Abstract
Executive function task (EF) deficits are hypothesized to underlie difficulties with self-regulation. However, tasks assessing EF impairments have only been weakly correlated with rating scales that index self-regulation difficulties. A community sample of children and youth aged between 8 and 20 years old were assessed longitudinally. Growth curve analyses and correlations were conducted to better understand how these two types of measures relate to one another across development, as well as the impact of age-related variance. EF was assessed using the Stroop Task and Trail Making test and behavioral ratings of self-regulation were captured using the SWAN scale. EF task performance improved steeply until age 14–15, whereas the SWAN Scale showed small age-related decreases. EF task performance was moderately correlated with age among 8–13-year-olds and to a lesser extent among 14–20-year-olds. SWAN scores were not significantly related to age in either group. Correlations were similar in an ADHD “at-risk” subgroup. EF task performance and parent ratings of attention regulation have different developmental trajectories, which may partly explain why correlations are low to modest in these samples. In particular, age-related variance is an important methodological consideration with significant implications for the assessment of self-regulation in children and youth with ADHD.
1. Introduction
The development of self-regulation is characterized in most models and taxonomies as a process whereby an individual acquires the ability to control behavior volitionally in the service of goals or situational expectations [,]. The capacity for self-regulation develops consistently across childhood, and there is accumulating evidence that it continues to develop at the cognitive [], behavioral [,] and neurobiological levels [,] well into adolescence. Executive function (EF) includes processes such as attention, working memory, planning/organizing and response inhibition, which are neurocognitive processes integral to self-regulation and daily functioning [,]. EF deficits are hypothesized to underlie difficulties with attention and impulsivity, behavior characteristic of neurodevelopmental disorders (NDDs), such as attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) [,,]. Despite EF’s integral role in NDDs and in the development of self-regulation generally, there are weak correlations between EF tasks and rating scales that index these types of difficulties [,,,]. To better understand the overlap and divergence among these two types of measures, the purpose of this study was to examine EF task performance and parent ratings of attention and behavior regulation in a community sample of children and youth who were followed longitudinally on three occasions. Developmental trajectories of these measures and correlations among them were examined to better understand whether EF task performance and parent ratings of attention and behavior regulation capture similar age-related variance. On the other hand, if these two sets of measures show differing developmental trajectories, it may explain the divergence reported in the literature.
1.1. Executive Function Task Performance
EFs represent a number of top-down neurocognitive processes required for goal-directed behavior [,]. These processes are important aspects of cognitive development that predict behavior in everyday life [,]. As these neurocognitive processes develop with age, children become increasingly competent in approaching problems, planning and organizing thoughts and behavior, maintaining goals in mind and acting on them and self-evaluation [,]. The past two decades of research support a model with three correlated but distinct EFs, namely, inhibition, set-shifting and working memory [,,]. Inhibition or inhibitory control refers to the ability to control attention, thought and behavior in the presence of interfering internal or external stimuli, to overcome automatic impulses and respond appropriately so that with increasing inhibitory control, one is able to better restrict and regulate impulsive behaviors [,]. Set-shifting, also known as cognitive flexibility, describes one’s ability to mentally shift from one task to another, utilizing alternative strategies and processing more than one source of information []. Updating and monitoring of working memory representations, or simply updating, is a working memory operation that requires replacing old information with new information relevant to the task at hand [], which is needed to hold out-of-sight information in mind, manipulate it and work with it to achieve goals and meet task demands [,,].
EF skills develop rapidly in the preschool years; however, performance has been reported to continue into late adolescence and peak in early adulthood [,,,,]. The developmental trajectory for the maturation of EF depends on prefrontal cortex engagement, particularly the dorsolateral region, to perform these high-level cognitive processes which are not considered fully developed until early adulthood [,,,]. Both speed and accuracy of inhibitory control continue to mature into adolescence []. Cognitive skills underlying the different facets of EF develop at different times. For example, the ability to delay a response (a skill strongly associated with the successful development of inhibitory control) appears to develop earlier than other EF skills. Set-shifting is the last of the three core EFs to emerge (around 7 to 9 years of age), and is thought to build on inhibition and working memory abilities [].
1.2. Behavioral Ratings of Attention and Impulse Regulation
The ability to regulate one’s behavior, impulse and attention is dependent on numerous underlying cognitive skills, such as EFs, that develop with age. The assessment of self-regulation skills in children has been frequently indexed by informant ratings of children’s functioning relative to peers their age (e.g., parent ratings or teacher ratings). Informant-based scales indexing self-regulation have typically focused on attention, hyperactivity and impulse regulation, including items relating to the observed cognitive, motor and impulse control of the child []. For example, behavior rating scales of this nature have been used to assess self-control longitudinally []. These scales are typically completed by parent or teacher informants, as child self-report is not usually considered reliable for assessing these behaviors [,].
Historically, we have been most interested in capturing deficits in these domains due to their role in developmental psychopathology and most clinical scales have been designed to do so. Nonetheless, attention and behavior regulation are critical aspects of healthy development and as such have been examined in community samples as well. For example, the Strengths and Weaknesses of ADHD Symptoms and Normal Behavior rating scale (SWAN) [], used in this study, allows the assessment of a child’s ability to regulate attention and hyperactivity/impulsivity along the full dimension []. The SWAN also differs from most behavior rating scales of this type as the items are worded using a competency-based rather than a weakness-based formulation as in the DSM-5 [] and has been studied extensively in community samples [,].
Longitudinally, parental ratings of attention, hyperactivity and impulsivity problems in a general population sample demonstrated decreasing hyperactivity with age and relative stability of inattention symptoms from early childhood through to late adolescence []. Developmental effects obtained by parent ratings on the SWAN and another ADHD scale using a non-clinical sample of 528 pairs of same-sex twins aged 6 to 9 and 488 pairs aged 12 to 20 years of age showed a similar effect []. In this cross-sectional study, younger children had more parent-reported problems than the older children on the SWAN for both inattention and hyperactivity–impulsivity subscales [].
1.3. Comparing Executive Task Performance and Parent Ratings of Attention and Behavior Regulation
Two main classes or types of measures have been used to index the development of self-regulation: (a) self- and informant-report questionnaires of behavior observed in real-world settings and (b) performance-based measures, such as executive function tasks [,,]. These classes parallel the performance-based versus rating scale distinction of EF discussed previously in the literature [,,]. Specifically, performance-based measures involve standardized procedures administered by an examiner and usually assess accuracy or response time. Rating measures of self-regulation involve an informant retroactively reporting on the frequency or severity of an individual’s difficulties carrying out everyday tasks and their behaviors related to self-regulation. It is also important to note here that the association between ratings of ADHD severity are significantly correlated with executive function ratings, ranging from r = 0.68 to 0.91 []. While both types of measures assess the aspects of self-regulation, there is accumulating evidence that they are also conceptually and operationally different [,,]. Specifically, performance-based measures of EF capture optimal performance situations because the parameters for task completion are determined externally by the examiner and are not left up to the participant. In contrast, on rating measures, participants estimate the frequency and typicality of how well they perform in day-to-day situations that are likely to engage executive processes. Their responses are not constrained by an external examiner and there are no explicit instructions to maximize or optimize their ratings. Interpretation of the task is left up to the rater, who must decide on instances from their everyday lives that map onto the questions asked.
