Application of a Brain–Computer Interface System with Visual and Motor Feedback in Limb and Brain Functional Rehabilitation after Stroke: Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. L-B300 EEG Acquisition and Rehabilitation Training System
2.3. Training and Evaluation Methods
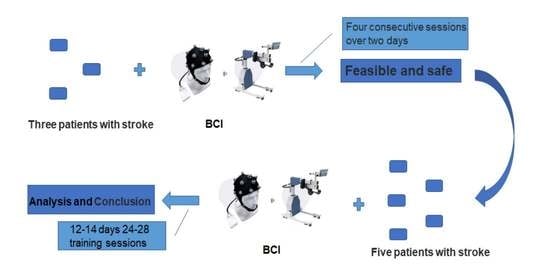
2.3.1. Feasibility and Safety Verification of the System
2.3.2. Effectiveness Evaluation of the System
2.4. Statistical Analysis
3. Results
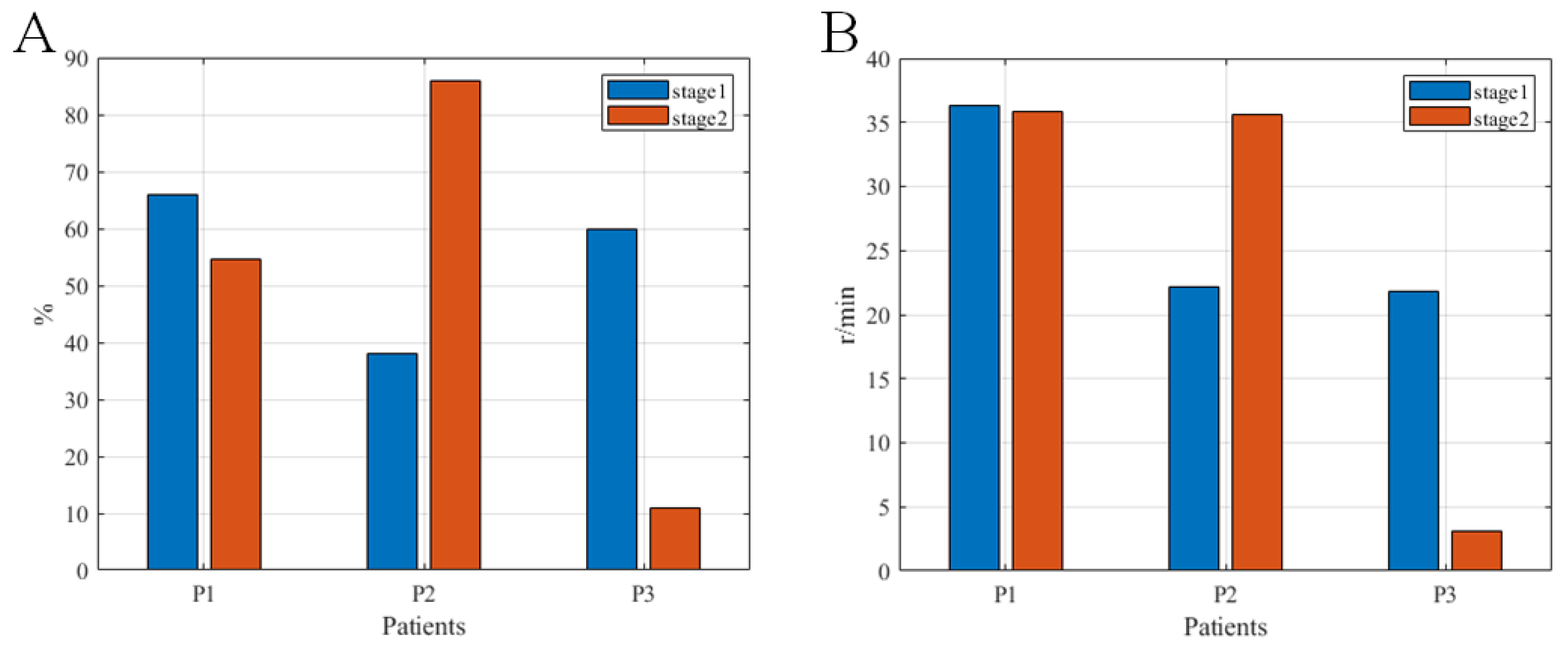
3.1. Feasibility and Safety Verification of the System
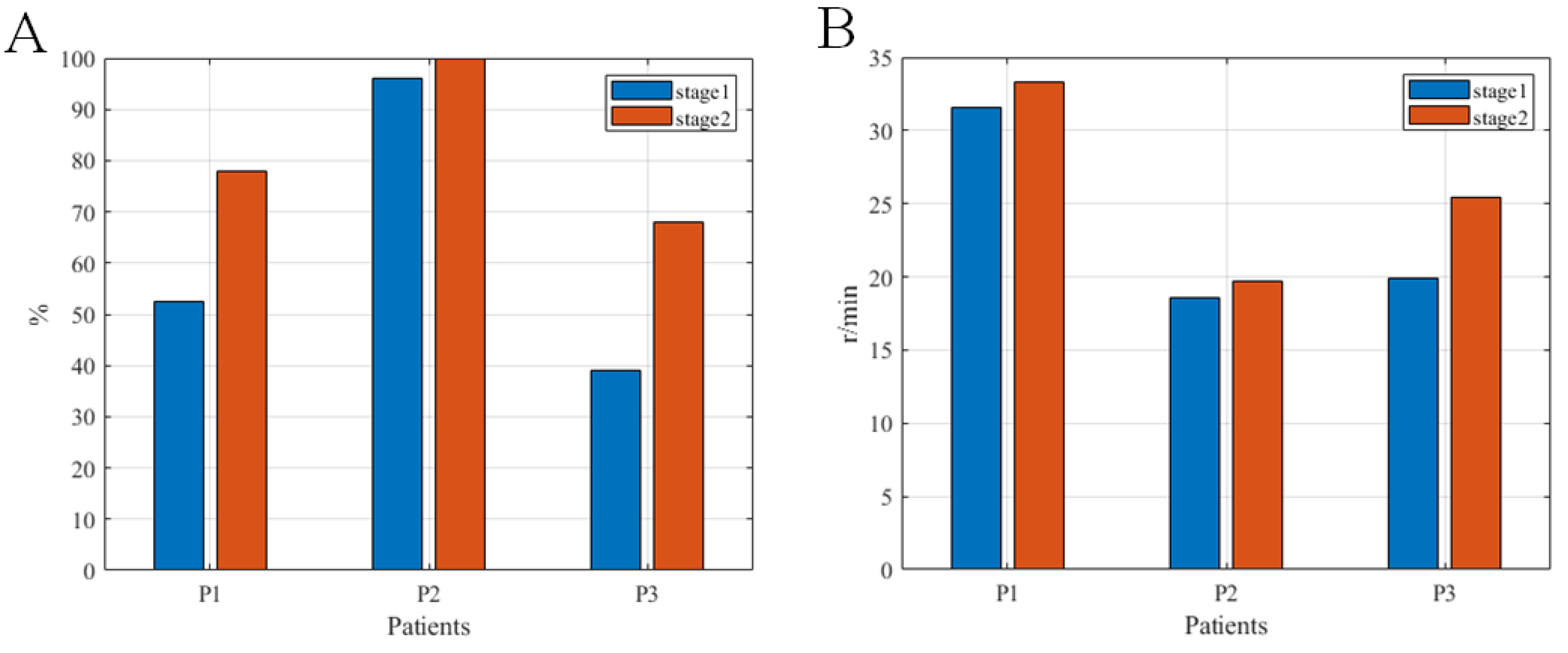
3.2. Rehabilitation Effects of the System
3.2.1. Clinical Indicators in the Limb Motor Function of Patients
3.2.2. The RMSs of the Limb Muscles on the Hemiplegic Side
3.2.3. Brain Function Test Results of Subjects
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Zhu, J.; Chen, W.; Wang, L.; Liu, S.; Li, Y.; Wang, L.; Liu, Y.; Yin, P. Cause-specific mortality for 240 causes in China during 1990–2013: A systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016, 387, 251–272. [Google Scholar] [CrossRef]
- Moraga, P.; GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar]
- Ono, T.; Shindo, K.; Kawashima, K.; Ota, N.; Ito, M.; Ota, T.; Mukaino, M.; Fujiwara, T.; Kimura, A.; Liu, M.; et al. Brain-Computer Interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Front. Neuroeng. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.; Alyasseri, Z.A.A.; Abdulkareem, K.H.; Ali, N.S.; Al-Mhiqani, M.N.; Guger, C. EEG feature fusion for motor imagery: A new robust framework towards stroke patients rehabilitation. Comput. Biol. Med. 2021, 137, 104799. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; Donnan, G.A.; Gorelick, P.B.; Hacke, W.; Cramer, S.C.; Kaste, M.; Fisher, M.; Brainin, M.; Buchan, A.M.; Lo, E.H.; et al. Stroke: Working toward a prioritized world agenda. Cerebrovasc. Dis. 2010, 30, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.K.; Ali, S.H.B.; Ahmad, S.A.; Chellappan, K.; Islam, M.; Escudero, J. Role of EEG as biomarker in the early detection and classification of dementia. Sci. World J. 2014, 2014, 906038. [Google Scholar] [CrossRef] [PubMed]
- Junior, V.A.D.S.; Santos, M.D.S.; Ribeiro, N.M.D.S.; Maldonado, I.L. Combining proprioceptive neuromuscular facilitation and virtual reality for improving sensorimotor function in stroke survivors: A randomized clinical trial. J. Cent. Nerv. Syst. Dis. 2019, 11, 593276910. [Google Scholar] [CrossRef] [PubMed]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.Z.; Lewek, M.D.; Sawicki, G.S. A neuromechanics-based powered ankle exoskeleton to assist walking post-stroke: A feasibility study. J. Neuroeng. Rehabil. 