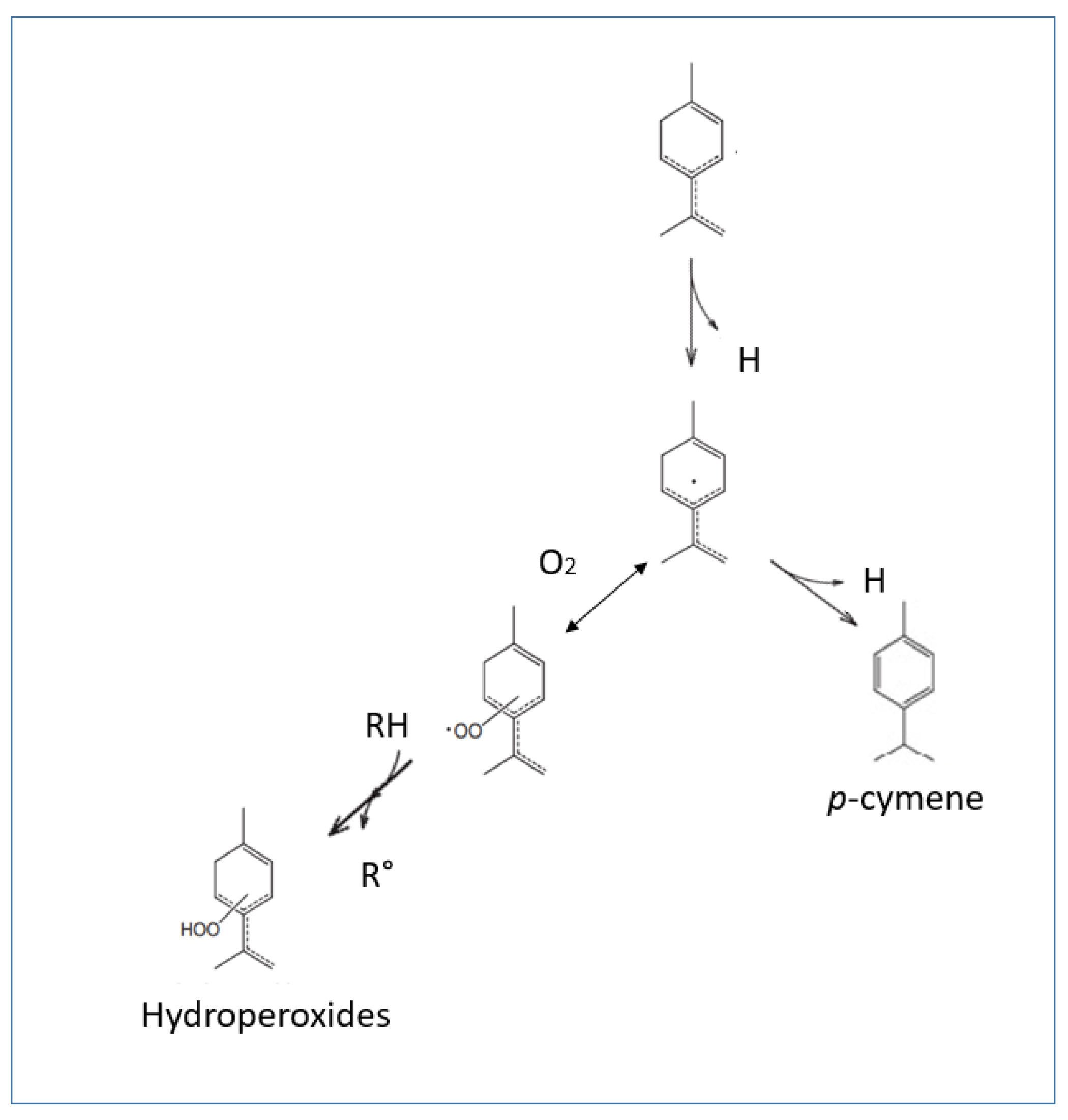
Antioxidant Activity and Mechanisms of Action
| Essential Oil (Plant Species) | Location (Plant Part) | Main Components | In Vitro Assay, Bioactivity | References |
|---|---|---|---|---|
| Sage (Salvia officinalis L.) | Saudi Arabia (leaves) | Camphor (20.3%), 1,8-cineole (15.0%), α-thujone (14.9%), viridiflorol (9.9%), carvone (6.2%), β-thujone (5.7%) | DPPH, IC50 = 970 µg/mL | [178] |
| Morocco (leaves) | Camphor (24.1%), α-thujone (21.4%), 1,8-Cineole (16.5%), α-pinene (11.2%), camphene (6.9%) | DPPH, IC50 = 2.12 mg/mL (> Q and AA); BCB, RC50 = 3.78 mg/mL (> Q and BHT); FRAP, EC50 = 2.98 mg/mL (> Q and AA) | [179] | |
| Spain (leaves and flowers) | Camphor (25.0%), 1,8-cineole (24.7%), camphene (7.6%), α-pinene (6.8 %), α-terpinyl acetate (6.0%) | DPPH, IC50 = 4.20 mg/mL (> AA and BHT); TBARS, EC50 = 35.56 mg/mL (> AA and BHT); FIC, EC50 = 7.16 mg/mL (< AA and BHT); Rancimat, AAI = 1.07 (< AA and BHT) | [167,180] | |
| Tunisia (leaves) | Camphor (25.1%), α-thujone (18.8%), 1,8-cineole (14.1%), viridiflorol (8.0%), β-thujone (4.5%), and β-caryophyllene (3.3%) | DPPH, IC50 = 6.7 mg/mL (> BHT); FRAP, IC50 = 28.4 mg/mL (> BHT); LAP inhibition, IC50 = 9.6 mg/mL (> α-tocopherol) | [181] | |
| Laurel (Laurus nobilis L.) | Morocco; (Flowers) | 1.8-Cineole (45.0%), α-caryophyllene (7.5%), germacradienol (6.1%), limonene (4.7%), α-pinene (3.0%), germacrene D (3.1%) | DPPH, IC50 = 82.01 µg/mL (> BHT and AA) | [182] |
| Turkey (leaves) | 1,8-Cineole (51.8%), α-terpinyl acetate (11.2%), sabinene (10.1%), α-terpineol (5.2%) | DPPH, IC50 = 59.2 µg/mL (> BHT and AA); BCB, RAA = 76.8 % (< BHT and AA) | [183] | |
| Lebanon (leaves) | 1,8-Cineole (35.2%), 1-p-menthen-8-ethyl acetate (13.5%), linalool (7.1%), sabinene (6.2%), α-pinene (5.7%) | DPPH, IC50 = 53.5 µg/mL (> AA); BCB, IC50 = 35.6–38.9 µg/mL (> PG) | [184] | |
| Lebanon (seeds) | β- Ocimene (21.8%), 1,8-cineole (9.4%), α-pinene (3.7%), β-pinene (2.1%) | DPPH, IC50 = 66.1 µg/mL (> AA); BCB, IC50 = 41.1–45.9 µg/mL (> PG) | [184] | |
| Coriander (Coriandrum sativum L.) | Slovakia (fruit) | β-Linalool (66.1%), camphor (8.3%), geranyl acetate (6.9%), cymene (6.4%) | DPPH, IC50 ~ 39.38 mg TEAC/L | [185] |
| Rosemary (Rosmarinus officinalis L.) | Morocco (leaves) | 1,8-Cineole (42.3%), camphor (14.8%), α-pinene (8.9%), β-pinene (6.3%), α-terpineol (5.6%), borneol (4.9%) | DPPH, IC50 = 4.82 mg/mL (> Q and AA); BCB, RC50 = 5.79 mg/mL (> Q and BHT); FRAP, EC50 = 5.62 mg/mL (> Q and AA) | [179] |
| Serbia (leaves) | Limonene (21.7%), camphor (21.6%), α-pinene (13.5%), Z-linalool oxide (10.8%) | DPPH, IC50 = 3.82 µL/mL (< BHT) | [186] | |
| Serbia (aerial parts) | 1,8-Cineole (43.8%), camphor (12.5%), α-pinene (11.5%), β-pinene (8.2%) | DPPH, IC50 = 77.6 μL/mL (= α-tocopherol) | [187] | |
| Conehead thyme (Thymus capitatus) | Morocco (aerial parts) | p-Cymene (18.9%), carvacrol (13.4%), geranyl acetate (12.2%), borneol (10.2%) | DPPH, IC50 = 103 µg/mL (> AA) | [188] |
| Tunisia (aerial parts) | Carvacrol (65.6–80.7%), p-cymene (4.8–8.9%), γ-terpinene (5.3–13.7%), β-caryophyllene (1.8–3.2%) | DPPH, IC50 ~ 250 µg/mL (< BHT); TBARS, IC50 < 100 µg/mL | [168] | |
| Algeria (leaves) | Thymol (51.2%), carvacrol (12.6%), γ-terpinene (10.3%) | DPPH, IC50 = 0.62 µg/mL (< BHA, AA, and thymol); FRAP, EC50 = 2.13 µg/mL (< BHA, AA, and thymol); TAC, EC50 = 0.78 µg/mL (< BHA, AA, and thymol) | [189] | |
| Black cumin (Nigella sativa) | Austria (seeds) | Thymoquinone (30–48%), p-cymene (7–15%), carvacrol (6–12%), 4-terpineol (2–7%), longifolene (1–8%), t-anethole (1–4%) | DPPH, IC50 = 460 µg/mL (> BHT, AA, Q, carvacrol, and thymoquinone); •OH, IC50 = 0.011 µg/mL (< carvacrol and thymoquinone) | [76] |
| Iran (seeds) | Thymoquinone (20.3 %), camphene (11.0 %), thymol (10.1 %), β-pinene (7.0 %), α-thujene (6.0%), γ-terpinene (5.1 %) | DPPH, IC50 = 19 µg/mL (> BHA, AA, and thymoquinone, but < α-thujene, β-pinene, p-cymene, and γ-terpinene) | [175] | |
| Tunisia (seeds) | p-Cymene (60.5%), α-thujene (6.9%), γ-terpinene (3.5%), thymoquinone (3.0%), β-pinene (2.4%), carvacrol (2.4%), terpinen-4-ol (2.1%) | DCFH (ROS production), IC50 = 1 µg/mL (< Q) | [176] | |