Age differences, or development, is another important factor to consider when trying to understand why correlations between these sets of tasks are low to modest. We know that EF and attention and behavior regulation are important skills that develop with age. However, we are yet to examine their developmental trajectories simultaneously. Such an examination would help us better understand the rate of development and how that impacts the association between these two sets of measures.
In this study, we examined whether differences in the rate of development of these skills might explain the low to modest association often reported between these measures. We expected that the performance of EF tasks would improve with age, consistent with the research showing an increase in cognitive abilities over development. We expected parent-reported impulse regulation to improve with age, whereas the ratings of attention were not expected to change with age. Finally, small to modest correlations between these measures were expected within the full sample, as seen in previous large sample studies and meta-analyses []. However, we also examined these correlations within different periods of development (8–13 years and 14–20 years) to further examine the effect size of these correlations in these different age groups.
2. Materials and Methods
2.1. Participants
The current study included data from a sample of children recruited from suburban and rural schools as part of a longitudinal research project. Time 1 and Time 2 data from this study are previously reported []. All available data were used. There were 204 children (110 males) at the first measurement occasion (Time 1), with ages ranging from 8 to 14 years old (M = 10.15, SD = 1.73). Follow-up data were collected twice at three-year intervals. Time 2 includes data from 156 participants (86 males), ranging from 10 to 18 years old (M = 13.23, SD = 1.84) and Time 3 data were from 134 participants (77 males) from 13 to 20 years old (M = 15.97, SD = 1.79). The estimated full-scale intelligence score for the sample at the first period of data collection was 108.19 (SD = 12.96), based on the Vocabulary and Matrix Reasoning subtests of the WASI (Wechsler, 1999). At Time 2, parents were asked to report their educational attainment. Of the 156 mothers for whom data were available, 48 (23.5%) had professional degrees, 83 (40.7%) completed college or university, 3 (1.5%) had some college or university education, 15 (7.4%) completed high school, 1 (0.5%) did not complete high school and 4 mothers did not report their educational status. Of the fathers, 42 (20.6%) had professional degrees, 67 (32.8%) completed college or university, 14 (6.9%) had some college or university education, 22 (10.8%) completed high school, 3 (1.5%) did not complete high school and 8 fathers did not report their educational status. At both follow-ups, sample retention was good (Time 2: n = 156, 76% of the total sample; Time 3: n = 135, 66% of the total sample).
2.2. Measures
2.2.1. Attention and Impulse Regulation
The SWAN rating scale [] was used to measure parent ratings of attention and impulse regulation. Parents were asked to rate their child’s behavior relative to same-aged peers for each of the 18 items using a seven-point scale ranging from far below average to far above average. Thus, total scores could range from 18 to 126. The SWAN has been reported to demonstrate good validity and reliability [,,,,]. The dependent variable was domain scores on inattention, hyperactivity, impulsivity and an overall SWAN score (total score). A higher score indicated better attention and behavioral regulation.
2.2.2. Executive Function: Inhibition
The Stroop Task [] was used to measure inhibition. There were three different conditions, each with 24 items arranged in a 4 × 6 matrix: a word reading condition, a color naming condition and an interference condition. The dependent variable of the Stroop Task was the total naming time (in seconds) for the interference condition minus the total naming time for the color condition. Lower scores indicate better inhibition skills.
2.2.3. Executive Function: Set-Shifting
The Trail Making test (TMT) [,] was used to measure set shifting. Part A required participants to connect 25 numbered circles in ascending order. Part B required participants to connect 12 lettered and 13 numbered circles, whereby the participant was instructed to alternate between numeric and alphabetic order, going from 1 to A to 2 to B to 3 to C, and so on. Both parts of the test were administered. Total completion time in seconds was recorded for both parts. To remove the effects of individual differences in processing speed, the set-shifting score was obtained by removing the time taken to complete Part A from Part B. Thus, lower scores are indicative of better set-shifting ability.
2.3. Procedure
Assessments were administered by trained graduate students and bachelor-level research assistants. Measures used in this study were part of a larger set of questionnaires and tasks administered at each time point. Parent consent and child assent were obtained before starting the study. The administration of task order was as follows: demographics form, WASI Vocabulary, WASI Matrices, Stroop and TMT. One parent completed the SWAN questionnaire for each child.
2.4. Data Analysis
The present analyses included data from the two EF tasks and the SWAN scale. There were 13 missing parents’ SWAN ratings at the baseline. Because there was considerable age heterogeneity within each time point, we modeled developmental trajectories of parent-reported attention and impulsivity as a function of age rather than the time point of data collection (i.e., the data are consistent with a cohort-sequential design) []. Specifically, because participant ages ranged from 8 to 20 years across Time 1 to Time 3, a long-term developmental trajectory could be approximated by combining the temporally overlapping repeated measures of youth observed at different ages. Thus, with only three time points of data collection, the age-based data were linked to form a common developmental trajectory spanning ages 8 to 20, albeit with substantial amounts of missing data within a given year of age. In fact, the sparseness of data at some ages necessitated collapsing age into the following six categories to facilitate convergence of model estimation: age 8–9 (age category 1; n = 90 observations; 49 males, 41 females), 10–11 (age category 2; n = 89 observations; 55 males; 34 females), 12–13 (age category 3; n = 112 observations; 57 males, 55 females), 14–15 (age category 4; n = 107 observations; 59 males; 48 females), 16–17 (age category 5; n = 60 observations; 36 males, 24 females) and 18–20 (age category 6; n = 28 observations; 13 males, 15 females).
All models were estimated using full information maximum likelihood; this procedure allows data from participants with incomplete data (including longitudinal dropouts) to be incorporated in the model estimation [], which is essential given that we organized the data according to the age categories described above. All models were estimated using Mplus (version 7.3). Overall, model fit was assessed using the standardized root mean square residual (SRMR) and the root mean square error of approximation (RMSEA), comparative fit index (CFI) and Tucker–Lewis index (TLI) calculated based on the robust chi-square statistic of Yuan and Bentler [], as implemented by Mplus. For RMSEA and SRMR, values < 0.08 are typically considered indicative of adequate model fit, whereas values of CFI and TLI > 0.90 indicate acceptable model fit.