2015, 12, 23. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, D.; Qian, W.; Xiao, X.; Guo, Z. Modeling and control of a cable-driven rotary series elastic actuator for an upper limb rehabilitation robot. Front. Neurorobot. 2020, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Awad, L.N.; Lewek, M.D.; Kesar, T.M.; Franz, J.R.; Bowden, M.G. These legs were made for propulsion: Advancing the diagnosis and treatment of post-stroke propulsion decits. J. Neuroeng. Rehabil. 2020, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Zulauf-Czaja, A.; Al-Taleb, M.K.H.; Purcell, M.; Petric-Gray, N.; Cloughley, J.; Vuckovic, A. On the way home: A BCI-FES hand therapy self-managed by sub-acute SCI participants and their caregivers: A usability study. J. Neuroeng. Rehabil. 2021, 18, 44. [Google Scholar] [CrossRef]
- Foong, R.; Ang, K.K.; Quek, C.; Guan, C.; Phua, K.S.; Kuah, C.W.K.; Deshmukh, V.A.; Yam, L.H.L.; Rajeswaran, D.K.; Tang, N. Assessment of the efficacy of EEG-based MI-BCI with visual feedback and EEG correlates of mental fatigue for upper-limb stroke rehabilitation. IEEE. Trans. Biomed. Eng. 2020, 67, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.; Chouhan, T.; Guan, C. BCI for stroke rehabilitation: Motor and beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.V.; Wong, Y.T. Neurobionics and the brain-computer interface: Current applications and future horizons. Med. J. Aust. 2017, 206, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, M.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors 2021, 21, 5746. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, K. Robotic arm control system based on augmented reality brain-computer interface and computer vision. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2021, 38, 483–491. [Google Scholar] [PubMed]
- Mihara, M.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Hino, T.; Miyai, I. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: A pilot study. Stroke 2013, 44, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Ephraim Joseph, G.J.; Kuah, C.W.; Chua, K.S. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.; Low, W.; Guan, C. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clin. EEG Neurosci. 2015, 46, 310–320. [Google Scholar] [CrossRef]
