Abstract
Background/Objectives: The main objective of this systematic review was to explore the role of magnetoencephalography (MEG) in the diagnosis, assessment, and monitoring of mild traumatic brain injury (mTBI) and post-concussion syndrome (PCS). We aimed to evaluate the potential of some MEG biomarkers in detecting subtle brain abnormalities often missed by conventional imaging techniques. Methods: A systematic review was conducted using 25 studies that administered MEG to examine mTBI and PCS patients. The quality of the studies was assessed based on selection, comparability, and outcomes. Studies were analyzed for their methodology, evaluated parameters, and the clinical implications of using MEG for mTBI diagnosis. Results: MEG detected abnormal brain oscillations, including increased delta, theta, and gamma waves and disruptions in functional connectivity, particularly in the default mode and frontoparietal networks of patients suffering from mTBI. MEG consistently revealed abnormalities in mTBI patients even when structural imaging was normal. The use of MEG in monitoring recovery showed significant reductions in abnormal slow-wave activity corresponding to clinical improvements. Machine learning algorithms applied to MEG data demonstrated high sensitivity and specificity in distinguishing mTBI patients from healthy controls and predicting clinical outcomes. Conclusions: MEG provides a valuable diagnostic and prognostic tool for mTBI and PCS by identifying subtle neurophysiological abnormalities. The high temporal resolution and the ability to assess functional brain networks make MEG a promising complement to conventional imaging. Future research should focus on integrating MEG with other neuroimaging modalities and standardizing MEG protocols for clinical use.
1. Introduction
It is currently accepted that traumatic brain injury (TBI) is one of the most important causes of physical and mental disability, affecting millions each year. The severity of TBI can range from mild to severe, with mild TBI (mTBI) or concussion being the most common form, accounting for approximately 90% of all TBIs [1]. Despite its prevalence, mTBI uniquely challenges clinical diagnosis due to the often subtle and transient nature of its symptoms, as well as the limitations of conventional imaging techniques in detecting mild brain injuries following concussions [2]. Mild TBI patients frequently experience quality-of-life-affecting and persistent cognitive, emotional, and physical symptoms, conventionally referred to as post-concussion syndrome (PCS). Given the limitations of traditional imaging techniques in diagnosing mTBI and PCS, there is a growing need for more sensitive and objective tools to detect and monitor the underlying neural dysfunction associated with these conditions [3].
Magnetoencephalography (MEG) has emerged as a promising neuroimaging tool for mTBI and PCS diagnosis [4]. Unlike structural imaging techniques, MEG provides a functional assessment of brain activity by measuring the magnetic fields generated by neuronal currents. With its high temporal resolution, MEG can capture dynamic brain activity in real time, offering valuable insights into the neural mechanisms disrupted by brain injury. Recent advances in MEG technology coupled with machine learning approaches have allowed for a more precise identification of functional abnormalities in mTBI patients, even in cases where conventional imaging methods fail to detect any structural damage [4]. MEG has been used to investigate various neural changes associated with mTBI, including alterations in oscillatory brain activity, disruptions in functional connectivity, and abnormal slow-wave generation. Several studies have demonstrated that MEG can detect abnormal low-frequency activity, particularly in the delta and theta frequency bands, which often indicate neural injury [5,6,7,8,9]. Moreover, MEG has shown promise in identifying biomarkers for mTBI, which could assist diagnosis, provide insight into the mechanisms underlying persistent symptoms, and guide personalized treatment approaches. In some cases, post-traumatic stress disorder (PTSD) co-occurs or is confounded with mTBI and PCS, as some of the symptoms are overlapping [10]. As a consequence, it is important that the differentiated diagnosis is performed with accuracy and the patients receive proper care.
The aim of this systematic review is to assess the role of MEG in the diagnosis and monitoring of mTBI and PCS by evaluating the sensitivity of MEG in detecting functional brain abnormalities, comparing its effectiveness with traditional imaging modalities, and exploring its potential as a diagnostic tool for differentiating mTBI from other conditions with overlapping symptoms, such as PTSD. Additionally, the potential use of MEG as a monitor for the recovery of mTBI patients and the application of machine learning techniques in improving diagnostic accuracy will be pursued as secondary aims. In this way, the present study will make significant contributions regarding the utility of MEG in the clinical management of mTBI and the unmet diagnostic needs as a consequence of the heterogeneity of both cohorts that have previously been assessed and the type of analyses that have been employed during the assessments, albeit considering the novel data and recent advances in the field.
2. Materials and Methods
2.1. Study Design
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered to the PROSPERO platform (reg. no. CRD42024598973). The PRISMA flowchart could be found at Figure 1. This review aimed to evaluate the use of MEG in the diagnosis of mTBI and PCS, focusing on its ability to detect neural abnormalities, track recovery, and distinguish mTBI from other neuropsychiatric conditions.
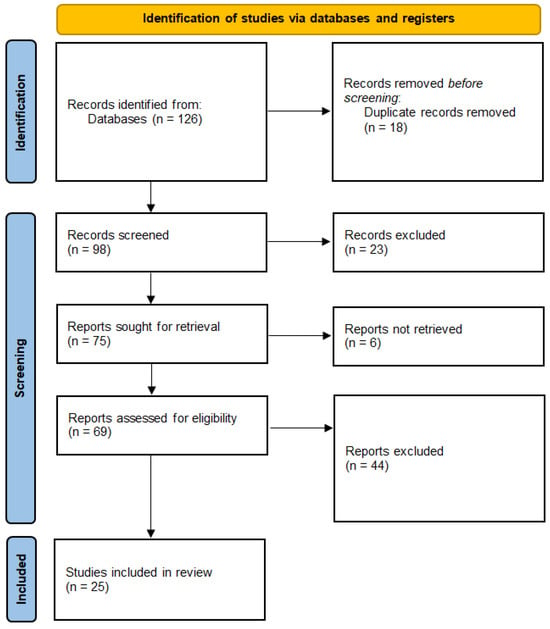
Figure 1.
PRISMA flowchart.