Relationships among variables were examined within two developmental age ranges, rather than by the time of data collection: childhood (ages 8–13; n = 194/182) and adolescence (ages 14–20; n = 143/142). These data were analyzed using IBM SPSS (version 27). If participants had more than one observation within the same age range (n = 146), scores were averaged across the data points. A total of 92 participants had two observations and 5 participants had three observations in the 8–13-year range, and 49 participants had two observations in the 14–20-year range. An “at-risk” subgroup was identified using an overall SWAN score cut-off at or below the 25th percentile (scores equal to or less than 75). A total of 85 participants were categorized as “at risk” for ADHD. The same correlations were carried out in this subgroup.
3. Results
3.1. Descriptive Statistics
Descriptive statistics are presented in Table 1 on each of the raw variable scores. At Time 1, parents reported their children to have well-developed attention and behavior regulation (SWAN total score M = 85.74, SD = 17.0), where the potential range is from 18 to 126. The 13 children with missing SWAN parent reports did not notably differ from the rest of the sample on the four cognitive measures as these 13 children were within the one standard deviation of the mean of the full sample of children who had no missing data.

Table 1.
Descriptive statistics of variables at the start of the study.
3.2. Trajectories of Parent-Reported Attention and Impulse Regulation
The mean of the SWAN total score displayed a linear trend across the six age categories, such that the means increased from ages 8–9 up to ages 18–20. Impulsivity, hyperactivity and inattention subscales exhibited a similar trend. As such, linear growth curve models were fitted to the data. The linear growth curve model for the SWAN total score is shown in Figure 1. The mean slope was significantly greater than 0 (0.81, p = 0.04), while the standard deviation of the slope factor was not significant (2.07, p = 0.45). These results suggest that parent-reported attention and impulse regulation show some improvement as children get older, but there are not substantial individual differences in the amount that the SWAN total score changes across age.
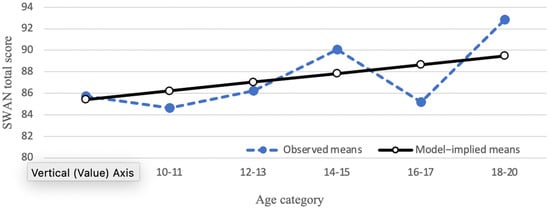
Figure 1.
Developmental trajectory of SWAN total score. Closed circles represent observed means; open circles represent model-implied means.
Linear growth curve models of the SWAN subscales are illustrated in Figure 2. The mean slopes were significantly greater than 0 for the impulsivity (0.16, p = 0.05) and hyperactivity (0.35, p = 0.02) subscales, but not the inattention subscale (0.31, p = 0.17). Similar to the SWAN total score, the standard deviation of the slope factor was not significant for impulsivity (0.53, p = 0.14), hyperactivity (0.17, p = 0.96) or inattention (1.29, p = 0.38) subscales. Thus, impulsivity and hyperactivity showed some improvement with age, whereas inattention did not. Similar to the SWAN total score, the results suggest that there is essentially no intra-individual heterogeneity in the amount that each of the subscale scores change across age.
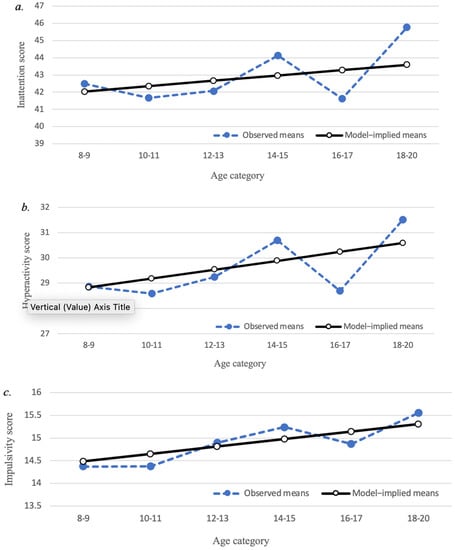
Figure 2.
Developmental trajectories of SWAN subscale scores. (a) Trajectory of inattention scores; (b) Trajectory of hyperactivity scores; (c) Trajectory of impulsivity scores. Closed circles represent observed means; open circles represent model-implied means.
3.3. Trajectories of EF Tasks
Linear growth curve models of EF measures are presented in Figure 3. The mean scores for each of the EF variables displayed a non-linear pattern across the six age categories described above. Specifically, for set-shifting (Trail Making test) and interference control (Stroop Task), mean scores decreased steadily (reflecting improving performance) up to age 14–15, then showed less steep decreases from ages 14–15 to ages 18–20. To represent this non-linear pattern, a piecewise linear latent growth model was estimated because of its interpretational advantages over alternative models for non-linear growth, such as a quadratic growth model []. Specifically, we estimated models with two separate linear segments of time, the first segment representing linear change from ages 8–9 to ages 14–15, and the second representing linear change from ages 14–15 to ages 18–20. Importantly, these models allow the linear slopes to differ across these two time segments, thereby representing the overall non-linear pattern. Furthermore, the models were specified so that the intercept factor represented the level of EF at ages 14–15 rather than the initial timepoint (ages 8–9).
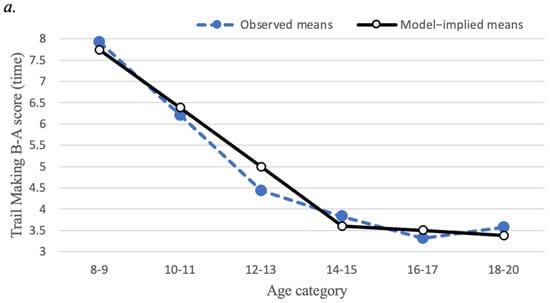
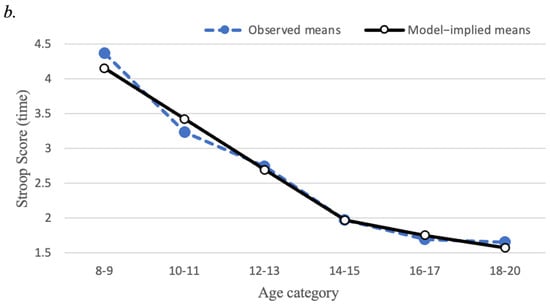
Figure 3.
Mean trajectories for executive function task performance (a) Trajectory of Trail Making Task Part B minus Part A time; (b) Trajectory of Stroop Task interference time. Closed circles represent observed means; open circles represent model-implied means. Score reflects time (in seconds), where less time indicates better performance.
For Trail Making Part B minus Part A time, the first linear slope factor mean of −1.39 (p < 0.001) indicated that Trail Making scores decreased steeply from ages 8–9 to ages 14–15. Next, the second linear slope factor mean of −0.14 indicated a less steep, non-significant (p = 0.38) average decrease from ages 14–15 to ages 18–20. The standard deviations of the first linear slope factor (SD = 1.07, p = 0.03) and second linear slope factor (SD = 1.09, p = 0.04) were both significant, suggesting that there are substantial individual differences in the amount that Trail Making scores change across age.