- Wang, C.; Phua, K.S.; Ang, K.K.; Guan, C.; Zhang, H.; Lin, R.; Chua, K.S.G.; Ang, B.T.; Kuah, C.W.K. A feasibility study of non-invasive motor-imagery BCI-based robotic rehabilitation for Stroke patients. In Proceedings of the 2009 4th International IEEE/EMBS Conference on Neural Engineering, Antalya, Turkey, 29 April–2 May 2009; pp. 271–274. [Google Scholar]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.C.; Bermúdez, I.; Badia, S. Efficacy and Brain Imaging Correlates of an Immersive Motor Imagery BCI-Driven VR System for Upper Limb Motor Rehabilitation: A Clinical Case Report. Front. Hum. Neurosci. 2019, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.L. Effects of a Brain-Computer Interface With Virtual Reality (VR) Neurofeedback: A Pilot Study in Chronic Stroke Patients. Front. Hum. Neurosci. 2019, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Mukaino, M.; Ushiba, J. Functional recovery in upper limb function in stroke survivors by using brain-computer interface A single case A-B-A-B design. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 265–268. [Google Scholar]
- Montag, M.; Paschall, C.; Ojemann, J.; Rao, R.; Herron, J. A Platform for Virtual Reality Task Design with Intracranial Electrodes. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 6659–6662. [Google Scholar]
- Chmura, J.; Rosing, J.; Collazos, S.; Goodwin, S.J. Classification of Movement and Inhibition Using a Hybrid BCI. Front. Neurorobot. 2017, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rodriguez, M.; Peters, J.; Hill, J.; Schölkopf, B.; Gharabaghi, A.; Grosse-Wentrup, M. Closing the sensorimotor loop: Haptic feedback facilitates decoding of motor imagery. J. Neural Eng. 2011, 8, 036005. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, O.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A.; et al. Brain-machine interface in chronic stroke rehabilitation: A controlled study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef]
- Cui, Z.; Fu, X.; Wan, X.; Li, J.; Chen, W.; Zhu, S.; Li, Y. The brain-computer interface based robot gives spinal cord injury patients a full-cycle active rehabilitation. In Proceedings of the 2021 9th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Korea, 22–24 February 2021; pp. 1–5. [Google Scholar]
- Ciesla, N.; Dinglas, V.; Fan, E.; Kho, M.; Kuramoto, J.; Needham, D. Manual muscle testing: A method of measuring extremity muscle strength applied to critically ill patients. J. Vis. Exp. 2011, 50, 2632. [Google Scholar] [CrossRef]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early rehabilitation after stroke: A narrative review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the international federation. The internanional federation of clinical nenrophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Cui, Z.; Li, Y.; Huang, S.; Wu, X.; Fu, X.; Liu, F.; Wan, X.; Wang, X.; Zhang, Y.; Qiu, H. BCI system with lower-limb robot improves rehabilitation in spinal cord injury patients through short-term training: A pilot study. Cogn. Neurodyn. 2022, 1–19. [Google Scholar] [CrossRef]