| Cade (Juniperus oxycedrus) | Tunisia (leaves) | β-Phellandrene (36.8%), α-terpinolene (13.2%), β-myrcene (9.1%), α-campholenal (9.0%), and p-cymene (6.0%) | DPPH, IC50 = 20.1 µg/mL (< BHT); FRAP, 15.9 μmol Fe2+/g | [190] |
| Turkey (needles) | Caryophyllene oxide (31.6%), α-pinene (24.3%), caryophyllene (10.0%) | DPPH, IC50 < 40 µg/mL (< BHA and BHT); FRAP, 179.6 µmol TX/g (< BHA and BHT) | [191] | |
| Turkey (cones) | β-Pinene (29.9%), α-pinene (26.6%), limonene (9.7%) | DPPH, IC50 < 40 µg/mL (< BHA and BHT); FRAP, 415.38 µmol TX/g (< BHA and BHT) | [191] | |
| Algeria (aerial parts) | α-Pinene (37.8%), abietadiene (8.3%), bulnesol (7.2%), manoyl oxide (5.0%), germacrene D (4.8%) | DPPH, IC50 = 91.25 mg/mL (> AA); FRAP, 0.97 µmol TX/g (< AA); ABTS, 5.82 µmol TX/g (< AA) | [192] | |
| Geranium (Pelargonium graveolens) | India (leaves) | Citronellol (37.0%), geraniol (18.0%), citronellyl formate (5.5%), linalool (4.1%), rose oxide (2.4%), geranyl formate (2.2%) | DPPH, IC50 = 18.02 μg/mL (> AA); NO, IC50 = 19.98 µg/mL (> AA) | [193] |
| Egypt (leaves) | β-Citronellol (35.9%), geraniol (11.7%), citronellyl formate (11.4%), linalool (9.6%), (+)-isomenthone (6.4%), σ-selinene (5.5%) | DPPH, IC50 = 6.2 μg/mL (> BHT); BCLA, IC50 = 4.1 μg/mL (> BHT) | [194] | |
| Oregano (Origanum vulgare) | Croatia (flowered tops and stalks) | Thymol (35.0%), carvacrol (32.0%), γ-terpinene (10.5.%), p-cymene (9.1%), α-terpinene (3.6%) | DPPH, IC50 = 0.5 mg/mL (> BHT, AA, and α-tocopherol, but = thymol); TBARS, AAI = 29.9% (< BHT, α-tocopherol, but = thymol and carvacrol) | [169] |
| Chile (aerial parts) | Thymol (15.9%), Z-sabinene hydrate (13.4%), γ-terpinene (10.6%), p-cymene (8.6%), linalyl acetate (7.2%), sabinene (6.5%), carvacrol methyl ether (5.6%), carvacrol (3.1%) | DPPH, IC50 = 4.75 mg/mL (> Q and TX); ABTS, 1252.7 µmol TX/g; FRAP, 270.5 µmol TX/g | [195] | |
| Algeria (aerial parts) | p-Cymene (25.6%), thymol (23.1%), carvacrol (20.3%), γ-terpinene (16.6%), α-terpinene (1.8%) | DPPH, IC50 = 0.461 mg/mL (> AA) | [166] | |
| White wormwood (Artemisia herba-alba) | Algeria (aerial parts) | Davanone D (49.5%), camphor (10%), trans-γ-cadinene (3.9%) | DPPH, IC50 = 2.61 mg/mL (> than AA); FRAP, 8.17 µmol TX/g (< AA); ABTS, 6.74 µmol TX/g (< AA) | [192] |
| Morocco (aerial parts) | β-Thujone (42.9%), chrysanthenone, α-terpineol (9.7%), α-thujone (5.4%), α-pinene (4.6%), bornyl acetate (2.4%) | DPPH, IC50 = 2.9 µg/mL (> BHT) | [196] | |
| Tunisia (aerial parts) | α-Thujone (17.6%), trans-sabinyl acetate (17.2%), chrysanthenone (8.3%), β-thujone (7.6%), germacrene D (7.0%) | DPPH, IC50 = 1.17 mg/mL (> TX); FRAP, 161.6 µmol Fe2+/g; BCB, 0.6 mg/mL (> BHT) | [197] |
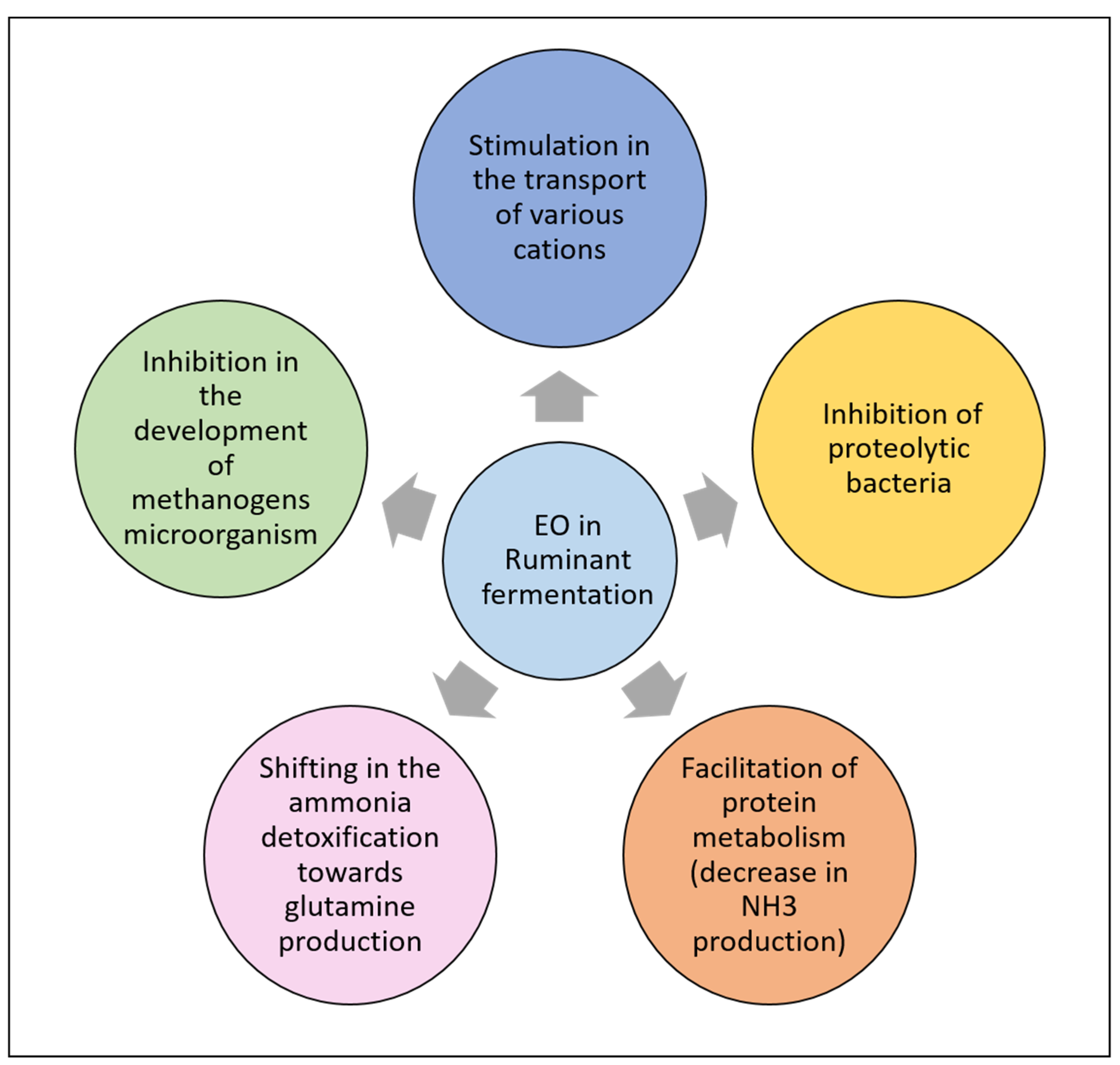
9. EOs and Rumen Fermentation
10. EOs in Animal Nutrition
11. EOs in Food Products
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| EO | Essential oil |
| LPS | Lipopolysaccharides |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| BCB | β-carotene bleaching |
| FRAP | Ferric-reducing antioxidant power |
| TBARS | Thiobarbituric acid-reactive species |
| FIC | Ferrous ion-chelating |
| BCLA | β-carotene-linoleic acid assay |
| AAI | Antioxidant activity index |
| TAC | Total antioxidant capacity |
| RAA | Relative antioxidant activity |
| TEAC | Trolox equivalent antioxidant capacity |
| LAP | Linoleic acid peroxidation |
| DCFH | 2′,7′-dichloro-fluorescin-diacetate |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| AA | Ascorbic acid |
| Q | Quercetin |
| PG | propyl gallate |
| TNF-α | Tumor necrosis factor-α |
| IL | Interleukin |
| DAO | Diamine oxidase |
| Ig | Immunoglobulin |
| ZO-1 | Zonula occludens-1 |
| NF-κB | Nuclear Factor Kappa B |
| BAX | Bcl-2 Associate X protein |
| TGF-β | Transforming growth factor beta |
| IFN | Interferon |
| GPx | Glutathione peroxidase |
| SOD | superoxide dismutase |
| ROS | Reactive oxygen species |
| FA | fatty acids |
| ADME | absorption distribution metabolism and excretion |
| IC50 | The half maximal inhibitory concentration |
References
- Lesage, M. Les Antibiorésistances en Élevage: Vers des Solutions Intégrées. MONTREUIL SOUS BOIS. Available online: https://agriculture.gouv.fr/sites/minagri/files/cep_analyse82_antibioresistances_en_elevage.pdf (accessed on 13 January 2021).