For the Stroop Task interference time, the growth model converged to a proper solution only after the variance parameters for the two slopes were fixed to zero. The first linear slope factor mean of −0.74 (p < 0.001) indicated that Stroop scores decreased steeply from ages 8–9 to 14–15 years of age. Next, the second linear slope factor mean of −0.21 (p < 0.001) indicated a less steep average decrease in Stroop scores from ages 14–15 to 18–20 years of age. Because the variances of the two linear slopes were fixed to zero, the model suggests that essentially there is no intra-individual heterogeneity in the amount that Stroop scores change across age.
3.4. Correlations
Correlations are reported in Table 2, Table 3 and Table 4. Age displayed a modest correlation with EF task performance in the full sample (Stroop: r = −0.63, p < 0.05; Trail Making: r = −0.55, p < 0.05), shown in Table 2. In the full sample, correlations between SWAN scores and EF tasks were mostly small to moderate (Stroop: r’s from 0.10 to −0.18; Trail Making: r’s from −0.16 to −0.28, p < 0.05); all were statistically significant except the relationship between Stroop and SWAN impulsivity (r = 0.10, ns). Parent rating of attention displayed the highest correlation with the EF tasks, as well as the total SWAN score. Age was not correlated with the SWAN ratings in the full sample (r’s from [0.03] to [0.12], ns).

Table 2.
Correlations between age, EF tasks and parent reports of impulse and attention regulation in the full sample.

Table 3.
Correlations between age, EF tasks and parent reports of impulse and attention regulation among 8–13-year-olds.

Table 4.
Correlations between age, EF tasks and parent reports of impulse and attention regulation among 14–20-year-olds.
Given the steep change in performance of the EF tasks from 8–9 to 12–13 years of age and the far less steep change from 14–15 to 18–20 years of age, correlations between these measures were examined separately in these two different periods of development. In the 8–13-year-old group shown in Table 3, Stroop and Trail Making scores continued to be significantly related to age in the expected direction (r = −0.25 and r = 0.35, p < 0.05), with older children demonstrating better interference and set-shifting than younger children. In the 14–20-year-old group shown in Table 4, the Stroop displayed a smaller significant effect size than with age, compared to the younger age group (r = −0.18, p < 0.05). The correlation with Trail Making being even smaller and not statistically significant (r = −0.16, ns). Consistent with the full sample, age was not correlated with parent-reported attention and impulse regulation. As shown in Table 4, age was not significantly correlated with SWAN ratings in the total sample (r’s from [0.03] to [0.12], ns), nor in childhood (r’s from [0.01] to [0.06], ns) or adolescence (r’s from [<0.01] to [0.03], ns).
Finally, the correlations between the EF tasks and SWAN ratings displayed somewhat different patterns in childhood and adolescence, as shown in Table 3 and Table 4. In childhood (8–13 years old), correlations were also small to moderate (Stroop: r’s from 0.12 to −0.26; Trail Making: r’s from −0.22 to −0.33, p < 0.05) and followed the same pattern as in the full sample whereby all relationships were significant except between Stroop and SWAN impulsivity (r = 0.12, ns). Among adolescents (14–20 years old), correlations between the SWAN scores were smaller and often did not reach statistical significance.
Overall, age was correlated with EF task performance in the 9–13-year-old and full samples, and EF tasks also displayed correlations with the SWAN scale in the 8–13-year-old and full samples However, age displayed a much lower effect size with the EF tasks in the 14–20-year-old sample, consistent with the trajectory analyses. In addition, the effect sizes between the EF tasks and SWAN rating were also very small in the 14–20-year-old sample. This overall pattern suggests that age-related variance likely underlies the correlations between EF tasks and SWAN ratings in the younger group. Given that age-related variance in these EF tasks seems to plateau in the older group, the correlations are much smaller between the EF tasks and SWAN ratings in this group, highlighting the lack of correspondence between EF tasks and the SWAN rating.
These same analyses were conducted separately for the group identified as at risk for ADHD. These correlations are shown in Table 2, Table 3 and Table 4. The findings for the ADHD risk group were parallel to the findings in the full sample, as well as across both age groups. In fact, the effect size correlations between the Trail Making and SWAN ratings were smaller for the full 14–20-year-old group than for the subset at-risk for ADHD, demonstrating this pattern even more clearly than in the full sample.
4. Discussion
We evaluated the extent to which EF task performance and parent rating measures capture age-related variance to better understand the divergence in these measures which have been theoretically and conceptually related. Overall, attention and impulse regulation as rated by parents using the SWAN improved from ages 8–9 to ages 18–20; however, the overall trajectory is quite flat and appears to be driven by the hyperactivity and impulsivity subscales. Parent ratings of inattention showed no statistically significant change with age. The pattern of impulsive and hyperactive behaviors improving with age and attention skills remaining constant has been previously demonstrated in the literature [,,,]. In contrast, both EF task performance measures demonstrate notable improvement with age, particularly from 8–9 to 14–15 years of age relative to 14–15 to 18–20 years of age. This trajectory of rapid age-related improvement across childhood that slows in later adolescence has been well documented [,,].
Based on these results, it is not surprising that age was unrelated to the SWAN total and subscale scores across developmental periods. Conversely, the opposite pattern was seen with age and EF task performance. Age was significantly correlated with the performance of both EF tasks in childhood (8–12-year-olds), and only with the Stroop in adolescents (13–15-year-olds). This is in line with the developmental trajectories of Stroop and Trail Making task performance. Findings were largely consistent in the ADHD risk group.
Regarding associations between these measures, SWAN scores were not consistently significantly correlated with EF performance in the full sample spanning 8 to 20 years of age. This finding aligns with the inconsistent and modest correlations reported between performance-based EF measures and behavioral rating scales in the literature [,,,,]. A review of informant reports and performance-based measures of executive function demonstrated that the median correlation was only 0.19 []. Similar results were found using a latent EF task performance variable, showing low correlations with both the Behavioral Rating Inventory of Executive Function (r = 0.11) and the Early Adolescent Temperament Questionnaire (r = 0.21) [].
Given the steep change in the performance of EF tasks from ages 8–9 to ages 12–13 and the far less steep change from 14–15 to 18–20 years of age, correlations between these measures were then examined separately in these two different periods of development. EF task performance was significantly correlated with the SWAN total score and subscale scores in childhood (8 to 13 years of age), except for impulsivity ratings and performance on the Stroop. In adolescence (14 to 20 years of age), correlations between the SWAN parent ratings and Stroop performance were non-significant. There were significant but small associations between most of the SWAN ratings (all except hyperactivity) and Trail Making performance in this age group. The variability in the size of correlation across developmental periods suggests a fundamental role of age.