- Mousavi, M.; Krol, L.R.; de Sa, V.R. Hybrid brain-computer interface with motor imagery and error-related brain activity. J. Neural Eng. 2020, 17, 056041. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; Cuchí, G.U. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1024–1033. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Thompson, P.; Beath, T.; Bell, J.; Jacobson, G.; Phair, T.; Salbach, N.M.; Wright, F.V. Test–retest reliability of the 10-metre fast walk test and 6-minute walk test in ambulatory school-aged children with cerebral palsy. Dev. Med. Child. Neurol. 2008, 50, 370–376. [Google Scholar] [CrossRef]
- Enright, P.L. The six-minute walk test. Resp. Care 2003, 48, 783–785. [Google Scholar]
- Taghizadeh, G.; Martinez-Martin, P.; Meimandi, M.; Habibi, S.A.H.; Jamali, S.; Dehmiyani, A.; Rostami, S.; Mahmuodi, A.; Mehdizadeh, M.; Fereshtehnejad, S. Barthel index and modified rankin scale: Psychometric properties during medication phases in idiopathic Parkinson disease. Ann. Phys. Rehabil. Med. 2020, 63, 500–504. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Cheng, M.; Alshahrani, Y.; Lemos, S. Comparison of machine learning methods in sEMG signal processing for shoulder motion recognition. Biomed. Signal Process. 2021, 68, 102577. [Google Scholar] [CrossRef]
- Kim, W.J.; Min, Y.S.; Yang, E.J.; Paik, N.J. Neuronavigated vs. Conventional Repetitive Transcranial Magnetic Stimulation Method for Virtual Lesioning on the Broca’s Area. Neuromodulation 2014, 17, 16–21. [Google Scholar] [CrossRef]
- Parker, R.I.; Hagan-Burke, S. Useful effect size interpretations for single case research. Behav. Ther. 2007, 38, 95–105. [Google Scholar] [CrossRef]
- Sheorajpanday, R.V.; Nagels, G.; Weeren, A.J.; De Surgeloose, D.; De Deyn, P.P. Additional value of quantitative EEG in acute anterior circulation syndrome of presumed ischemic origin. Clin. Neurophysiol. 2010, 121, 1719–1725. [Google Scholar] [CrossRef]
- Sheorajpanday, R.V.; Nagels, G.; Weeren, A.J.; van Putten, M.J.; De Deyn, P.P. Quantitative EEG in ischemic stroke: Correlation with functional status after 6 months. Clin. Neurophysiol. 2011, 122, 874–883. [Google Scholar] [CrossRef]