2.2. Search Strategy
A comprehensive literature search was conducted across multiple electronic databases, including PubMed, Scopus, Web of Science, and Embase, to identify relevant studies published up until September 2024. The search terms included a combination of keywords and medical subject headings (MeSHs) such as “magnetoencephalography”, “MEG”, “mild traumatic brain injury”, “mTBI”, “concussion”, “post-concussion syndrome”, “PCS”, “functional connectivity”, and “brain oscillations”, separated by Boolean operators (e.g., “magnetoencephalography” OR “MEG” AND “concussion” OR “mild traumatic brain injury” OR “mTBI”; “mTBI” OR “PCS” AND “MEG” AND “functional connectivity”; “mTBI” OR “PCS” AND “MEG” AND “functional connectivity”; “mTBI” OR “PCS” AND “MEG” AND “brain oscillations”; and so on). The search was limited to English-written, human studies. Furthermore, their reference lists were searched for additional studies.
2.3. Studies Selection
2.3.1. Inclusion Criteria
- Population: Patients diagnosed with mTBI or PCS.
- Intervention: Use of MEG for assessing brain function, either at rest or during cognitive tasks.
- Outcomes: MEG-based biomarkers, such as abnormal oscillatory activity, functional connectivity disruptions, and slow-wave detection, in relation to diagnosing or monitoring mTBI or PCS.
- Study Design: Observational (e.g., case–control and cohort studies) and experimental (e.g., clinical trials) designs.
2.3.2. Exclusion Criteria
- Studies not using MEG as the primary neuroimaging tool.
- Studied not reporting results specific to mTBIs.
- Review articles, meta-analyses, editorials, or opinion papers.
2.4. Data Extraction
The titles and abstracts of all retrieved articles were independently screened by two reviewers. Full texts of potentially relevant studies were reviewed for eligibility based on the inclusion and exclusion criteria, and for the selected studies, the following data were extracted:
- Study characteristics: author names, publication year, study design, and sample size.
- Participant characteristics: age, sex, and specific diagnosis (mTBI, PCS).
- MEG protocols: frequency bands analyzed (e.g., delta, theta, alpha, beta, gamma), resting-state versus task-based MEG, and analysis methods (e.g., functional connectivity, source localization).
- Outcomes: key MEG findings, including abnormal brain oscillations, connectivity changes, and correlations with cognitive or behavioral measures.
By its unique characteristics, MEG could detect the patterns of the five main types of brain wave oscillations as a result of synchronized neuronal activity. Each type of brain waves is associated with different physiological functions: delta (0.5–4 Hz)— sleep brain waves, which are increased in brain injuries and cognitive and attention deficits; theta (4 Hz)—deep relaxation brain waves, which are severely changed in insomnia; alpha (8–12 Hz)—awake, relaxed-state brain waves, which decrease with the occurrence of anxiety, high stress, and insomnia; beta (12–35 Hz)—active and attentive physiological-state brain waves, which are increased in anxiety and stress and decreased in attention deficit and depression; and gamma (>35 Hz)—concentration waves in which changes could suggest cognitive impairments [11,12,13,14,15].
A third reviewer provided additional evaluation when disagreements between reviewers occurred.
2.5. Data Synthesis and Analysis
Due to the heterogeneity of the studies in terms of study design, population, and MEG analysis methods, a quantitative meta-analysis was not feasible. Instead, a narrative synthesis of the findings was conducted. The results were organized into key themes, including the following:
- MEG biomarkers for mTBI: Detection of abnormal oscillations (e.g., delta and theta waves) and their association with cognitive dysfunction.
- MEG functional connectivity analysis: Disruptions in neural networks, particularly in the default mode network (DMN) and thalamocortical circuitry.
- MEG for differentiating mTBI from PTSD: Studies that explored MEG’s ability to distinguish mTBI from PTSD based on distinct brain activity patterns.
- MEG as a tool for tracking recovery: The role of MEG in monitoring changes in brain function over time and its correlation with clinical outcomes.
2.6. Quality Assessment
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) for observational studies and the Cochrane Risk of Bias tool for clinical trials [16,17]. Each study was evaluated on standard criteria, such as sample representativeness, comparability between groups, and clarity of outcome measurement.
2.6.1. Assessment of Observational Studies Using the Newcastle–Ottawa Scale (NOS)
The Newcastle–Ottawa Scale was used to evaluate the quality of observational studies based on three major domains: selection, comparability, and outcome. Each study was scored on a scale from 0 to 9, with higher scores indicating better methodological quality.
- Selection of Participants. Most of the studies included in this review had well-defined inclusion criteria for patients with mTBI or PCS. Mainly participants from clinical settings were recruited, ensuring the representativeness of the target population. However, a few studies had small sample sizes, which may have affected the generalizability of their findings.
- Comparability of Groups. Several studies included control groups of healthy participants (healthy controls, HCs) or individuals with orthopedic injuries (orthopedic trauma controls, OTCs) to allow for the comparison of MEG results. The studies that matched HCs based on age, sex, and education were rated highly for comparability. Some studies failed to control for confounding factors, such as co-occurring conditions—i.e., post-traumatic stress disorder (PTSD)—which could have introduced bias into the results.
- Outcome Measurement. The outcome measurement for most studies was based on objective MEG biomarkers, such as abnormal oscillatory activity, functional connectivity, and slow-wave detection. Many studies used automated MEG analysis techniques, reducing the likelihood of observer bias. However, some studies relied on subjective cognitive assessments, which could have introduced variability in outcome reporting. A brief overview of the standard techniques for the analysis of MEG outcomes was previously addressed by multiple technical studies [15,18,19,20].
2.6.2. Assessment of Randomized Controlled Trials Using the Cochrane Risk of Bias Tool
The Cochrane Risk of Bias tool was applied to the randomized controlled trials (RCTs) included in this review. This tool assesses the risk of bias across six domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases.
- Selection Bias. Randomization methods were generally well reported in the included RCTs. The majority of studies used appropriate randomization procedures, such as computer-generated random sequences, to assign participants to different intervention groups (e.g., MEG assessment versus other diagnostic tools). However, a few studies did not adequately describe their randomization process, leading to an unclear risk of selection bias.
- Performance Bias. Blinding of participants and personnel was rarely implemented in the studies, particularly in those using MEG as a diagnostic tool. While blinding is challenging in diagnostic studies, the lack of blinding could have introduced performance bias in studies where subjective outcomes (such as cognitive performance) were measured alongside MEG data.
- Detection Bias. Detection bias was minimized in most studies by the use of objective MEG biomarkers (e.g., frequency band analysis and source localization). Automated MEG analysis tools reduced the likelihood of bias in outcome detection. However, in studies that used cognitive tests as secondary outcomes, there was a potential for detection bias, especially if assessors were not blinded to the intervention groups.