- Wall, B.A.; Mateus, A.L.P.; Marshall, L.; Pfeiffer, D.U.; Lubroth, J.; Ormel, H.J.; Otto, P.; Patriarchi, A. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Available online: http://www.fao.org/publications/card/fr/c/d5f6d40d-ef08-4fcc-866b-5e5a92a12dbf/ (accessed on 14 February 2021).
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, G.F.; Zhu, B. The antibiotic resistome: Gene flow in environments, animals and human beings. Front. Med. 2017, 11, 161–168. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Simitzis, P.E. Enrichment of Animal Diets with Essential Oils—A Great Perspective on Improving Animal Performance and Quality Characteristics of the Derived Products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Shah, A.M.; Shah, A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine mastitis: An appraisal of its alternative herbal cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Jean, B. Pharmacognosy Phytochemistry, Medicinal Plants, 2nd ed; Lavoisier: Paris, France, 2008; Volume 934. [Google Scholar]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Zhao, X.; Meng, R.; Liu, Z.; Chen, X.; Guo, N. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Fernandes RV de, B.; Guimarães, I.C.; Ferreira, C.L.R.; Botrel, D.A.; Borges, S.V.; de Souza, A.U. Microencapsulated Rosemary (Rosmarinus officinalis) Essential Oil as a Biopreservative in Minas Frescal Cheese. J. Food Process. Preserv. 2017, 41, e12759. [Google Scholar] [CrossRef]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Carmona, M.; Zalacain, A. Dairy matrix effect on the transference of rosemary (Rosmarinus officinalis) essential oil compounds during cheese making. J. Sci. Food Agric. 2015, 95, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. (2007, May). Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef]
- Jang, I.S.; Ko, Y.H.; Kang, S.Y.; Lee, C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Cetin, E.; Yibar, A.; Yesilbag, D.; Cetin, I.; Cengiz, S.S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 2016, 57, 780–787. [Google Scholar] [CrossRef]
- Adaszýnska-Skwirzýnska, M.; Szczerbínska, D. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. 2019, 98, 358–365. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X.G. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Boyle, W. Spices and Essential Oils as Presservatives. In The American Perfumer and Essential Oil Review 66; 1955; pp. 25–28. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=902045 (accessed on 30 January 2021).
- Luebbe, K.M.; Stalker, L.A.; Klopfenstein, T.J.; Funston, R.N. Influence of weaning date and late gestation supplementation on beef system productivity I: Animal performance. Transl. Anim. Sci. 2019, 3, 1492–1501. [Google Scholar] [CrossRef]
- Funston, R.N.; Summers, A.F. Effect of prenatal programming on heifer development. Vet. Clin. Food Anim. Pract. 2013, 29, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Kasaian, J.; Asili, J.; Iranshahi, M. Sulphur-containing compounds in the essential oil of Ferula alliacea roots and their mass spectral fragmentation patterns. Pharm. Biol. 2016, 54, 2264–2268. [Google Scholar] [CrossRef]
- Guenther, E. The Essential Oils; D. Van Nostrand: New York, NY, USA, 1948. [Google Scholar]
- Van de Braak, S.A.A.J.; Leijten, G.C.J.J. Essential Oils and Oleoresins: A Survey in the Netherlands and other Major Markets in the European Union; CBI, Centre for the Promotion of Imports from Developing Countries: Rotterdam, The Netherlands, 1999; p. 116. [Google Scholar]
- Shelef, L.A.; Jyothi, E.K.; Bulgarellii, M.A. Growth of Enteropathogenic and Spoilage Bacteria in Sage-Containing Broth and Foods. J. Food Sci. 1984, 49, 737–740. [Google Scholar] [CrossRef]
- Nychas, G.J.E. Natural antimicrobials from plants. In New Methods of Food Preservation; Gould, G.W., Ed.; Blackie Academic and Professional: London, UK, 1995; pp. 58–89. [Google Scholar] [CrossRef]
- Tuley de Silva, K. A Manual on the Essential Oil Industry; United Nations Industrial Development Organization, Vienna, Austria, 1996.