- Carelli, L.; Solca, F.; Faini, A.; Meriggi, P.; Sangalli, D.; Cipresso, P.; Riva, G.; Ticozzi, N.; Ciammola, A.; Silani, V.; et al. Brain-Computer Interface for Clinical Purposes: Cognitive Assessment and Rehabilitation. Biomed. Res. Int. 2017, 2017, 1695290. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Gong, Q.; Liu, K.; Li, H. Analysis of Behavioural Characteristics Related to Unintentional Injury in Southeast Chinese Adolescents: Evidence from a School-Based Survey. Int. J. Environ. Res. Public Health 2017, 14, 241. [Google Scholar] [CrossRef]
- Cakar, E.; Akyuz, G.; Durmus, O.; Bayman, L.; Yagci, I.; Karadag-Saygi, E.; Gunduz, O.H. The relationships of motor-evoked potentials to hand dexterity, motor function, and spasticity in chronic stroke patients: A transcranial magnetic stimulation study. Acta Neurol. Belg. 2016, 116, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Kindred, J.H.; Cash, J.J.; Ergle, J.B.; Charalambous, C.C.; Wonsetler, E.C.; Bowden, M.G. Comparing cortico-motor hotspot identification methods in the lower extremities post-stroke: MEP amplitude vs. latency. Neurosci. Lett. 2021, 754, 135884. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Herrera, A.; Gaunt, R.; Collinger, J. Bidirectional brain-computer interfaces. Handb. Clin. Neurol. 2020, 168, 163–181. [Google Scholar]
- Brown, R.E.; Bligh, T.W.B.; Garden, J.F. The Hebb Synapse Before Hebb: Theories of Synaptic Function in Learning and Memory Before, With a Discussion of the Long-Lost Synaptic Theory of William McDougall. Front. Behav. Neurosci. 2021, 15, 732195. [Google Scholar] [CrossRef]



| Subject | Age | Gender | Sites of Injury | Course of Disease(d) | BCI Treatment Times |
|---|---|---|---|---|---|
| 1 | 58 | Male | Left basal ganglia cerebral hemorrhage | 34 | 4 |
| 2 | 31 | Male | Right basal ganglia cerebral hemorrhage | 127 | 4 |
| 3 | 55 | Female | Right basal ganglia cerebral infarction | 30 | 4 |
| 1′ | 34 | Male | Left cerebellar hemisphere hemorrhage | 41 | 26 |
| 2′ | 44 | Male | Left basal ganglia cerebral hemorrhage | 37 | 26 |
| 3′ | 51 | Male | Left basal ganglia cerebral infarction | 18 | 28 |
| 4′ | 52 | Female | left basal ganglia cerebral infarction | 15 | 24 |
| 5′ | 58 | Male | Left basal ganglia, left paraventricular cerebral infarction | 41 | 26 |
| Assessment Item | Before | After | Difference | Cohen’s w |
|---|---|---|---|---|
| Sitting balance ability | 2.4 ± 0.9 | 2.8 ± 0.5 | 0.4 ± 0.55 | 1.22 ** |
| Upper limb FMA | 22.2 ± 10.1 | 30.2 ± 8.9 | 8.0 ± 5.61 | 5.18 ** |
| Lower limb FMA | 19.6 ± 10.1 | 25.0 ± 5.7 | 5.4 ± 5.18 | 5.44 ** |
| 10 m walking speed | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.04 ± 0.03 | 0.16 |
| 6MWD | 155.6 ± 50.0 | 167.0 ± 48.9 | 11.4 ± 4.30 | 2.29 ** |
| MBI | 65.0 ± 7.1 | 72.0 ± 7.6 | 7.0 ± 2.70 | 2.06 ** |