- Attrition Bias. Most studies reported low rates of participant dropout, and reasons for dropout were typically well documented. Studies with long follow-up periods, however, had higher rates of attrition, which could have influenced the results. The effect of attrition bias was generally low, as intention-to-treat analyses were applied in most cases.
- Reporting Bias. Selective reporting of outcomes was minimal in the included studies, as most trials were pre-registered and reported all pre-specified outcomes. However, a few studies failed to report secondary outcomes, raising the potential for reporting bias.
2.7. Statistical Considerations
The characteristics of the included studies were summarized using descriptive statistics. A qualitative comparison of MEG results across different study designs and populations was conducted to highlight consistent patterns in the use of MEG for diagnosing mTBI and PCS.
3. Results
3.1. Summary of Quality Findings
3.1.1. Overall Quality
The majority of the studies included in this review demonstrated moderate-to-high methodological quality. Most studies received favorable scores for participant selection and the use of objective MEG outcome measures, although some suffered from issues related to small sample sizes and lack of control for confounding factors.
3.1.2. Key Strengths
- ○
- The use of MEG as an objective high-resolution functional neuroimaging tool minimized observer and detection biases in many studies.
- ○
- Studies that incorporated machine learning techniques in MEG analysis enhanced the precision of mTBI diagnosis and reduced subjective variability.
3.1.3. Key Limitations
- ○
- A lack of blinding in many studies and small sample sizes in some resulted in an increased risk of bias.
- ○
- Studies that did not control for co-occurring conditions, such as PTSD or psychiatric disorders, may have confounded the findings related to mTBI and MEG biomarkers.
A total of 24 studies exploring the use of MEG in detecting functional and connectivity changes in mTBI, its correlation with cognitive impairments, and its potential as a biomarker for diagnosing mTBI and monitoring recovery were included.
3.2. MEG in mTBI Diagnosis and Biomarker Development
We found various studies [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] (Table 1) demonstrating the potential utility of MEG in mTBI and PCS diagnosis, particularly in detecting functional brain abnormalities that were not visible by conventional imaging techniques, such as MRI and CT scans. Moreover, more than one study conducted by Huang et al. [21,22,23,24,25,26] showed that MEG slow-wave abnormalities in delta (1–4 Hz) and theta (4–7 Hz) frequencies correlated with cognitive and emotional symptoms in mTBI patients, paving the way for identifying imaging biomarkers.

Table 1.
Studies focused on MEG for mTBI diagnosis and biomarker development.
MEG also revealed abnormalities in white matter that were undetected by structural imaging. Alhourani et al. [27] and Rowland et al. [28] further supported the diagnostic utility of MEG by identifying disruptions in functional connectivity, particularly in the DMN. These disruptions were linked to long-term cognitive deficits in mTBI patients, highlighting the potential of MEG to uncover neural communication deficits that underlie PCS [27,28].
3.3. Functional Connectivity Changes in mTBI
MEG was found to be effective in detecting large-scale network changes in mTBI. Diwakar et al. [29] and Zhang et al. [31] demonstrated altered functional connectivity in frontal, temporal, and parietal regions in mTBI patients during resting-state and cognitive tasks. Specifically, MEG revealed connectivity-related changes in different brain regions, including the prefrontal cortex and medial temporal lobes. These changes were correlated with cognitive impairments, such as attention deficits and executive dysfunction. Particularly, Zhang et al. [31] concluded that beta wave patterns could predict cognitive dysregulations after mTBI.
Dunkley et al. [32] and Zhang et al. [33] reported beta-band oscillatory dysfunction in mTBI patients. Reduced beta-band power and connectivity were found in the thalamocortical circuits, which correlated with impairments in cognitive processing and motor control (Table 2).

Table 2.
Functional connectivity changes in mTBI.
3.4. MEG in Differentiating mTBI from PTSD
Several studies explored the role of MEG in differentiating mTBI from PTSD, a common comorbid condition (Table 3). Rowland et al. [28], Zhang et al. [33], and Popescu et al. [40] showed that MEG can differentiate between mTBI and PTSD based on differences in neural oscillations and network connectivity. MEG scans revealed distinct alterations in theta, alpha, and high gamma oscillations in the amygdala, hippocampus, and temporal regions, which were more pronounced in PTSD than mTBI.

Table 3.
MEG for differentiating mTBI from PTSD.
These findings suggested that MEG may provide a valuable diagnostic tool for distinguishing between mTBI and PTSD, particularly when symptoms overlap, as conventional neuroimaging techniques often fail to differentiate between these conditions.
3.5. MEG as a Tool for Monitoring Recovery in mTBI
MEG has been shown to be a useful tool for monitoring recovery in mTBI patients (Table 4). Li et al. [34], Antonakakis et al. [36], and Lawton et al. [37] reported improvements in functional connectivity and reductions in abnormal slow-wave activity over time in mTBI patients, suggesting that MEG can track neural recovery. These studies demonstrated that MEG can detect improvements in brain connectivity patterns, which correlate with cognitive and behavioral recovery in mTBI patients.

Table 4.
MEG for monitoring recovery in mTBI.
Furthermore, Huang et al. [26] employed machine learning models to predict recovery based on MEG-derived neural activity. These models demonstrated high sensitivity and specificity in predicting mTBI outcomes, with improvements in functional connectivity correlating with better neuropsychological performance over time.
3.6. Cognitive and Behavioral Correlates of MEG Findings
Multiple studies linked MEG findings with cognitive and behavioral performance in mTBI patients (Table 5). Diwakar et al. [29] and Pang et al. [36] showed that alterations in brain connectivity detected by MEG were associated with deficits in mental flexibility, attentional control, and information processing speed. Patients with mTBI exhibited reduced functional connectivity in the prefrontal cortex and parietal regions, which correlated with impairments in executive functioning and processing speed.

Table 5.
Cognitive and behavioral correlates of MEG findings.
In addition, Lawton et al. [37] demonstrated improvements in cognitive functions such as working memory, attention, and problem-solving skills following movement-based training, as detected by MEG.
3.7. MEG’s Role in Identifying Subtle Brain Injury
MEG’s ability to detect subtle brain injuries that are not visible on MRI or CT scans was highlighted in several studies (Table 6). Huang et al. [21] and Zhang et al. [33] demonstrated that MEG could reveal subtle changes in brain activity, particularly in the beta and gamma bands, which were associated with cognitive impairments in mTBI patients. Also, Rier et al. [39] described and compared the neuroimaging techniques with regard to the subtle brain injuries occurring as a result of mTBI concluding that MEG could detect small brain injuries with great accuracy and specificity, as compared to other neuroscans.