One explanation for the small and inconsistent relationship between EF task performance and parent ratings is their differential ability to capture developmental change. We know from prior research that the performance of task-based assessments is highly influenced by age. Moreover, there is evidence that age represents a large portion of the common variance between tasks assessing intellectual abilities and EF tasks in developmental samples. A recent study found that controlling for age eliminated the relationship between EF task performance and intellectual abilities []. These findings demonstrate that age-related variance is an important common feature of task-based cognitive ability measures. On the other hand, parent ratings of attention and impulse regulation are a qualitatively different measure that may not capture age-related variance in any manner.
Methodologically, it is important to consider that for SWAN, parents are asked to rate their child’s behavior relative to other children of the same age. When parents rate how well their child can sustain attention, they may say “above average” when the child is eight, but also indicate the same rating at age ten. The instructions for the SWAN scale do not provide any developmental or age-based reference as part of the assessment. The instructions for this scale are very similar to many scales of this type, as shown in Table 5.

Table 5.
Rating scales of attention regulation and executive functions: Availability of age-based norms and informant instructions.
In the instructions to raters, many of these scales do not provide any developmental reference point (such as: “rate your child compared to other children of the same age”), and instead ask parents to rate the child’s behavior over a recent period of time (e.g., the last six months). Instead of assessing any age-related changes, these scales focus on deficits or difficulties in attentional and self-regulation skills. Thus, even methodologically, rating scales do not capture age-related variance. One possible direction may be to integrate developmental anchors or to explicitly ask the rater to consider age-related differences based on a particular period of time, such as making a current rating relative to an earlier period for each item. However, even if such instructions were provided, this would still be methodologically flawed as individual raters will likely differ in their personal reference points for what is expected at different periods of development. Overall, the same pattern of findings was obtained for what was defined as an ADHD risk group, based on the bottom 25th percentile of this community sample of children. While this cut-off was based on identifying the children who were least well developed in attention and impulse regulation, this may be considered a limitation of the current study. However, SWAN scores have been used to identify children at risk for ADHD based on more elaborate cut-off metrics [].
The SWAN does not have published normative data available and to the best of our knowledge, no research has specifically investigated age effects for this scale. One study comparing the SWAN to another ADHD rating scale notes age-related differences, whereby children in the younger age group (6- to 9-year-olds) were rated as more impaired on the SWAN than children in the older age group (12- to 20-year-olds) []. However, the largest difference in group mean scores on the inattention and hyperactivity/impulsivity subscales was 0.33, which is comparable to the small changes across developmental periods found in the present study. Several scales of this type do not have age-based norms (see Table 5). However, for those scales in Table 5 that do have age-based norms, it is not clear whether these age-based norms necessarily suggest age-related differences in the item ratings of these scales.
The present results suggest parent ratings of behavior and EF task performance do not converge within and across development as we might expect if they were indeed measuring the same construct. The small and variable relationships between these measures raise important questions about how we define and measure behaviors related to attention and self-regulation. Based on current findings and trends in the literature, we are positing that a differential ability to capture age-related variance may explain, in part, the weak correlations obtained between the EF tasks and the SWAN scale in developmental samples. For the SWAN rating scale, parents are effectively asked to control for age in their ratings by explicitly asking them to compare their child’s behavior to other children the same age. Due to this scale property, they may continue to rate their child as “average” or “below average” despite a change in the frequency of behaviors over time. Alternatively, EF task performance is based on objective indicators, such as accuracy and reaction time, which have been shown to be developmentally sensitive with the performance of these tasks improving steeply throughout childhood and leveling off in adolescence. This trajectory looks very different compared to the parent ratings of behavior.
4.1. Considerations for ADHD Assessment
It is important to place these findings into the larger context, including the consideration of theoretical implications and translational applications for the assessment of ADHD. Most explanatory models for ADHD have focused on EF deficits [], which has led to the understanding of EF as critical to developmental improvement in attention and impulse regulation. Structural and functional brain imaging research supports a relationship between ADHD and EF deficits. Findings suggest that specific areas of the brain that are highly related to executive function processes (e.g., frontostriatal and frontoparietal networks) are underactive in those with ADHD []. There is also evidence for delays in cortical maturation [,,] and decreased volume of these regions []. However, similar to the behavioral research, findings from neuroimaging and neurocognitive studies that focus on precise neuropsychological deficits and brain regions involved in ADHD are not always consistent. The magnitude, direction, localization, laterality and clinical significance of the functional and structural abnormalities differ from study to study [,,]. In addition to the neural bases of behavior, there is emerging evidence that psychophysiological processes (i.e., anatomical–functional interplay among central and peripheral nervous systems) may play a role in psychiatric conditions [] which may further explain the lack of consistency in the literature. Indeed, there has been considerable progress and change within the field of ADHD with the accumulation of studies documenting the relationships between these measures and advances in our understanding of the complexity and heterogeneity in the presentation of symptoms among individuals with ADHD [], as well as the issues related to the diagnostic taxonomies we use [].
The focus of the current paper was on measurement issues and understanding the implications for the models and assessment of ADHD. The diagnosis of ADHD has been primarily based on criteria from the DSM-5 or ICD-11, which are conventionally assessed using clinical interviews and rating scales []. The manner in which we operationalize and measure each symptom/criterion has significant implications for the scientific precision of measuring the underlying processes and mechanisms, but also for the individual in whether they do or do not meet criteria for the diagnosis of ADHD. Specifically, our findings suggest that EFs show developmental effects, but the parent ratings of attention/impulse regulation for the SWAN do not. Consistent with other reviews, the current findings suggest that these two types of measures assess different levels of analysis and they should not be considered as equivalent or interchangeable [,,]. This is similar to the case of EF tasks and EF ratings, where the measures should not be interpreted as parallel or interchangeable, despite both carrying the label “EF” [].
The performance of EF tasks provides information regarding how well the individual behaves and manages in an optimal and highly structured testing environment with considerable direction and guidance from an examiner. This is consistent with the distinction that has been made in the psychometric literature between optimal or maximal performance situations and typical performance situations [,,,,,,]. Optimal performance situations include standardized testing situations in which task interpretation is determined by the examiner. Here, the examinee is instructed to maximize performance and often receives feedback to ensure that maximal performance is obtained. The goals and expectations are clearly laid out for the examinee in these testing situations. With age, children demonstrate measurably better performance on these tasks given the growth of cognitive capacities, such as those measured in EF tasks.