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Juglal, S.; Govinden, R.; Odhav, B. Spice oils for the control of co-occurring mycotoxin-producing fungi. J. Food Prot. 2002, 65, 683–687. [Google Scholar] [CrossRef]
- Pandey, R.; Kalra, A.; Tandon, S.; Mehrotra, N.; Singh, H.N.; Kumar, S. Essential Oils as Potent Source of Nematicidal Compounds. J. Phytopathol. 2000, 148, 501–502. [Google Scholar] [CrossRef]
- Pessoa, L.M.; Morais, S.M.; Bevilaqua, C.M.L.; Luciano, J.H.S. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet. Parasitol. 2002, 109, 59–63. [Google Scholar] [CrossRef]
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z.G. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 1992, 48, 616–619. [Google Scholar] [CrossRef]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Oregano Essential Oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Weinheim, Germany, 2001; p. 293. [Google Scholar]
- Crosthwaite, D. UK trade within the flavour and fragrance industry. In Proceedings of the International Federation of Essential Oils and Aroma Trades—21st International Conference on Essential Oils and Aroma’s 1998; IFEAT: London, UK, 1998; pp. 6–12. [Google Scholar]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the essential oil of Melaleuca alternifolia. Lett. Appl. Microbiol. 1993, 16, 49–55. [Google Scholar] [CrossRef]
- KBauer und, D. Garbe: Common Fragrance and Flavour Materials: Preparation, Properties and Uses; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Poolman, B.; Smid, E.J. S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crop. Prod. 1995, 4, 23–31. [Google Scholar] [CrossRef]
- Manabe, A.; Nakayama, S.; Sakamoto, K. Effects of Essential Oils on Erythrocytes and Hepatocytes from Rats and Dipalmitoyl Phosphatidylcholine-Liposomes. Jpn. J. Pharmacol. 1987, 44, 77–84. [Google Scholar] [CrossRef]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 3rd ed.; John Wiley & Sons: Weinheim, Germany, 2008; Available online: https://www.wiley.com/en-us/Common+Fragrance+and+Flavor+Materials%3A+Preparation%2C+Properties+and+Uses%2C+3rd+Edition-p-9783527612376 (accessed on 1 February 2021).
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (Tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Van Krimpen, M.M.; Binnendijk, G. RopadiarR as alter252 S. Burt. International Journal of Food Microbiology 94, 223–253. Retrieved from native for antimicrobial growth promoter in diets of weanling pigs. Lelystad, Praktijkonderzoek Veehouderij. May 2001; ISSN 0169-3689. p. 14. [Google Scholar]
- Ilsley, S.; Miller, H.; Greathead, H.; Kamel, C. Herbal sow diets boost pre-weaning growth. Pig Progress 2002, 18, 8–10. [Google Scholar]
- Mendoza-Yepes, M.J.; Sanchez-Hidalgo, L.E.; Maertens, G.; Marin-Iniesta, F. Inhibition Of Listeria Monocytogenes And Other Bacteria By A Plant Essential Oil (Dmc) In Spanish Soft Cheese. J. Food Saf. 1997, 17, 47–55. [Google Scholar] [CrossRef]
- Cutter, C.N. Antimicrobial effect of herb extracts against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella Typhimurium associated with beef. J. Food Prot. 2000, 63, 601–607. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Comparison of Chemical Composition and Biological Properties of Essential Oils Obtained by Hydrodistillation and Steam Distillation of Laurus nobilis L. Plant Foods Hum. Nutr. 2020, 75, 495–504. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2009, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, N.; Abert Vian, M.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Özel, M.Z.; Göǧüş, F.; Lewis, A.C. Comparison of direct thermal desorption with water distillation and superheated water extraction for the analysis of volatile components of Rosa damascena Mill. using GCxGC-TOF/MS. Anal. Chim. Acta 2006, 566, 172–177. [Google Scholar] [CrossRef]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Mattana, A.; Usai, M. Composition and in vitro antimicrobial activity of the essential oil of thymus herba-barona loisel growing wild in sardinia. J. Essent. Oil Res. 2000, 12, 516–522. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venancio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Academic Press: Cambridge, MA, USA, 2017; pp. 379–419. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn essential oils of Greek oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastelic, J.; Milos, M. The impact of both the season of collection and drying on the volatile constituents of Origanum vulgare L. ssp. hirtum grown wild in Croatia. Int. J. Food Sci. Technol. 2001, 36, 649–654. [Google Scholar] [CrossRef]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of Salinity and Water Stress on the Essential Oil Components of Rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
- Nejad Ebrahimi, S.; Hadian, J.; Ranjbar, H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294. [Google Scholar] [CrossRef]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Hammami, M.; Marzouk, B. Effects of growing region and maturity stages on oil yield and fatty acid composition of coriander (Coriandrum sativum L.) fruit. Sci. Hortic. 2009, 120, 525–531. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 39–44. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Lens-Lisbonne, C.; Cremieux, A.; Maillard, C.; Balansard, G. Methodes d’evaluation de l’activite´ antibacterienne des huiles essentielles: Application aux essences de thym et de cannelle. J. De Pharm. De Belg. 1987, 42, 297–302. [Google Scholar]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Chelghoum, C. Essential oil composition of Juniperus oxycedrus growing in Algeria. Pharm. Biol. 2006, 44, 1–6. [Google Scholar] [CrossRef]
- Rana, V.S.; Juyal, J.P.; Blazquez, M.A. Chemical constituents of essential oil of Pelargonium graveolens leaves. Int. J. Aromather. 2002, 12, 216–218. [Google Scholar] [CrossRef]
- Lawrence, B.M. The botanical and chemical aspects of oregano. Perfum. Flavorist 1984, 9, 41–51. [Google Scholar]
- Prudent, D.; Perineau, F.; Bessiere, J.M.; Michel, G.M.; Baccou, J.C. Analysis of the essential oil of wild oregano from martinique (Coleus aromaticus Benth.)—Evaluation of its bacteriostatic and fungistatic properties. J. Essent. Oil Res. 1995, 7, 165–173. [Google Scholar] [CrossRef]
- Charai, M.; Mosaddak, M.; Faid, M. Chemical Composition and Antimicrobial Activities of Two Aromatic Plants: Origanum majorana L. and O. compactum Benth. J. Essent. Oil Res. 1996, 8, 657–664. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and cytotoxic activities of Origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Demetzos, C.; Perdetzoglou, D.K.; Tan, K. Composition and antimicrobial studies of the oils of Origanum calcaratum juss and O. scabrum boiss. et Heldr. from Greece. J. Essent. Oil Res. 2001, 13, 460–462. [Google Scholar] [CrossRef]
- Bourgou, S.; Tammar, S.; Salem, N.; Mkadmini, K.; Msaada, K. Phenolic Composition, Essential Oil, and Antioxidant Activity in the Aerial Part of Artemisia Herba-Alba from Several Provenances: A Comparative Study. Int. J. Food Prop. 2016, 19, 549–563. [Google Scholar] [CrossRef]
- Hernandez, E. Essential Oils | Distillation. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 2739–2744. [Google Scholar] [CrossRef]
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2005, 10, 813–829. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. Lwt Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Taylor, L. The Healing Power of Rainforest Herbs: A Guide to Understanding and Using Herbal Medicinals; Square One Publishers: New York, NY, USA, 2005. [Google Scholar]
- Selim, S. Antimicrobial activity of essential oils against vancomycin-resistant enterococci (vre) and Escherichia coli o157:h7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. (Eds.) Encyclopedia of Analytical Science, 2nd ed.; Elsevier Inc: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 2019; pp. 554–561. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Özbek, Z.A.; Ergönül, P.G. A Review on Encapsulation of Oils. Celal Bayar Üniversitesi Fen Bilimleri Dergisi 2017, 13, 293–309. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Anis, A.; Pal, K. Review on Encapsulation of Vegetable Oils: Strategies, Preparation Methods, and Applications. Polym. Plast. Technol. Eng. 2016, 55, 291–311. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Nehme, R.; Blel, W.; Montillet, A.; Bellettre, J.; Marchal, L. Production of oil in water emulsions in microchannels at high throughput: Evaluation of emulsions in view of cosmetic, nutraceutical or pharmaceutical applications. Chem. Eng. Process. Process Intensif. 2021, 161, 108301. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Almeida, F.N.; Htoo, J.K.; Thomson, J.; Stein, H.H. Amino acid digestibility in camelina products fed to growing pigs. Can. J. Anim. Sci. 2013, 93, 335–343. [Google Scholar] [CrossRef]
- Ghalem, B.R.; Zouaoui, B. Microbiological, physico-chemical and sensory quality aspects of yoghurt enriched with Rosmarinus officinalis oil. Afr. J. Biotechnol. 2013, 12, 192–198. [Google Scholar] [CrossRef]
- Shah, B.; Davidson, P.M.; Zhong, Q. Encapsulation of eugenol using Maillard-type conjugates to form transparent and heat stable nanoscale dispersions. Lwt Food Sci. Technol. 2012, 49, 139–148. [Google Scholar] [CrossRef]
- Benjemaa, M.; Neves, M.A.; Falleh, H.; Isoda, H.; Ksouri, R.; Nakajima, M. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Ind. Crop. Prod. 2018, 113, 414–421. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Chapeau, A.L.; Bertrand, N.; Briard-Bion, V.; Hamon, P.; Poncelet, D.; Bouhallab, S. Coacervates of whey proteins to protect and improve the oral delivery of a bioactive molecule. J. Funct. Foods 2017, 38, 197–204. [Google Scholar] [CrossRef]
- Hajjali, H. Assemblage Nanoparticules Lipidiques Solides-Polysaccharide: Étude des Propriétés Physico-Chimiques Pour la Vectorisation d’un Polyphénol. Université de Lorraine, France 2015. Available online: http://www.culture.gouv.fr/culture/infos-pratiques/droits/protection.htm (accessed on 4 January 2021).