Table 6.
MEG’s role in detecting subtle brain injury.
These findings suggest that MEG can detect brain dysfunction at a finer level of detail, providing valuable insights into the neurophysiological basis of mTBI.
3.8. Applications of Machine Learning and Deep Learning in MEG
Several studies applied machine learning algorithms to MEG data to improve diagnostic accuracy and predict recovery in mTBI patients (Table 7). Huang et al. [24] and Zhang et al. [33] employed machine learning models to classify mTBI patients based on MEG-derived connectivity patterns. These models achieved high diagnostic accuracy, sensitivity, and specificity, indicating that machine learning approaches can enhance the diagnostic capabilities of MEG.

Table 7.
Studies applying machine learning to MEG data for mTBI diagnosis.
4. Discussion
This systematic review aimed to highlight the significant role of MEG in the diagnosis and assessment of mTBI and PCS. MEG offers advanced neuroimaging scans with high temporal resolution, enabling the detection of subtle neural abnormalities that are often missed by traditional structural imaging modalities, such as MRI and CT scans. Thus, we explored a wide range of studies, each demonstrating MEG’s utility in identifying biomarkers for mTBI and PCS, particularly through measures of abnormal oscillatory activity and functional connectivity disruptions.
Since one of the first studies suggesting the possible use of MEG in detecting functional abnormalities of mTBI brain and providing wave patterns for mTBI by comparison to healthy controls [46], the interest for MEG in mTBI diagnosis significantly increased. Recently, a comprehensive review aimed at evaluating the potential of MEG detections in differentiating between mTBI and PTSD, yet the study concluded that the utility of MEG is mostly limited to research in TBI [47]. However, Allen et al [6] documented the use of MEG in mTBI diagnosis routines for adults by systematic review, but concluded that the data they collected had increased heterogeneity, thus not appropriate for meta-analyses. The efforts to improve the potential of MEG as a diagnosis and prognosis tool in mTBI were accompanied by complementing MEG with other powerful tools, such as other imaging scans (EEG, [48]), advanced cross-correlational analyses (synchronous neural interactions test [49]), or artificial intelligence (deep learning, Table 7). Further interest regarding MEG was paid not only for documenting differences in regional brain activation in mTBI, as compared to control brains, but also in timing of activation during relevant cognitive tasks [50]. In this context, the interest on MEG currently tends towards its possible use in detecting more subtle brain alterations, e.g. those resulted from sub-concussive impacts (head impacts not receiving a mTBI diagnosis) [51,52].
Overall, the results of this systematic review demonstrate that MEG is a powerful tool for diagnosing mTBI, monitoring recovery, and understanding the neurophysiological basis of PCS. MEG’s ability to detect subtle brain injuries, to identify functional connectivity changes, and to correlate with cognitive performance makes it a valuable addition to conventional neuroimaging techniques. However, further research is needed to standardize MEG protocols and validate its use in clinical practice.
4.1. MEG as a Diagnostic Tool for mTBI
Several studies provided strong evidence supporting the use of MEG in diagnosing mTBI, particularly through the detection of abnormal slow-wave activity. Huang et al. [21] identified abnormal low-frequency magnetic activity (1–4 Hz) in mTBI patients, which was linked to underlying axonal injury, as detected by DTI. This finding aligns with the results from other studies that observed increased delta and theta waves activity in mTBI patients, further demonstrating the sensitivity of MEG to pathological changes in brain activity that are not captured by conventional imaging techniques.
The ability of MEG to detect functional disruptions in specific brain regions also shows promising potential in identifying cognitive impairments associated with mTBI. Hung et al. [41] and Zhang et al. [33] reported significant increases in delta and theta oscillatory activities in frontal and temporal regions correlated with cognitive deficits in attention and executive function. Similarly, Popescu et al. [40] found that reduced alpha-band power in prefrontal regions was linked to the severity of PTSD symptoms in individuals with mTBI.
Moreover, recent relevant data suggested that newly developed artificial intelligence tools (machine learning) could be successfully used in increasing the potential of MEG as a diagnosis tool in mTBI. Thus, in some of the studies that were included in this systematic review, it was demonstrated that the classification of mTBIs and recovery prediction based on connectivity patterns analysis pipelines were improved [24,26,31,33,39,40,44]. In this context, Aaltonen et al. [53] compared three different machine learning-assisted algorithms for diagnosis accuracy and found that their performance was higher than the traditional evaluation of resting-state MEG power spectra in patients that were affected by mTBI at less than 2 months before the scan. It is important to note that machine learning-assisted evaluation of MEG spectra could be used a diagnosis tool in both adults and children, as seen in the studies we evaluated in this report. However, there could be differences between the MEG spectra by function of age and time from injury in mTBI patients, as suggested by some reports. Moreover, Safar et al. [54] suggested that machine learning-assisted evaluation of MEG spectra could also be employed in detecting neural functioning disturbances in chronic TBI and prolonged post-concussive syndrome, but they draw attention to the differences they observed in children and adolescents affected by chronic TBI—theta and gamma bands changes, subtle delta band changes, and alpha band peaks—in contrast to the discussed changes seen in adults.
4.2. Functional Connectivity and Network Disruptions
Beyond the identification of abnormal brain oscillations, MEG has proven effective in analyzing functional connectivity, particularly in mTBI patients. Functional connectivity disruptions, especially in the DMN and frontoparietal networks, were consistently reported across multiple studies. Alhourani et al. [27] used MEG to reveal significant reductions in functional connectivity across cortical regions involved in the DMN, suggesting that disruptions in this network may underline the cognitive and attentional deficits commonly observed in mTBI patients. Furthermore, Zhang et al. [33] showed that MEG could differentiate mTBI from other conditions, such as PTSD, by revealing distinct patterns of connectivity in specific brain regions.
Graph theoretical analyses of functional connectivity have also been useful in identifying network-level disruptions in mTBI patients. Kaltiainen et al. [42,43] and Itallina et al. [44] demonstrated that individuals with mTBI exhibited reduced local efficiency in brain networks, which is indicative of altered information processing. This supports the hypothesis that mTBI leads to widespread disruptions in functional networks contributing to cognitive and behavioral symptoms.