Alternatively, typical performance situations are far less constrained and there are no explicit instructions to maximize performance. Often, participants are left to interpret the task and determine for themselves what is required or expected of them. Ratings of EF assess typical performance. In the assessment of child ADHD, it is common for different informants to provide information on how well the child manages in less-structured environments relative to the testing situation, such as a classroom with several other children and in the home setting where there is likely even less structure than in the classroom. These ratings provide an assessment of how well the child executes their goals and manages their behavior without explicit guidance. Both domains are useful and valuable in the assessment of ADHD, but they provide different types of information in the context of a clinical assessment. While this type of performance may also change based on age, such differences are not measured by rating tools.
4.2. Limitations and Future Directions
These findings should be viewed with certain limitations in mind. We only assessed two of the three defining EF processes [] in addition to the SWAN scale; thus, it will be important for future research to replicate the results with other measures. Additionally, our sample was also relatively high functioning, which may impact the variability in the rate of change and our ability to detect different trajectories.
Should researchers and clinicians continue to use behavior ratings and performance-based tasks interchangeably, the lack of age-related variance in behavior ratings must be addressed. One possible solution is to integrate developmental considerations into measures of behavior, such as in the instructions, items or in the rating scale. Task instructions or each item could provide explicit instructions to determine a current rating relative to an earlier period in development. The rating or response scale could also include specific reference to whether the behavior was displayed at the current time relative to an earlier period. However, even if such instructions were provided, this would still be methodologically flawed as individual raters will differ in their personal reference points for what is expected at different periods of development. Thus, individual differences in behavior, but not age-related differences, are assessed on these rating measures.
5. Conclusions
This study included an examination of developmental trajectories of EF task performance and parent ratings of attention and impulse regulation in a community sample. Furthermore, we demonstrated how different methods used for measuring self-regulation do not necessarily converge within and across development, and highlighted the challenges associated with assessing the relationship among performance-based tasks and parent-reported measures. The small growth in the ratings of overall attention and impulse regulation as opposed to more rapid growth seen in EF, at least early in development, demonstrated the differential nature of the developmental trajectories of behavioral and cognitive aspects of self-regulation. Age-related differences in cognitive ability tasks, such as EF tasks, have been consistently demonstrated in the literature. This growth in capacity and efficiency of processing with age is expected, for example, a 10-year-old will likely have better inhibitory control and be more accurate in solving complex abstract puzzles than a six-year-old. However, in the case of behavior rating scales, the scores and ratings cannot be expected to track age-related changes. One might expect that there may be some age-related differences in children’s behavior, but methodologically, there is no reason to expect that rating scales will capture any age-related differences. Understanding these different indicators of self-regulation can inform the development of early prevention and targeted treatment strategies. For example, informing educators on what to expect within the classroom and parents on what to expect from their child’s development of regulation over time, especially for at-risk and ADHD populations.
Author Contributions
Conceptualization, R.E.L. and M.E.T.; methodology, R.E.L., J.R., D.B.F. and M.E.T.; formal analysis, R.E.L. and D.B.F.; writing—original draft preparation, R.E.L. and M.E.T.; writing—review and editing, R.E.L., J.R., D.B.F., R.M., B.F.A. and M.E.T.; visualization, R.E.L. and D.B.F.; supervision, M.E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Social Sciences and Humanities Research Council (SSHRC; Grant File Numbers 410-2006-2330 and 435-2013-1359).
Institutional Review Board Statement
The study was approved by the Institutional Review Board of York University (certificate # 2018-181; initial approval 7 November 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We do not have consent from participants to make data publicly available or to post data on an online repository.
Acknowledgments
The authors wish to thank all of the research assistants and trainees who helped with data collection and administration of this study: Mohamed Al-Haj, Armita Hosseini, Jill Shuster and Geoff Sorge.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nigg, J.T. Annual research review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 2017, 58, 361–383. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008, 28, 78–106. [Google Scholar] [CrossRef] [Green Version]
- Monahan, K.C.; Steinberg, L.; Cauffman, E.; Mulvey, E.P. Trajectories of antisocial behavior and psychosocial maturity from adolescence to young adulthood. Dev. Psychol. 2009, 45, 1654–1668. [Google Scholar] [CrossRef] [Green Version]
- Dahl, R.E. Adolescent brain development: A period of vulnerabilities and opportunities. Ann. N. Y. Acad. Sci. 2004, 1021, 1–22. [Google Scholar] [CrossRef]
- Luna, B.; Garver, K.E.; Urban, T.A.; Lazar, N.A.; Sweeney, J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004, 75, 1357–1372. [Google Scholar] [CrossRef]
- Carlson, S.; Zelazo, P.; Faja, S. Executive functions. In The Oxford Handbook of Developmental Psychology, Vol. 1: Body and Mind; Zelazo, P.D., Ed.; Oxford University Press: Oxford, UK, 2012; Volume 1, pp. 706–743. [Google Scholar]
- Clark, C.; Prior, M.; Kinsella, G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J. Child Psychol. Psychiatry 2002, 43, 785–796. [Google Scholar] [CrossRef]
- Craig, F.; Margari, F.; Legrottaglie, A.R.; Palumbi, R.; de Giambattista, C.; Margari, L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2016, 12, 1191–1202. [Google Scholar] [CrossRef] [Green Version]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Halse, M.; Steinsbekk, S.; Hammar, Å.; Wichstrøm, L. Longitudinal relations between impaired executive function and symptoms of psychiatric disorders in childhood. J. Child Psychol. Psychiatry 2022. [Google Scholar] [CrossRef]
- McAuley, T.; Chen, S.; Goos, L.; Schachar, R.; Crosbie, J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J. Int. Neuropsychol. Soc. JINS 2010, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.E.; West, R.F.; Stanovich, K.E. Practitioner review: Do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry 2013, 54, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Gustavson, D.E. Do rating and task measures of control abilities assess the same thing? Curr. Dir. Psychol. Sci. 2022, 31, 262–271. [Google Scholar] [CrossRef]
- Snyder, H.R.; Friedman, N.P.; Hankin, B.L. Associations between task performance and self-report measures of cognitive control: Shared versus distinct abilities. Assessment 2021, 28, 1080–1096. [Google Scholar] [CrossRef]
- Benedek, M.; Jauk, E.; Sommer, M.; Arendasy, M.; Neubauer, A.C. Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence 2014, 46, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Best, J.R.; Miller, P.H.; Jones, L.L. Executive functions after age 5: Changes and correlates. Dev. Rev. 2009, 29, 180–200. [Google Scholar] [CrossRef] [Green Version]
- Jurado, M.B.; Rosselli, M. The elusive nature of executive functions: A review of our current understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Craik, F.I.; Booth, L. Executive function across the life span. Acta Psychol. 2004, 115, 167–183. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Lehto, J.E.; Juujarvi, P.; Kooistra, L.; Pulkkinen, L. Dimensions of executive functioning: Evidence from children. Br. J. Dev. Psychol. 2003, 21, 59–80. [Google Scholar] [CrossRef]