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef]
- Kulkarni, M.; Greiser, U.; O’Brien, T.; Pandit, A. Liposomal gene delivery mediated by tissue-engineered scaffolds. Trends Biotechnol. 2010, 28, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Setya, S.; Talegaonkar, S. Nanoemulsions:formulation methods and stability aspects. World J. Pharm. Pharm. Sci. 2014, 3, 2214–2228. [Google Scholar]
- Liu, C.H.; Yu, S.Y. Cationic nanoemulsions as non-viral vectors for plasmid DNA delivery. Colloids Surf. B Biointerfaces 2010, 79, 509–515. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to prepare and stabilize very small nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Alhaj, N.A.; Shamsudin, M.N.; Alipiah, N.M.; Zamri, H.F.; Bustamam, A.; Ibrahim, S.; Abdullah, R. Characterization of Nigella sativa L. essential oil-loaded solid lipid nanoparticles. Am. J. Pharmacol. Toxicol. 2010, 5, 52–57. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Talbaoui, A.; Khouchlaa, A.; Charfi, S.; Abrini, J.; Dakka, N. Résistance aux antibiotiques et mécanismes d’action des huiles essentielles contre les bactériesResistance to antibiotics and mechanisms of action of essential oils against bacteria. Phytothérapie 2017. [Google Scholar] [CrossRef]
- Scotti, R.; Stringaro, A.; Nicolini, L.; Zanellato, M.; Boccia, P.; Maggi, F.; Gabbianelli, R. Effects of Essential Oils from Cymbopogon spp. and Cinnamomum verum on Biofilm and Virulence Properties of Escherichia coli O157:H7. Antibiotics 2021, 10, 113. [Google Scholar] [CrossRef]
- Assane, I.M.; Valladão, G.M.R.; Pilarski, F. Chemical composition, cytotoxicity and antimicrobial activity of selected plant-derived essential oils against fish pathogens. Aquac. Res. 2021, 52, 793–809. [Google Scholar] [CrossRef]
- Iseppi, R.; Tardugno, R.; Brighenti, V.; Benvenuti, S.; Sabia, C.; Pellati, F.; Messi, P. Phytochemical Composition and In Vitro Antimicrobial Activity of Essential Oils from the Lamiaceae Family against Streptococcus agalactiae and Candida albicans Biofilms. Antibiotics 2020, 9, 592. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A Comparative Study of the in Vitro Antimicrobial and Synergistic Effect of Essential Oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with Antimicrobial Drugs: New Approach for Health Promoting Products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 2020, 272. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, onion, and cinnamon essential oil anti-biofilms’ effect against listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Chandrakanthan, M.; Handunnetti, S.M.; Sirimal, G.; Premakumara, A.; Kathirgamanathar, S. Topical Anti-Inflammatory Activity of Essential Oils of Alpinia calcarata Rosc., Its Main Constituents, and Possible Mechanism of Action. Evid. Based Complementary Altern. Med. 2020, 2020. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Sang Cho, K.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Inouye, S.; Abe, S. Nouvelle approache de l’aromathérapie anti-infectieuse. Phytotherapie 2007, 5, 2–4. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Anti-inflammatory Activity of Medicinal Plants: Present Status and Future Perspectives. In Botanical Leads for Drug Discovery; Springer: Singapore, 2020; pp. 67–92. [Google Scholar] [CrossRef]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia mill. Essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 2011, 166–167, 338–355. [Google Scholar] [CrossRef]
- Manzanilla, E.G.; Nofrarias, M.; Anguita, M.; Castillo, M.; Perez, J.F.; Martin-Orue, S.M.; Kamel, C.; Gasa, J. Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2006, 84, 2743–2751. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xiang, Q.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Improves Intestinal Morphology and Expression of Tight Junction Proteins Associated with Modulation of Selected Intestinal Bacteria and Immune Status in a Pig Model. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhao, Q.; Wang, H.; Wen, L.; Li, W.; Zhang, X.; Lin, W.; Li, H.; Xie, Q.; Wang, Y. Effects of antibacterial peptide combinations on growth performance, intestinal health, and immune function of broiler chickens. Poult. Sci. 2020, 99, 6481–6492. [Google Scholar] [CrossRef]
- Liu, S.D.; Song, M.H.; Yun, W.; Lee, J.H.; Kim, H.B.; Cho, J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019, 98, 2026–2033. [Google Scholar] [CrossRef]
- Montironi, I.D.; Reinoso, E.B.; Paullier, V.C.; Siri, M.I.; Pianzzola, M.J.; Moliva, M.; Campra, N.; Bagnis, G.; LaRocque-de-Freitas, I.F.; Decote-Ricardo, D.; et al. Minthostachys verticillata essential oil activates macrophage phagocytosis and modulates the innate immune response in a murine model of Enterococcus faecium mastitis. Res. Vet. Sci. 2019, 125, 333–344. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, R.; Marchesini, G.; Plaizier, J.C.; Li, S.; Khafipour, E.; Ricci, R.; Andrighetto, I.; Segato, S. Use of dicarboxylic acids and polyphenols to attenuate reticular pH drop and acute phase response in dairy heifers fed a high grain diet. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Keane, O.M. Symposium review: Intramammary infections—Major pathogens and strain-associated complexity. J. Dairy Sci. 2019, 102, 4713–4726. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet. Sci. 2020. [Google Scholar] [CrossRef]
- Hogeveen, H.; Van Der Voort, M. Assessing the economic impact of an endemic disease: The case of mastitis. Oie Rev. Sci. Tech. 2017, 36, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Nielen, M.; De Roos, A.P.W.; Van Hoorne, R.; de Jong, G.; Lam, T.J.G.M.; Van Werven, T.; Hogeveen, H. Production loss due to new subclinical mastitis in Dutch dairy cows estimated with a test-day model. J. Dairy Sci. 2009, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Rokana, N.; Chandra, M.; Singh, B.P.; Gulhane, R.D.; Gill, J.P.S.; Ray, P.; Puniya, A.K.; Panwar, H. Antimicrobial resistance: Its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 2018, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapong, S.; Khunkitti, W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011, 91. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Kher, M.N.; Sheth, N.R.; Bhatt, V.D. In Vitro Antibacterial Evaluation of Terminalia chebula as an Alternative of Antibiotics against Bovine Subclinical Mastitis. Anim. Biotechnol. 2019, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.K.; Zadoks, R.N.; Gaskins, C.T. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet. Microbiol. 2005, 107, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; El Rammouz, R.; Jammal, B.; Sleiman, M. In vitro and in vivo antimicrobial activity of two essential oils Thymus vulgaris and Lavandula angustifolia against bovine Staphylococcus and Streptococcus mastitis pathogen. Middle East J. Agric. Res. 2015, 4, 975–983. [Google Scholar]
- Cho, B.W.; Cha, C.N.; Lee, S.M.; Kim, M.J.; Park, J.Y.; Yoo, C.Y.; Son, S.E.; Kim, S.; Lee, H.J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Kammerer, M.; Le Guenic, M.; Roussel, P.; Linclau, O.; Cartaud, G.; Tainturier, D.; Larrat, M.; Bareille, N. Le traitement des mammites cliniques de la vache laitière par des huiles essentielles. Innov. Agron. 2009, 4, 79–83. [Google Scholar]
- Corre, C. L’utilisation de L’aromathérapie Dans les Élevages Français: État des Lieux, Efficacité et Limites. Available online: https://hal.inrae.fr/hal-02788515/document (accessed on 1 February 2021).