4.3. MEG as a Tool for Monitoring Recovery
In addition to its diagnostic capabilities, MEG has shown potential as a tool for monitoring recovery from mTBI. Several longitudinal studies included in this review reported improvements in MEG-derived biomarkers over time, paralleling clinical recovery. Huang et al. [23] found that reductions in abnormal slow-wave activity measured by MEG were associated with improvements in cognitive performance. These findings suggested that MEG could be used to track neural recovery and assess the effectiveness of interventions in mTBI patients. Moreover, studies have explored the use of MEG in predicting long-term outcomes in mTBI patients. Vakorin et al. [45] employed machine learning techniques to analyze MEG connectivity patterns and reported high-accuracy predictions of the severity of PCS. This highlights the potential for MEG to inform clinical decisions regarding prognosis and rehabilitation strategies.
4.4. Limitations of MEG in mTBI Diagnosis
Despite the promising findings, some limitations of MEG should be considered (Table 8). While MEG offers high temporal resolution, its spatial resolution is lower compared to other imaging techniques, such as functional MRI. This limitation could affect the precision of localizing brain regions involved in mTBI-related abnormalities. Furthermore, MEG is a relatively costly and less accessible neuroimaging technique, limiting its use in clinical practice. However, some of these limitations are currently addressed while developing new improved MEG systems, such as optically pumped magnetometer-assisted MEG, which enables the possible development of point-of-care testing systems with less expensive use and maintenance [55].

Table 8.
Limitations of MEG in mTBI diagnosis.
However, there could be some limitations regarding the use of MEG in mTBI diagnosis taking into account the demographic and clinical characteristics of the patients. Several studies have demonstrated that brain wave patterns could vary depending on physiological states (i.e., age, sex) [56,57,58,59] and in mTBI [60]. In this context, the studies we included in this analysis presented reports of brain activity in both children and adults. The findings suggested several differences regarding the variability of changes in brain wave patterns, concerning the wave types and the brain areas affected (Table 1). However, these differences could also be the results of the varied mechanisms of injury, as [25] demonstrated by comparing blast and non-blast mTBI cases for differences in brain activity. In this context, according to the included studies, most of the children/adolescents suffered from mTBIs that were due to falls or sports accidents, while the origin of injury in adults was more heterogeneous. Moreover, we observed that many studies focused on evaluating the changes in brain waves activity in males, rather than in females. This could be a result of the increased prevalence of concussions in men, as compared to women [61]. Further studies should focus on analyzing the possible differences that occur in mTBI cases based on age, sex, mechanism of injury, and other variables that could be implicated in brain activity variability, as demonstrated by several studies [62,63,64,65] for sex and age differences after mTBI in behavior and brain anatomy.
Moreover, the heterogeneity in study designs, MEG scan processing methods, and patient populations posed a challenge in comparing results across studies. Future research should aim to standardize MEG protocols for mTBI assessment to facilitate cross-study comparisons and improve the reliability of MEG as a diagnostic tool.
4.5. Implications for Clinical Practice and Future Perspectives
The results of this review demonstrate the potential of MEG as a valuable tool in the diagnosis and monitoring of mTBI and PCS. By providing objective biomarkers of brain function, MEG could complement traditional imaging modalities, which often fail to detect the subtle neural changes associated with mTBI. Furthermore, the use of machine learning techniques in conjunction with MEG data shows promise in improving diagnostic accuracy and predicting clinical outcomes. For instance, to overcome the limitation of lower spatial resolution, as compared to other imaging techniques, Antonakakis et al. [48] described a possible combination between MEG and EEG and analyzed the possibility to improve connectivity mapping by adding MRI with diffusion tensor imaging.
Future perspectives that are opened by this study (Table 9) include the necessity for larger, multi-centered studies that could enable the validation of the previous findings. Additionally, further exploration of MEG’s potential to differentiate between mTBI and co-occurring conditions, such as PTSD, could improve diagnostic precision and patient management. Developing more cost-effective and accessible MEG technologies will also be essential for translating these research findings into clinical practice.

Table 9.
Future perspectives.
4.6. Limitations of the Current Systematic Review
Except for the limitations of MEG as a diagnosis tool in mTBI, as presented in the selected studies and discussed above, this study has several limitations. For instance, the studies that were evaluated in accordance with the selection criteria reported a small number of patients and healthy controls with varying demographic characteristics (mainly age and mTBI source). In this context, further studies could concentrate on large-scale evaluations of mTBI patients and populations in order to provide more comprehensive results. Another important limitation was the heterogeneity of the parameters used in MEG evaluation, as well as in filtering the imaging data for noise, signal power, and connectivity type. However, this study was unable to perform a meta-analysis of the data, similarly to a previous report by Allen et al. [6], which failed to demonstrate the use of MEG in mTBI diagnosis routines for adults. Further meta-analyses could be performed only after a rigorous filtering of the collected data and by eliminating as many variables as possible.
5. Conclusions
MEG is a promising tool for the diagnosis and monitoring of mTBI and PCS. Its ability to detect abnormal brain oscillations and functional connectivity disruptions offers unique insights into the neural mechanisms underlying mTBI-associated brain damage and its long-term consequences. Significant reports endorsed the potential of MEG to assist in diagnosis and prognosis. The sensitivity of detecting abnormal brain oscillations and functional connectivity disruptions could provide significant advantages in understanding the subtle neural impairments often missed by traditional imaging methods. Also, several studies have demonstrated the correlation between MEG biomarkers and cognitive and behavioral symptoms that could accommodate differentiated diagnosis—between mTBI and overlapping or co-occurring conditions—and monitoring recovery. Despite its limitations, mainly concerning standardization and accessibility, our analysis showed that MEG could play a crucial role in improving the diagnosis, treatment, and prognosis of mTBI.