| Assessment Item | Examined Position | Before | After | Difference | Cohen’s w |
|---|---|---|---|---|---|
| RMS (μV) | Biceps brachii | 2.9 ± 1.1 | 3.0 ± 1.1 | 0.2 ± 0.09 | 0.25 |
| Triceps brachii | 6.8 ± 7.7 | 7.0 ± 7.9 | 0.2 ± 0.25 | 0.20 | |
| Flexor digitorum | 0.6 ± 0.5 | 0.6 ± 0.4 | 0.0 ± 0.07 | 0.11 | |
| Extensor digitorum | 0.7 ± 0.8 | 0.7 ± 0.8 | 0.0 ± 0.01 | 0.06 | |
| Abductor pollicis brevis | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.0 ± 0.03 | 0.08 | |
| Quadriceps femoris | 18.5 ± 12.5 | 19.0 ± 12.8 | 0.5 ± 0.68 | 0.35 * | |
| Hamstring muscle | 15.1 ± 8.0 | 15.5 ± 8.2 | 0.4 ± 0.35 | 0.24 | |
| Anterior tibial muscle | 9.0 ± 6.4 | 9.1 ± 6.5 | 0.2 ± 0.14 | 0.14 | |
| Triceps surae | 10.0 ± 6.5 | 10.8 ± 7.6 | 0.9 ± 1.06 | 0.71 ** |
| Assessment Item | Examined Position | Testing Indicator | Before | After | Difference | Cohen’s w |
|---|---|---|---|---|---|---|
| MEP | M1 area on the non-lesion side | Latent period (ms) | 42.1 ± 8.3 | 39.5 ± 8.6 | −2.6 ± 2.17 | 1.11 ** |
| Amplitude (10−5) | 32.7 ± 10.9 | 36.2 ± 9.0 | 3.6 ± 3.78 | 2.41 ** | ||
| M1 area on the lesion side | Latent period (ms) | 14.4 ± 5.7 | 13.8 ± 5.9 | −0.6 ± 0.39 | 0.44 * | |
| Amplitude (10−5) | 20.0 ± 7.7 | 23.9 ± 7.8 | 3.7 ± 3.89 | 2.83 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Cui, Z.; Yu, Y.; Mao, J.; Xu, J.; Ji, L.; Kan, X.; Shen, X.; Li, X.; Zhu, S.; et al. Application of a Brain–Computer Interface System with Visual and Motor Feedback in Limb and Brain Functional Rehabilitation after Stroke: Case Report. Brain Sci. 2022, 12, 1083. https://doi.org/10.3390/brainsci12081083
Gao W, Cui Z, Yu Y, Mao J, Xu J, Ji L, Kan X, Shen X, Li X, Zhu S, et al. Application of a Brain–Computer Interface System with Visual and Motor Feedback in Limb and Brain Functional Rehabilitation after Stroke: Case Report. Brain Sciences. 2022; 12(8):1083. https://doi.org/10.3390/brainsci12081083
Chicago/Turabian StyleGao, Wen, Zhengzhe Cui, Yang Yu, Jing Mao, Jun Xu, Leilei Ji, Xiuli Kan, Xianshan Shen, Xueming Li, Shiqiang Zhu, and et al. 2022. "Application of a Brain–Computer Interface System with Visual and Motor Feedback in Limb and Brain Functional Rehabilitation after Stroke: Case Report" Brain Sciences 12, no. 8: 1083. https://doi.org/10.3390/brainsci12081083
APA StyleGao, W., Cui, Z., Yu, Y., Mao, J., Xu, J., Ji, L., Kan, X., Shen, X., Li, X., Zhu, S., & Hong, Y. (2022). Application of a Brain–Computer Interface System with Visual and Motor Feedback in Limb and Brain Functional Rehabilitation after Stroke: Case Report. Brain Sciences, 12(8), 1083. https://doi.org/10.3390/brainsci12081083