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2018, 61. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Hernandez, J.; Pereira, V.; Benedito, J.L. Update about Nutritional Strategies in Feedlot for Preventing Ruminal Acidosis. Adv. Zool. Res. 2012, 4, 1–84. [Google Scholar]
- Younsi, F.; Mehdi, S.; Aissi, O.; Rahali, N.; Jaouadi, R.; Boussaid, M.; Roura, E. Review on in vivo and in vitro methods evaluation of antioxidant activity. Flavour Fragr. J. 2020, 25, 282. [Google Scholar] [CrossRef]
- Amaral, A.B.; SILVA, M.V.D.; LANNES, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors a review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukić, N.; Orčić, D.; Lesjak, M.; Šibul, F. Essential oils as powerful antioxidants: Misconception or scientific fact. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1218, pp. 187–208. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bouhaddouda, N.; Aouadi, S.; Labiod, R. Evaluation of chemical composition and biological activities of essential oil and methanolic extract of Origanum vulgare L. ssp. glandulosum (DESF.) Ietswaart from Algeria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 104–112. [Google Scholar]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential oils and aromatic plants in animal feeding a European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Yannakopoulos, A.L.; Fletouris, D.J.; Tserveni-Goussi, A.S.; Fortomaris, P.D. Effect of Dietary Thyme on the Oxidative Stability of Egg Yolk. J. Agric. Food Chem. 1997, 45, 3711–3716. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br. J. Nutr. 2000, 83, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Cava, R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: Contradictory effects in different types of frankfurters. Meat Sci. 2006, 72, 348–355. [Google Scholar] [CrossRef]
- Takayama, C.; de-Faria, F.M.; de Almeida, A.C.A.; Dunder, R.J.; Manzo, L.P.; Socca, E.A.R.; Luiz-Ferreira, A. Chemical composition of Rosmarinus officinalis essential oil and antioxidant action against gastric damage induced by absolute ethanol in the rat. Asian Pac. J. Trop. Biomed. 2016, 6, 677–681. [Google Scholar] [CrossRef]
- Kazemi, M. Phytochemical Composition, Antioxidant, Anti-inflammatory and Antimicrobial Activity of Nigella sativa L. Essential Oil. J. Essent. Oil Bear. Plants 2014, 17, 1002–1011. [Google Scholar] [CrossRef]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S. Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Sultan, M.T.; Butt, M.S.; Karim, R.; Ahmad, N.; Ahmad, R.S.; Ahmad, W. Nigella sativa fixed and essential oil improves antioxidant status through modulation of antioxidant enzymes and immunity. Pak. J. Pharm. Sci. 2015, 28, 589–595. [Google Scholar] [PubMed]
- El Jery, A.; Hasan, M.; Rashid, M.M.; Al Mesfer, M.K.; Danish, M.; Ben Rebah, F. Phytochemical characterization, and antioxidant and antimicrobial activities of essential oil from leaves of the common sage Salvia officinalis L. from Abha, Saudi Arabia. Asian Biomed. 2020, 14, 261–270. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Presti, V.L.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical composition, antioxidant capacity and antibacterial action of five Moroccan essential oils against Listeria monocytogenes and different serotypes of Salmonella enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruíz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Chemical Composition of the Essential Oils Obtained From Some Spices Widely Used in Mediterranean Region. Acta Chim. Slov. 2007, 54, 921–926. [Google Scholar]
- Ben Khedher, M.R.; Ben Khedher, S.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. Excli J. 2017, 16, 160–173. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; El Ghouizi, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical Composition, Antioxidant Activity, and Antifungal Effects of Essential Oil from Laurus nobilis L. Flowers Growing in Morocco. J. Food Qual. 2020, 2020, 8819311. [Google Scholar] [CrossRef]
- Yilmaz, E.S.; Timur, M.; Aslim, B. Antimicrobial, Antioxidant Activity of the Essential Oil of Bay Laurel from Hatay, Turkey. J. Essent. Oil Bear. Plants 2013, 16, 108–116. [Google Scholar] [CrossRef]
- Saab, A.M.; Tundis, R.; Loizzo, M.R.; Lampronti, I.; Borgatti, M.; Gambari, R.; Menichini, F.; Esseily, F.; Menichini, F. Antioxidant and antiproliferative activity of Laurus nobilis L. (Lauraceae) leaves and seeds essential oils against K562 human chronic myelogenous leukaemia cells. Nat. Prod. Res. 2012, 26, 1741–1745. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Ouariachi, E.; Paolini, J.; Bouyanzer, A.; Tomi, P.; Hammouti, B.; Salghi, R.; Costa, J. Chemical composition and antioxidant activity of essential oils and solvent extracts of Thymus capitatus (L.) Hoffmanns and link from Morocco. J. Med. Plant Res. 2011, 5, 5773–5778. [Google Scholar]
- Goudjil, M.B.; Zighmi, S.; Hamada, D.; Mahcene, Z.; Bencheikh, S.E.; Ladjel, S. Biological activities of essential oils extracted from Thymus capitatus (Lamiaceae). S. Afr. J. Bot. 2020, 128, 274–282. [Google Scholar] [CrossRef]
- Riahi, L.; Chograni, H.; Ziadi, S.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Chemical profiles and antioxidant activities of the essential oils of two medicinal plant species grown in Tunisia. J. Essent. Oil Res. 2013, 25, 324–329. [Google Scholar] [CrossRef]
- Sahin Yaglioglu, A.; Eser, F.; Yaglioglu, M.S.; Demirtas, I. The antiproliferative and antioxidant activities of the essential oils of Juniperus species from Turkey. Flavour Fragr. J. 2020, 35, 511–523. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical Composition, Antioxidant, Anti-Tyrosinase, Anti-Cholinesterase and Cytotoxic Activities of Essential Oils of Six Algerian Plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef]
- Lohani, A.; Mishra, A.K.; Verma, A. Cosmeceutical potential of geranium and calendula essential oil: Determination of antioxidant activity and in vitro sun protection factor. J. Cosmet. Dermatol. 2019, 18, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Lairini, S.; Farah, A.; Taghzouti, K.; Lalami, A.E.O. Antioxidant and Antibacterial Activities of Artemisia herba-alba Asso Essential Oil from Middle Atlas, Morocco. Phytotherapie 2018, 16, S48–S54. [Google Scholar] [CrossRef]
- Younsi, F.; Mehdi, S.; Aissi, O.; Rahali, N.; Jaouadi, R.; Boussaid, M.; Messaoud, C. Essential Oil Variability in Natural Populations of Artemisia campestris (L.) and Artemisia herba-alba (Asso) and Incidence on Antiacetylcholinesterase and Antioxidant Activities. Chem. Biodivers. 2017, 14, e1700017. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Noirot, V.; Moncouldon, R.; Sauvant, D.; Bayourthe, C. Effet d’une Supplémentation en Huiles Essentielles et Composés D’huiles Essentielles Chez le Ruminant: Analyse Statistique. Rev. Med. Vet. 2007, 158, 587–597. Available online: https://www.researchgate.net/publication/281798515_Effet_d’une_supplementation_en_huiles_essentielles_et_composes_d’huiles_essentielles_chez_le_ruminant_analyse_statistique (accessed on 1 February 2021).