Author Contributions
Conceptualization, methodology, and formal analysis, I.M., D.K. and F.E.P.; investigation, data curation, and writing—original draft preparation, I.M., D.K., F.E.P., I.-M.B. and A.C.; writing—review and editing, I.-M.B. and A.C.; visualization, I.M. and I.-M.B.; supervision, I.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jackson, W.T.; Starling, A.J. Concussion Evaluation and Management. Med. Clin. N. Am. 2019, 103, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bielanin, J.P.; Metwally, S.A.H.; Paruchuri, S.S.; Sun, D. An overview of mild traumatic brain injuries and emerging therapeutic targets. Neurochem. Int. 2024, 172, 105655. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.N.; Cross, D.J. Brain Trauma Imaging. J. Nuclear Med. 2023, 64, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.R.; Huang, M. Magnetoencephalography in the diagnosis of concussion. Prog. Neurol. Surg. 2014, 28, 94–111. [Google Scholar] [CrossRef]
- Davenport, E.M.; Urban, J.E.; Vaughan, C.; DeSimone, J.C.; Wagner, B.; Espeland, M.A.; Powers, A.K.; Whitlow, C.T.; Stitzel, J.D.; Maldjian, J.A. MEG measured delta waves increase in adolescents after concussion. Brain Behav. 2022, 12, e2720. [Google Scholar] [CrossRef]
- Allen, C.M.; Halsey, L.; Topcu, G.; Rier, L.; Gascoyne, L.E.; Scadding, J.W.; Furlong, P.L.; Dunkley, B.T.; das Nair, R.; Brookes, M.J.; et al. Magnetoencephalography abnormalities in adult mild traumatic brain injury: A systematic review. NeuroImage Clin. 2021, 31, 102697. [Google Scholar] [CrossRef]
- Desjardins, M.; Drisdelle, B.L.; Lefebvre, C.; Gagnon, J.F.; De Beaumont, L.; Jolicoeur, P. Interhemispheric differences in P1 and N1 amplitude in EEG and MEG differ across older individuals with a concussion compared with age-matched controls. Psychophysiology 2021, 58, e13751. [Google Scholar] [CrossRef]
- Krieger, D.; Shepard, P.; Soose, R.; Puccio, A.; Beers, S.; Schneider, W.; Kontos, A.P.; Collins, M.W.; Okonkwo, D.O. MEG-Derived Symptom-Sensitive Biomarkers with Long-Term Test-Retest Reliability. Diagnostics 2021, 12, 84. [Google Scholar] [CrossRef]
- Suri, A.K.; Lipton, M.L. Neuroimaging of brain trauma in sports. Handb. Clin. Neurol. 2018, 158, 205–216. [Google Scholar] [CrossRef]
- Stein, M.B.; McAllister, T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatry 2009, 166, 768–776. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Technological Basics of EEG Recording and Operation of Apparatus. In Introduction to EEG- and Speech-Based Emotion Recognition; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–50. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Adetunji, C.O.; Dare, A.; Adeyomoye, O.; Adeniyi, M.J.; Enoch, A. Neural signaling and communication using machine learning. In Artificial Intelligence for Neurological Disorders; Chapter 15; Academic Press: Cambridge, MA, USA, 2023; pp. 245–260. [Google Scholar]
- Hoshi, H.; Hirata, Y.; Fukasawa, K.; Kobayashi, M.; Shigihara, Y. Oscillatory characteristics of resting-state magnetoencephalography reflect pathological and symptomatic conditions of cognitive impairment. Front. Aging Neurosci. 2024, 16, 1273738. [Google Scholar] [CrossRef] [PubMed]
- Afnan, J.; von Ellenrieder, N.; Lina, J.M.; Pellegrino, G.; Arcara, G.; Cai, Z.; Hedrich, T.; Abdallah, C.; Khajehpour, H.; Frauscher, B.; et al. Validating MEG source imaging of resting state oscillatory patterns with an intracranial EEG atlas. NeuroImage 2023, 274, 120158. [Google Scholar] [CrossRef] [PubMed]
- Fred, A.L.; Kumar, S.N.; Kumar Haridhas, A.; Ghosh, S.; Purushothaman Bhuvana, H.; Sim, W.K.; Vimalan, V.; Givo, F.A.; Jousmäki, V.; Padmanabhan, P.; et al. A Brief Introduction to Magnetoencephalography (MEG) and Its Clinical Applications. Brain Sci. 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Gierisch, J.M.; Beadles, C.; Shapiro, A.; McDuffie, J.; Cunningham, N.; Bradford, D.; Strauss, J.; Callahan, M.; Chen, M.; Hemminger, A.; et al. Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness [Internet]. Washington (DC): Department of Veterans Affairs (US); 2014 Oct. Appendix B, Newcastle-Ottawa Scale Coding Manual for Cohort Studies. Available online: https://www.ncbi.nlm.nih.gov/books/NBK299087/ (accessed on 4 December 2024).
- Higgins, J.P.T.; Altman, D.G.; Gatzsche, P.C.; Jani, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Gross, J.; Baillet, S.; Barnes, G.R.; Henson, R.N.; Hillebrand, A.; Jensen, O.; Jerbi, K.; Litvak, V.; Maess, B.; Oostenveld, R.; et al. Good practice for conducting and reporting MEG research. NeuroImage 2013, 65, 349–363. [Google Scholar] [CrossRef]
- Cichy, R.M.; Pantazis, D. Multivariate pattern analysis of MEG and EEG: A comparison of representational structure in time and space. NeuroImage 2017, 158, 441–454. [Google Scholar] [CrossRef]
- Singh, S.P. Magnetoencephalography: Basic principles. Ann. Indian. Acad. Neurol. 2014, 17 (Suppl. S1), S107–S112. [Google Scholar] [CrossRef]
- Huang, M.X.; Theilmann, R.J.; Robb, A.; Angeles, A.; Nichols, S.; Drake, A.; D’Andrea, J.; Levy, M.; Holland, M.; Song, T.; et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 2009, 26, 1213–1226. [Google Scholar] [CrossRef]
- Huang, M.X.; Nichols, S.; Robb-Swan, A.; Angeles-Quinto, A.; Harrington, D.L.; Drake, A.; Huang, C.W.; Song, T.; Diwakar, M.; Risbrough, V.B.; et al. MEG Working Memory N-Back Task Reveals Functional Deficits in Combat-Related Mild Traumatic Brain Injury. Cereb. Cortex 2019, 29, 1953–1968. [Google Scholar] [CrossRef]