- Baggetta, P.; Alexander, P.A. Conceptualization and operationalization of executive function. Mind Brain Educ. 2016, 10, 10–33. [Google Scholar] [CrossRef]
- Jewsbury, P.A.; Bowden, S.C.; Strauss, M.E. Integrating the switching, inhibition, and updating model of executive function with the Cattell-Horn-Carroll model. J. Exp. Psychol. Gen. 2016, 145, 220–245. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G.J. Developments in the concept of working memory. Neuropsychology 1994, 8, 485–493. [Google Scholar] [CrossRef]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Engle, R.W.; Tuholski, S.W.; Laughlin, J.E.; Conway, A.R.A. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. J. Exp. Psychol. Gen. 1999, 128, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Muller, U.; Frye, D.; Marcovitch, S.; Argitis, G.; Boseovski, J.; Chiang, J.K.; Hongwanishkul, D.; Schuster, B.V.; Sutherland, A. The development of executive function in early childhood. Monogr. Soc. Res. Child Dev. 2003, 68, vii-137. [Google Scholar] [CrossRef]
- Best, J.R.; Miller, P.H. A developmental perspective on executive function. Child Dev. 2010, 81, 1641–1660. [Google Scholar] [CrossRef] [Green Version]
- Carlson, S.M. Developmentally sensitive measures of executive function in preschool children. Dev. Neuropsychol. 2005, 28, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Bunge, S.A.; Zelazo, P.D. A brain-based account of the development of rule use in childhood. Curr. Dir. Psychol. Sci. 2006, 15, 118–121. [Google Scholar] [CrossRef]
- Diamond, A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In Principles of Frontal Lobe Function; Oxford University Press: New York, NY, USA, 2002; pp. 466–503. [Google Scholar]
- Durston, S.; Davidson, M.C.; Tottenham, N.; Galvan, A.; Spicer, J.; Fossella, J.A.; Casey, B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006, 9, 1–8. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Hiraki, K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. USA 2009, 106, 6017–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achenbach, T.M.; Rescorla, L. Aseba School-Age Forms & Profiles; Aseba Burlington, Vt: Burlington, VT, USA, 2001. [Google Scholar]
- Moffitt, T.E.; Arseneault, L.; Belsky, D.; Dickson, N.; Hancox, R.J.; Harrington, H.; Houts, R.; Poulton, R.; Roberts, B.W.; Ross, S.; et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. USA 2011, 108, 2693–2698. [Google Scholar] [CrossRef] [Green Version]
- Pelham, W.E., Jr.; Fabiano, G.A.; Massetti, G.M. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J. Clin. Child Adolesc. Psychol. 2005, 34, 449–476. [Google Scholar] [CrossRef]
- Smith, B.H.; Pelham, W.E.; Gnagy, E.; Molina, B.; Evans, S. The reliability, validity, and unique contributions of self-report by adolescents receiving treatment for attention-deficit/hyperactivity disorder. J. Consult. Clin. Psychol. 2000, 68, 489–499. [Google Scholar] [CrossRef]
- Swanson, J.M.; Schuck, S.; Porter, M.M.; Carlson, C.; Hartman, C.A.; Sergeant, J.A.; Clevenger, W.; Wasdell, M.; McCleary, R.; Lakes, K.; et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: History of the SNAP and the SWAN rating scales. Int. J. Educ. Psychol. Assess. 2012, 10, 51–70. [Google Scholar] [PubMed]
- Arnett, A.B.; Pennington, B.F.; Friend, A.; Willcutt, E.G.; Byrne, B.; Samuelsson, S.; Olson, R.K. The SWAN captures variance at the negative and positive ends of the ADHD symptom dimension. J. Atten. Disord. 2013, 17, 152–162. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-V); American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Lakes, K.D.; Swanson, J.M.; Riggs, M. The reliability and validity of the English and Spanish Strengths and Weaknesses of ADHD and Normal behavior rating scales in a preschool sample: Continuum measures of hyperactivity and inattention. J. Atten. Disord. 2012, 16, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, J.R.; Cuffe, S.P.; Cai, B.; Visser, S.N.; Forthofer, M.S.; Bottai, M.; Ortaglia, A.; McKeown, R.E. Persistence of parent-reported ADHD symptoms from childhood through adolescence in a community sample. J. Atten. Disord. 2016, 20, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, D.A.; Bennett, K.S.; Levy, F.; Sergeant, J.; Swanson, J. A twin study of attention-deficit/hyperactivity disorder dimensions rated by the strengths and weaknesses of ADHD-symptoms and normal-behavior (SWAN) scale. Biol. Psychiatry 2007, 61, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Mischel, W.; Shoda, Y.; Rodriguez, M.I. Delay of gratification in children. Science 1989, 244, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Kochanska, G.; Coy, K.C.; Murray, K.T. The development of self-regulation in the first four years of life. Child Dev. 2001, 72, 1091–1111. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.L.; Kern, M.L. A meta-analysis of the convergent validity of self-control measures. J. Res. Personal. 2011, 45, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Barkley, R.A.; Murphy, K.R. Impairment in occupational functioning and adult ADHD: The predictive utility of executive function (EF) ratings versus EF tests. Arch. Clin. Neuropsychol. 2010, 25, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.L.; Smyth, H.L.; Bissett, P.G.; Canning, J.R.; Eisenberg, I.W.; Enkavi, A.Z.; Gonzalez, O.; Kim, S.J.; Metcalf, S.A.; Muniz, F.; et al. Correlation database of 60 cross-disciplinary surveys and cognitive tasks assessing self-regulation. J. Personal. Assess. 2020, 103, 238–245. [Google Scholar] [CrossRef]
- Toplak, M.E.; West, R.F.; Stanovich, K.E. Rational thinking and cognitive sophistication: Development, cognitive abilities, and thinking dispositions. Dev. Psychol. 2014, 50, 1037–1048. [Google Scholar] [CrossRef] [Green Version]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Reitan, R.M. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955, 19, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Nesselroade, J.R.; Baltes, P.B. History and rationale of longitudinal research. In Longitudinal Research in the Study of Behavior and Development; Nesselroade, J.R., Baltes, P.B., Eds.; Academic Press: New York, NY, USA, 1979; pp. 1–39. [Google Scholar]
- Bollen, K.A.; Curran, P.J. Latent Curve Models: A Structural Equation Approach; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yuan, K.-H.; Bentler, P.M. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol. Methodol. 2000, 30, 165–200. [Google Scholar] [CrossRef]
- Flora, D.B. Specifying piecewise latent trajectory models for longitudinal data. Struct. Equ. Modeling-A Multidiscip. J. 2008, 15, 513–533. [Google Scholar] [CrossRef]
- Vergunst, F.; Tremblay, R.E.; Galera, C.; Nagin, D.; Vitaro, F.; Boivin, M.; Côté, S.M. Multi-rater developmental trajectories of hyperactivity–impulsivity and inattention symptoms from 1.5 to 17 years: A population-based birth cohort study. Eur. Child Adolesc. Psychiatry 2019, 28, 973–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biederman, J.; Mick, E.; Faraone, S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am. J. Psychiatry 2000, 157, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Dopfner, M.; Hautmann, C.; Gortz-Dorten, A.; Klasen, F.; Ravens-Sieberer, U.; The BELLA Study Group. Long-term course of ADHD symptoms from childhood to early adulthood in a community sample. Eur. Child Adolesc. Psychiatry 2015, 24, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pingault, J.-B.; Viding, E.; Galéra, C.; Greven, C.U.; Zheng, Y.; Plomin, R.; Rijsdijk, F. Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry 2015, 72, 651–658. [Google Scholar] [CrossRef]
- Eisenberg, I.W.; Bissett, P.G.; Zeynep Enkavi, A.; Li, J.; MacKinnon, D.P.; Marsch, L.A.; Poldrack, R.A. Uncovering the structure of self-regulation through data-driven ontology discovery. Nat. Commun. 2019, 10, 2319. [Google Scholar] [CrossRef] [Green Version]
- Rizeq, J.; Flora, D.B.; Toplak, M.E. Changing relations among cognitive abilities across development: Implications for measurement and research. Clin. Neuropsychol. 2017, 31, 1353–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conners, C.K. Conners 3rd Edition: Manual; Multi-Health Systems: North Tonawanda, NY, USA, 2008. [Google Scholar]
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale-5 for Children and Adolescents: Checklists, Norms, and Clinical Interpretation; The Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- NICHQ Vanderbilt Assessment Scales, 3rd ed.; American Academy of Pediatrics: Itasca, IL, USA, 2019.