- Arzola-Alvarez, C.; Hume, M.E.; Anderson, R.C.; Latham, E.A.; Ruiz-Barrera, O.; Castillo-Castillo, Y.; Olivas-Palacios, A.L.; Felix-Portillo, M.; Armendariz-Rivas, R.L.; Arzola-Rubio, A.; et al. Influence of sodium chlorate, ferulic acid, and essential oils on Escherichia coli and porcine fecal microbiota. J. Anim. Sci. 2020, 98, 1–9. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C. Chemical constituents and antimicrobial and antioxidant potentials of essential oil and acetone extract of Nigella sativa seeds. J. Sci. Food Agric. 2005, 85, 2297–2306. [Google Scholar] [CrossRef]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yun, W.; Lee, J.H.; Lee, C.H.; Kwak, W.K.; Cho, J.H. Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J. Anim. Sci. Technol. 2017, 59. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Effects of Essential Oils on Rumen Fermentation, Microbial Ecology and Ruminant Production. Asian J. Anim. Vet. Adv. 2011, 6, 416–428. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Oh, J.; Hristov, A.N. Effects of plant-derived bio-active compounds on rumen fermentation, nutrient utilization, immune response, and productivity of ruminant animals. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1218, pp. 167–186. [Google Scholar] [CrossRef]
- Kim, H.; Jung, E.; Lee, H.G.; Kim, B.; Cho, S.; Lee, S.; Kwon, I.; Seo, J. Essential oil mixture on rumen fermentation and microbial community—An in vitro study. Asian-Australas. J. Anim. Sci. 2019, 32, 808–814. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Jafari, S.; Ebrahimi, M.; Goh, Y.M.; Rajion, M.A.; Jahromi, M.F.; Al-Jumaili, W.S. Manipulation of rumen fermentation and methane gas production by plant secondary metabolites (saponin, tannin and essential oil)—A review of ten-year studies. Ann. Anim. Sci. 2019, 19, 3–29. [Google Scholar] [CrossRef]
- Torres, R.N.S.; Moura, D.C.; Ghedini, C.P.; Ezequiel, J.M.B.; Almeida, M.T.C. Meta-analysis of the effects of essential oils on ruminal fermentation and performance of sheep. Small Rumin. Res. 2020, 189. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, K.; Li, C.; Jiao, T.; Wu, J.; Wei, Y.; Tian, K.; Li, C.; Tang, D.; Davis, D.I.; et al. Ruminal metagenomic analyses of goat data reveals potential functional microbiota by supplementation with essential oil-cobalt complexes. BMC Microbiol. 2019, 19, 30. [Google Scholar] [CrossRef]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Ouellet, D.R.; Chiquette, J.; Chouinardt, P.Y. Effects of essential oils on digestion, ruminai fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage or corn silage. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants1. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Ratika, K.; James Singh, R.K. Plant Derived Essential Oil in Ruminant Nutrition—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1747–1753. [Google Scholar] [CrossRef]
- Noirot, V.; Moncoulon, R.; Sauvant, D.; Bayourthe, C. Effet d’une supplémentation en huiles essentielles et composés d’huiles essentielles chez le ruminant: Analyse statistique. Rev. De Médecine Vétérinaire 2007, 158, 589–597. [Google Scholar]
- Vargas, J.E.; Andrés, S.; López-Ferreras, L.; Snelling, T.J.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Dietary supplemental plant oils reduce methanogenesis from anaerobic microbial fermentation in the rumen. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Günal, M.; Pinski, B.; AbuGhazaleh, A.A. Evaluating the effects of essential oils on methane production and fermentation under in vitro conditions. Ital. J. Anim. Sci. 2017, 16, 500–506. [Google Scholar] [CrossRef]
- Smeti, S.; Hajji, H.; Mekki, I.; Mahouachi, M.; Atti, N. Effects of dose and administration form of rosemary essential oils on meat quality and fatty acid profile of lamb. Small Rumin. Res. 2018, 158, 62–68. [Google Scholar] [CrossRef]
- Benchaar, C. Diet supplementation with thyme oil and its main component thymol failed to favorably alter rumen fermentation, improve nutrient utilization, or enhance milk production in dairy cows. J. Dairy Sci. 2021, 104, 324–336. [Google Scholar] [CrossRef]
- Rivaroli, D.C.; Guerrero, A.; Valero, M.V.; Zawadzki, F.; Eiras, C.E.; del Mar Campo, M.; Sañudo, C.; Jorge, A.M.; do Prado, I.N. Effect of essential oils on meat and fat qualities of crossbred young bulls finished in feedlots. Meat Sci. 2016, 121, 278–284. [Google Scholar] [CrossRef]
- De Oliveira Monteschio, J.; de Souza, K.A.; Vital, A.C.P.; Guerrero, A.; Valero, M.V.; Kempinski, E.M.B.C.; Barcelos, V.C.; Nascimento, K.F.; do Prado, I.N. Clove and rosemary essential oils and encapsuled active principles (eugenol, thymol and vanillin blend) on meat quality of feedlot-finished heifers. Meat Sci. 2017, 130, 50–57. [Google Scholar] [CrossRef]
- Harris, S.E.; Huff-Lonergan, E.; Lonergan, S.M.; Jones, W.R.; Rankins, D. Antioxidant status affects color stability and tenderness of calcium chloride-injected beef. J. Anim. Sci. 2001, 79, 666–677. [Google Scholar] [CrossRef][Green Version]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.M.P.S.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential oils encapsulated in chitosan microparticles against Candida albicans biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaiegh, S.B.; Morshedy, S.A.; Mansour, A.T.; Ahmed, M.H.; Zahran, S.M.; Alnemr, T.M.; Sallam, S.M.A. Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods. Sustainability 2020, 12, 9123. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Anele, U.Y.; Abdel-Wahed, A.M.; Abdou, A.R.; Khattab, I.M. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Anim. Feed Sci. Technol. 2021, 271, 114760. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Anim. Feed Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- Morsy, T.A.; Kholif, S.M.; Matloup, O.H.; Abdo, M.M.; El-Shafie, M.H. Impact of Anise, Clove and Juniper Oils as Feed additives on the productive performance of lactating goats. Int. J. Dairy Sci. 2012, 7, 20–28. [Google Scholar] [CrossRef]
- Kholif, A.E.; Kassab, A.Y.; Azzaz, H.H.; Matloup, O.H.; Hamdon, H.A.; Olafadehan, O.A.; Morsy, T.A. Essential oils blend with a newly developed enzyme cocktail works synergistically to enhance feed utilization and milk production of Farafra ewes in the subtropics. Small Rumin. Res. 2018, 161, 43–50. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Buendía, L.; Soto, S.; Ros, M.; Antolinos, V.; Navarro, L.; Sánchez, M.J.; Martínez, G.B.; López, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khorram, S.B.; Sohrabi, M. Antioxidant Activity of Essential Oils in Foods. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 247–265. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on color and texture deterioration. J. Food Sci. 2005, 70, c427–c432. [Google Scholar] [CrossRef]
- Bozkurt, H. Comparison of the effects of sesame and Thymbra spicata oil during the manufacturing of Turkish dry-fermented sausage. Food Control 2007, 18, 149–156. [Google Scholar] [CrossRef]
- Martín-Sánchez, A.M.; Chaves-López, C.; Sendra, E.; Sayas, E.; Fenández-López, J.; Pérez-Álvarez, J.Á. Lipolysis, proteolysis and sensory characteristics of a Spanish fermented dry-cured meat product (salchichón) with oregano essential oil used as surface mold inhibitor. Meat Sci. 2011, 89, 35–44. [Google Scholar] [CrossRef]