- Huang, M.X.; Robb Swan, A.; Angeles Quinto, A.; Huang, J.W.; De-la-Garza, B.G.; Huang, C.W.; Hesselink, J.R.; Bigler, E.D.; Wilde, E.A.; Max, J.E. Resting-State Magnetoencephalography Source Imaging Pilot Study in Children with Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 994–1001. [Google Scholar] [CrossRef]
- Huang, M.X.; Huang, C.W.; Harrington, D.L.; Nichols, S.; Robb-Swan, A.; Angeles-Quinto, A.; Le, L.; Rimmele, C.; Drake, A.; Song, T.; et al. Marked Increases in Resting-State MEG Gamma-Band Activity in Combat-Related Mild Traumatic Brain Injury. Cereb. Cortex 2020, 30, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Nichols, S.; Robb, A.; Angeles, A.; Drake, A.; Holland, M.; Asmussen, S.; D’Andrea, J.; Chun, W.; Levy, M.; et al. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. NeuroImage 2012, 61, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Angeles-Quinto, A.; Robb-Swan, A.; De-la-Garza, B.G.; Huang, C.W.; Cheng, C.K.; Hesselink, J.R.; Bigler, E.D.; Wilde, E.A.; Vaida, F.; et al. Assessing Pediatric Mild Traumatic Brain Injury and Its Recovery Using Resting-State Magnetoencephalography Source Magnitude Imaging and Machine Learning. J. Neurotrauma 2023, 40, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Alhourani, A.; Wozny, T.A.; Krishnaswamy, D.; Pathak, S.; Walls, S.A.; Ghuman, A.S.; Krieger, D.N.; Okonkwo, D.O.; Richardson, R.M.; Niranjan, A. Magnetoencephalography-based identification of functional connectivity network disruption following mild traumatic brain injury. J. Neurophysiol. 2016, 116, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.A.; Stapleton-Kotloski, J.R.; Alberto, G.E.; Rawley, J.A.; Kotloski, R.J.; Taber, K.H.; Godwin, D.W. Contrasting Effects of Posttraumatic Stress Disorder and Mild Traumatic Brain Injury on the Whole-Brain Resting-State Network: A Magnetoencephalography Study. Brain Connect. 2017, 7, 45–57. [Google Scholar] [CrossRef]
- Diwakar, M.; Harrington, D.L.; Maruta, J.; Ghajar, J.; El-Gabalawy, F.; Muzzatti, L.; Corbetta, M.; Huang, M.X.; Lee, R.R. Filling in the gaps: Anticipatory control of eye movements in chronic mild traumatic brain injury. NeuroImage Clin. 2015, 8, 210–223. [Google Scholar] [CrossRef]
- Robb Swan, A.; Nichols, S.; Drake, A.; Angeles, A.; Diwakar, M.; Song, T.; Lee, R.R.; Huang, M.X. Magnetoencephalography Slow-Wave Detection in Patients with Mild Traumatic Brain Injury and Ongoing Symptoms Correlated with Long-Term Neuropsychological Outcome. J. Neurotrauma 2015, 32, 1510–1521. [Google Scholar] [CrossRef]
- Zhang, J.; Safar, K.; Emami, Z.; Ibrahim, G.M.; Scratch, S.E.; da Costa, L.; Dunkley, B.T. Local and large-scale beta oscillatory dysfunction in males with mild traumatic brain injury. J. Neurophysiol. 2020, 124, 1948–1958. [Google Scholar] [CrossRef]
- Dunkley, B.T.; Da Costa, L.; Bethune, A.; Jetly, R.; Pang, E.W.; Taylor, M.J.; Doesburg, S.M. Low-frequency connectivity is associated with mild traumatic brain injury. NeuroImage Clin. 2015, 7, 611–621. [Google Scholar] [CrossRef]
- Zhang, J.; Emami, Z.; Safar, K.; McCunn, P.; Richardson, J.D.; Rhind, S.G.; da Costa, L.; Jetly, R.; Dunkley, B.T. Teasing apart trauma: Neural oscillations differentiate individual cases of mild traumatic brain injury from post-traumatic stress disorder even when symptoms overlap. Transl. Psychiatry 2021, 11, 345. [Google Scholar] [CrossRef]
- Li, L.; Arakaki, X.; Harrington, M.; Zouridakis, G. Source Connectivity Analysis Can Assess Recovery of Acute Mild Traumatic Brain Injury Patients. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3165–3168. [Google Scholar] [CrossRef]
- Pang, E.W.; Dunkley, B.T.; Doesburg, S.M.; da Costa, L.; Taylor, M.J. Reduced brain connectivity and mental flexibility in mild traumatic brain injury. Ann. Clin. Transl. Neurol. 2015, 3, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Papanicolaou, A.C.; Zouridakis, G. Mining cross-frequency coupling microstates from resting state MEG: An application to mild traumatic brain injury. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5513–5516. [Google Scholar] [CrossRef]
- Lawton, T.; Huang, M.X. Dynamic cognitive remediation for a Traumatic Brain Injury (TBI) significantly improves attention, working memory, processing speed, and reading fluency. Restor. Neurol. Neurosci. 2019, 37, 71–86. [Google Scholar] [CrossRef]
- Shah-Basak, P.P.; Urbain, C.; Wong, S.; da Costa, L.; Pang, E.W.; Dunkley, B.T.; Taylor, M.J. Concussion Alters the Functional Brain Processes of Visual Attention and Working Memory. J. Neurotrauma 2018, 35, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Rier, L.; Zamyadi, R.; Zhang, J.; Emami, Z.; Seedat, Z.A.; Mocanu, S.; Gascoyne, L.E.; Allen, C.M.; Scadding, J.W.; Furlong, P.L.; et al. Mild traumatic brain injury impairs the coordination of intrinsic and motor-related neural dynamics. NeuroImage Clin. 2021, 32, 102841. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Hughes, J.D.; Popescu, E.A.; Riedy, G.; Degraba, T.J. Reduced prefrontal MEG alpha-band power in mild traumatic brain injury with associated posttraumatic stress disorder symptoms. Clin. Neurophysiol. 2016, 127, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Vandewouw, M.; Emami, Z.; Bells, S.; Rudberg, N.; da Costa, L.; Dunkley, B.T. Memory retrieval brain-behavior disconnection in mild traumatic brain injury: A magnetoencephalography and diffusion tensor imaging study. Hum. Brain Mapp. 2022, 43, 5296–5309. [Google Scholar] [CrossRef]
- Kaltiainen, H.; Liljeström, M.; Helle, L.; Salo, A.; Hietanen, M.; Renvall, H.; Forss, N. Mild Traumatic Brain Injury Affects Cognitive Processing and Modifies Oscillatory Brain Activity during Attentional Tasks. J. Neurotrauma 2019, 36, 2222–2232. [Google Scholar] [CrossRef]