- Swanson, J. SNAP-IV Scale; University of California Child Development Center: Irvine, CA, USA, 1995. [Google Scholar]
- Brown, T.E. Brown Attention-Deficit Disorder Scales (ADDScales): For Children and Adolescent: Manual; Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Form & Profiles; University of Vermont, Research Centre for Children, Youth & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Reynolds, C.R.; Kamphaus, R.W. BASC-3: Behaviour Assessment System for Children, 3rd ed.; NCS Pearson, Inc.: Bloomington, MN, USA, 2015. [Google Scholar]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Bracken, B.A.; Boatwright, B.S. Clinical Assessment of Attention Deficit (Manual); PAR Press: Lutz, FL, USA, 2005. [Google Scholar]
- Gioia, G.; Isquith, P.K.; Guy, S.C.; Kenworth, L. Behavior Rating Inventory of Executive Function (BRIEF), 2nd ed.; PAR Inc.: Lutz, FL, USA, 2015. [Google Scholar]
- Thorell, L.B.; Nyberg, L. The Childhood Executive Functioning Inventory (CHEXI): A new rating instrument for parents and teachers. Dev. Neuropsychol. 2008, 33, 536–552. [Google Scholar]
- Thorell, L.B.; Lazarević, N.; Milovanović, I.; Bugarski Ignjatović, V. Psychometric properties of the Teenage Executive Functioning Inventory (TEXI): A freely available questionnaire for assessing deficits in working memory and inhibition among adolescents. Child Neuropsychol. 2020, 26, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Barkley, R.A. Barkley Deficits in Executive Functioning Scale—Children and Adolescents (BDEFS-CA); Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Delis, D. Delis Rating of Executive Function (D-REF); Pearson: Bloominton, MN, USA, 2012. [Google Scholar]
- Burton, C.L.; Wright, L.; Shan, J.; Xiao, B.; Dupuis, A.; Goodale, T.; Shaheen, S.M.; Corfield, E.C.; Arnold, P.D.; Schachar, R.J.; et al. SWAN scale for ADHD trait-based genetic research: A validity and polygenic risk study. J. Child Psychol. Psychiatry 2019, 60, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Biederman, J.; Valera, E.M.; Bush, G.; Kaiser, J.; Kennedy, D.N.; Caviness, V.S.; Faraone, S.V.; Seidman, L.J. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb. Cortex 2007, 17, 1364–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.; Gilliam, M.; Liverpool, M.; Weddle, C.; Malek, M.; Sharp, W.; Greenstein, D.; Evans, A.; Rapoport, J.; Giedd, J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: Support for a dimensional view of attention deficit hyperactivity disorder. Am. J. Psychiatry 2011, 168, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nigg, J.T. What Causes ADHD?: Understanding What Goes Wrong and Why; Guilford Publications: New York, NY, USA, 2009; p. 422. [Google Scholar]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Rosch, K.S.; Crocetti, D.; Hirabayashi, K.; Denckla, M.B.; Mostofsky, S.H.; Mahone, E.M. Reduced subcortical volumes among preschool-age girls and boys with ADHD. Psychiatry Res. Neuroimaging 2018, 271, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Silk, T.J.; Genc, S.; Anderson, V.; Efron, D.; Hazell, P.; Nicholson, J.M.; Kean, M.; Malpas, C.B.; Sciberras, E. Developmental brain trajectories in children with ADHD and controls: A longitudinal neuroimaging study. BMC Psychiatry 2016, 16, 59. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef]
- Stein, D.J.; Szatmari, P.; Gaebel, W.; Berk, M.; Vieta, E.; Maj, M.; de Vries, Y.A.; Roest, A.M.; de Jonge, P.; Maercker, A.; et al. Mental, behavioral and neurodevelopmental disorders in the ICD-11: An international perspective on key changes and controversies. BMC Med. 2020, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, P.L. Intelligence, attention, and learning: Maximal and typical performance. Curr. Top. Hum. Intell. 1994, 4, 1–27. [Google Scholar]
- Ackerman, P.L. A theory of adult intellectual development: Process, personality, interests, and knowledge. Intelligence 1996, 22, 227–257. [Google Scholar] [CrossRef]
- Ackerman, P.L.; Kanfer, R. Cognitive, affective, and conative aspects of adult intellect within a typical and maximal performance framework. In Motivation, Emotion, and Cognition; Routledge: London, UK, 2004; pp. 133–156. [Google Scholar]
- Ackerman, P.L.; Heggestad, E.D. Intelligence, personality, and interests: Evidence for overlapping traits. Psychol. Bull. 1997, 121, 219. [Google Scholar] [CrossRef]
- Cronbach, L.J. Essentials of Psychological Testing; Harper: New York, NY, USA, 1949. [Google Scholar]
- Matthews, G.; Zeidner, M.; Roberts, R.D. Emotional Intelligence: Science and Myth; MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Stanovich, K.E. Distinguishing the reflective, algorithmic, and autonomous minds: Is it time for a tri-process theory? In Two Minds: Dual Processes and Beyond; Evans, J., Frankish, K., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 55–88. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).