- Alsaiqali, M.; El-Shibiny, A.; El-Shibiny, A.; Adel, M.; Abdel-Samie, M.; Ghoneim, S. Use of Some Essential Oils as Antimicrobial Agents to Control Pathogenic Bacteria in Beef Burger. World J. Dairy Food Sci. 2016, 11, 109–120. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.F. Photo-oxidation and Photo-toxicity of Crude and Refined Oils. Spill Sci. Technol. Bull. 2003, 8, 157–162. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Alinkina, E.S.; Fatkulina, L.D.; Vorob’eva, A.K. Oregano essential oil as an inhibitor of higher fatty acid oxidation. Prikl. Biokhimiia I Mikrobiol. 2011, 47, 490–494. [Google Scholar] [CrossRef]
- Özcan, M.M.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar] [CrossRef]
- Gavahian, M.; Hashemi, S.M.; Khaneghah, A.M.; Tehrani, M.A. Ohmically extracted Zenyan essential oils as natural antioxidant in mayonnaise. Int. Food Res. J. 2013, 20, 3189–3195. [Google Scholar]
- Ben Jemaa, M.; Falleh, H.; Saada, M.; Oueslati, M.; Snoussi, M.; Ksouri, R. Thymus capitatus essential oil ameliorates pasteurization efficiency. J. Food Sci. Technol. 2018, 55, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Elama, C.; Alayoubi, M.; Jazzar, M. FA-R Effect of Different Essential Oils on the Shelf Life of Concentrated Yogurt. Annu. Res. Rev. Biol. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M.; et al. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant activities of Satureja cilicica essential oil in butter and in vitro. J. Food Eng. 2007, 79, 1391–1396. [Google Scholar] [CrossRef]
- Castillo, C.; Pereira, V.; Abuelo, Á.; Hernández, J. Effect of Supplementation with Antioxidants on the Quality of Bovine Milk and Meat Production. Sci. World J. 2013, 2013, 616098. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Castro-Montoya, J.; Peiren, N.; Cone, J.W.; Zweifel, B.; Fievez, V.; De Campeneere, S. In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation. Livest. Sci. 2015, 180, 134–142. [Google Scholar] [CrossRef]
- Tóth, T.; Körmöndi, M.; Tkovács, A.; Csavajda, É.; Trombitás, M.; Tóthi, R. Effect of essential oil supplement on milk yield and composition of lactating dairy cows. Krmiva 2017, 59, 15–19. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Karatzia, M.A.; Dovas, C.I.; Filioussis, G.; Papadopoulos, E.; Kiossis, E.; Arsenopoulos, K.; Papadopoulos, T.; Boscos, C.; Karatzias, H. Evaluation of the in-field efficacy of oregano essential oil administration on the control of neonatal diarrhea syndrome in calves. Res. Vet. Sci. 2017, 115, 478–483. [Google Scholar] [CrossRef]
- Froehlich, K.A.; Abdelsalam, K.W.; Chase, C.; Koppien-Fox, J.; Casper, D.P. Evaluation of essential oils and prebiotics for newborn dairy calves1. J. Anim. Sci. 2017, 95, 3772–3782. [Google Scholar] [CrossRef]
- Akbarian-Tefaghi, M.; Ghasemi, E.; Khorvash, M. Performance, rumen fermentation and blood metabolites of dairy calves fed starter mixtures supplemented with herbal plants, essential oils or monensin. J. Anim. Physiol. Anim. Nutr. 2018, 102, 630–638. [Google Scholar] [CrossRef]

| Latin Name of Plant Source | Major Components | Approximate % Composition | References |
|---|---|---|---|
 Salvia officinalis L. Credit: Dusan Baksa | Camphor α-Pinene β-Pinene 1,8-Cineole α-Thujone | 6–15% 4–5% 2–10% 6–14% 20–42% | [68] |
 Laurus nobilis Credit: Stefano | 1,8-Cineol Borneol | 51.63–63.19% 5.8–12.80% | [69] |
 Coriandrum sativum Credit: Forest & Kim Starr | Linalool | 40–70% | [70,71] |
 Rosmarinus officinalis Credit: Peter Stenzel | α-Pinene Bornyl acetate Camphor 1,8-Cineole | 2–25% 0–17% 2–14% 3–89% | [72,73,74] |
 Thymus vulgaris Credit: Ralf Wimmer | Thymol Carvacrol δ-Terpinene p-Cymene | 10–64% 76.1% 2–31% 10–56% | [59,63,66,72,75] |
 Nigella sativa Credit: Eran Finkle | Thymoquinone Carvacrol p-Cymene | 27–45% 7–11.6% 7–15% | [76] |
 Juniperus officinalis Credit: Zoran Radosavljevic | Β-Phellandrene α-Terpinyl acetate α-Pinene α-Phellandreneinene Manonyl oxide | 4.9–23.8% 4.6–15.5% 28–60% 0.8–17.7% 0.4–14.4% | [77] |
 Pelargonium graveolens Credit: Forest & Kim Starr | Citronellol Geraniol Linalool Citronellyl formate p-Menthone | 33.6% 26.8% 10.5% 9.7% 6% | [78] |
 Origanum vulgare Credit: Koromelana.yandex.ru | Carvacrol Thymol γ-Terpinene | Trace–80% Trace–64% 2–52% | [40,68,72,79,80,81,82,83] |
 Artemisia herba alba Credit: Peganum | Limonene Fenchol α-Thujone Camphor Nordavanone | 4–9% 7–14% 11–14% Trace–30% 1–9% | [84] |
| EOs | Bacteria | MIC | References |
|---|---|---|---|
| Cymbopogon spp. and Cinnamomum verum | Escherichia coli | 0.075% to 0.3% (v/v) and 0.0075% (v/v), respectively | [116] |
| A. sativum, C. verum, O. basilicum, S. aromaticum and T. vulgaris | Aeromonas hydrophila, A. jandaei and Citrobacter freundii isolated from diseased freshwater fish | MIC ≤ 500 µg/mL | [117] |
| Lavandula x intermedia | Streptococcus agalactiae and Candida albicans | MIC 9–18 µg/mL for Streptococcus MIC 9–18 µg/mL for Candida | [118] |
| Mentha arvensis | Streptococcus agalactiae and Candida albicans | MIC 18–36 µg/mL for Streptococcus MIC 18–144 µg/mL for Candida | [118] |
| P. armeniaca | M. luteus, S. aureus and E. coli | MIC 23.4 µg/mL for M. luteus MIC 23.4 µg/mL for S. aureus MIC 11.7 µg/mL for E. coli | [119] |
| L. nobilis | M. luteus, S. aureus and B. subtilis | MIC 22.2 µg/mL for M. luteus MIC 5.55 µg/mL for S. aureus MIC 1.39 µg/mL for B. subtilis | [119] |
| M. alternifolia | K. pneumoniae, P. aerginosa, and E coli | MIC 0.5 and 4 μg/mL | [120] |
| T. vulgaris | K. pneumoniae, P. aerginosa, and E coli | MIC 1 to 16 μg/mL | [120] |
| Garlic, cinnamon, and onion | L. monocytogenes | MIC respectively 100, 100, and 25 μg/mL | [121] |
| EO | Polymer | Food | Results | Reference |
|---|---|---|---|---|
| Laurus nobilis and Rosmarinus officinalis | Polyvinyl alcohol | Chicken breast fillets | Inhibition of the lipid oxidation up to 68%. Beneficial effect on both the pH and color parameters of the fillets during storage. | [232] |
| Rosemary and cinnamon EO | Polyethylene films | Pacific white shrimp | Prolonged and retained shrimp freshness and shelf life | [233] |
| Carvacrol, oregano, and cinnamon | Cardboard tray | Cherry tomato | Tomato color and firmness were highly maintained during storage. The shelf life was extended up to 4 days | [234] |
| Cumin EO | Shahri Balangu seed mucilage | Beef | Expand the shelf life of the beef | [235] |
| Oregano | Edible coating: mandarin fiber and sodium alginate | Low-fat cheese | Decontamination of external pathogens such as Staphylococcus aureus | [236] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. https://doi.org/10.3390/antiox10020330
Nehme R, Andrés S, Pereira RB, Ben Jemaa M, Bouhallab S, Ceciliani F, López S, Rahali FZ, Ksouri R, Pereira DM, et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants. 2021; 10(2):330. https://doi.org/10.3390/antiox10020330
Chicago/Turabian StyleNehme, Ralph, Sonia Andrés, Renato B. Pereira, Meriem Ben Jemaa, Said Bouhallab, Fabrizio Ceciliani, Secundino López, Fatma Zohra Rahali, Riadh Ksouri, David M. Pereira, and et al. 2021. "Essential Oils in Livestock: From Health to Food Quality" Antioxidants 10, no. 2: 330. https://doi.org/10.3390/antiox10020330
APA StyleNehme, R., Andrés, S., Pereira, R. B., Ben Jemaa, M., Bouhallab, S., Ceciliani, F., López, S., Rahali, F. Z., Ksouri, R., Pereira, D. M., & Abdennebi-Najar, L. (2021). Essential Oils in Livestock: From Health to Food Quality. Antioxidants, 10(2), 330. https://doi.org/10.3390/antiox10020330