- Kaltiainen, H.; Helle, L.; Liljeström, M.; Renvall, H.; Forss, N. Theta-Band Oscillations as an Indicator of Mild Traumatic Brain Injury. Brain Topogr. 2018, 31, 1037–1046. [Google Scholar] [CrossRef]
- Itälinna, V.; Kaltiainen, H.; Forss, N.; Liljeström, M.; Parkkonen, L. Using normative modeling and machine learning for detecting mild traumatic brain injury from magnetoencephalography data. PLoS. Comput. Biol. 2023, 19, e1011613. [Google Scholar] [CrossRef]
- Vakorin, V.A.; Doesburg, S.M.; da Costa, L.; Jetly, R.; Pang, E.W.; Taylor, M.J. Detecting Mild Traumatic Brain Injury Using Resting State Magnetoencephalographic Connectivity. PLoS Comput. Biol. 2016, 12, e1004914. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Zouridakis, G.; Rezaie, R.; Babajani-Feremi, A.; Papanicolaou, A.C. Functional connectivity changes detected with magnetoencephalography after mild traumatic brain injury. NeuroImage Clin. 2015, 9, 519–531. [Google Scholar] [CrossRef]
- Peitz, G.W.; Wilde, E.A.; Grandhi, R. Magnetoencephalography in the Detection and Characterization of Brain Abnormalities Associated with Traumatic Brain Injury: A Comprehensive Review. Med. Sci. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Antonakakis, M.; Schrader, S.; Wollbrink, A.; Oostenveld, R.; Rampp, S.; Haueisen, J.; Wolters, C.H. The effect of stimulation type, head modeling, and combined EEG and MEG on the source reconstruction of the somatosensory P20/N20 component. Hum. Brain Mapp. 2019, 40, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, D.R.; Engdahl, B.E.; Leuthold, A.; Georgopoulos, A.P. Assessing Recovery from Mild Traumatic Brain Injury (Mtbi) using Magnetoencephalography (MEG): An Application of the Synchronous Neural Interactions (SNI) Test. J. Neurol. Neuromed. 2020, 5, 28–34. [Google Scholar] [CrossRef]
- da Costa, L.; Robertson, A.; Bethune, A.; MacDonald, M.J.; Shek, P.N.; Taylor, M.J.; Pang, E.W. Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Proskovec, A.L.; Shah, B.R.; Yu, F.F.; Achilleos, M.; Maldjian, J.A.; Davenport, E.M. Magnetoencephalography and Mild Traumatic Brain Injury. Adv. Clin. Radiol. 2020, 2, 341–350. [Google Scholar] [CrossRef]
- Solar, K.G.; Ventresca, M.; Zamyadi, R.; Zhang, J.; Jetly, R.; Vartanian, O.; Rhind, S.G.; Dunkley, B.T. Repetitive subconcussion results in disrupted neural activity independent of concussion history. Brain Commun. 2024, 6, fcae348. [Google Scholar] [CrossRef]
- Aaltonen, J.; Heikkinen, V.; Kaltiainen, H.; Salmelin, R.; Renvall, H. Sensor-level MEG combined with machine learning yields robust classification of mild traumatic brain injury patients. Clin. Neurophysiol. 2023, 153, 79–87. [Google Scholar] [CrossRef]
- Safar, K.; Zhang, J.; Emami, Z.; Gharehgazlou, A.; Ibrahim, G.; Dunkley, B.T. Mild traumatic brain injury is associated with dysregulated neural network functioning in children and adolescents. Brain Commun. 2021, 3, fcab044. [Google Scholar] [CrossRef]
- Mardell, L.C.; Spedden, M.E.; O’Neill, G.C.; Tierney, T.M.; Timms, R.C.; Zich, C.; Barnes, G.R.; Bestmann, S. Concurrent spinal and brain imaging with optically pumped magnetometers. J. Neurosci. Methods 2024, 406, 110131. [Google Scholar] [CrossRef]
- Geerligs, L.; Renken, R.J.; Saliasi, E.; Maurits, N.M.; Lorist, M.M. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral. Cortex 2015, 25, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Erhardt, E.B.; Damaraju, E.; Gruner, W.; Segall, J.M.; Silva, R.F.; Havlicek, M.; Rachakonda, S.; Fries, J.; Kalyanam, R.; et al. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011, 5, 2. [Google Scholar] [CrossRef]
- Yener, G.; Kıyı, İ.; Düzenli-Öztürk, S.; Yerlikaya, D. Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study. Brain Sci. 2024, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cheung, V.C.K.; Chan, R.H.M. Aging amplifies sex differences in low alpha and low beta EEG oscillations. bioRxiv 2024, 603949. [Google Scholar] [CrossRef]
- Bittencourt-Villalpando, M.; van der Horn, H.J.; Maurits, N.M.; van der Naalt, J. Disentangling the effects of age and mild traumatic brain injury on brain network connectivity: A resting state fMRI study. NeuroImage Clin. 2021, 29, 102534. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.S.; Kim, J.H.; Yoon, S.H.; Lee, S.; Park, K.-J.; Ha, S.-K.; Choi, J.-G.; Jo, K.-W.; Kim, J.; Kang, S.H.; et al. Gender differences in adult traumatic brain injury according to the Glasgow coma scale: A multicenter descriptive study. Chin. J. Traumatol. 2021, 24, 333–343. [Google Scholar] [CrossRef]
- Wågberg, S.; Stålnacke, B.M.; Magnusson, B.M. Gender and Age Differences in Outcomes after Mild Traumatic Brain Injury. J. Clin. Med. 2023, 12, 4883. [Google Scholar] [CrossRef]
- Levin, H.S.; Temkin, N.R.; Barber, J.; Nelson, L.D.; Robertson, C.; Brennan, J.; Stein, M.B.; Yue, J.K.; Giacino, J.T.; McCrea, M.A.; et al. Association of Sex and Age with Mild Traumatic Brain Injury–Related Symptoms: A TRACK-TBI Study. JAMA Netw. Open 2021, 4, e213046. [Google Scholar] [CrossRef]
- Vakhtin, A.A.; Zhang, Y.; Wintermark, M.; Ashford, J.W.; Furst, A.J. Distant histories of mild traumatic brain injury exacerbate age-related differences in white matter properties. Neurobiol. Aging 2021, 107, 30–41. [Google Scholar] [CrossRef]
- Starkey, N.J.; Duffy, B.; Jones, K.; Theadom, A.; Barker-Collo, S.; Feigin, V.; BIONIC8 Research Group. Sex differences in outcomes from mild traumatic brain injury eight years post-injury. PLoS ONE 2022, 17, e0269101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).