Higher Plasma Fibrinogen Level at Admission Is Associated with Post-Stroke Depression at Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Measurement
2.3. Measurement of Systemic Low-Grade Inflammation Markers
2.4. Psychological Measurement
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Predictive of the Occurrence of Depression at Discharge
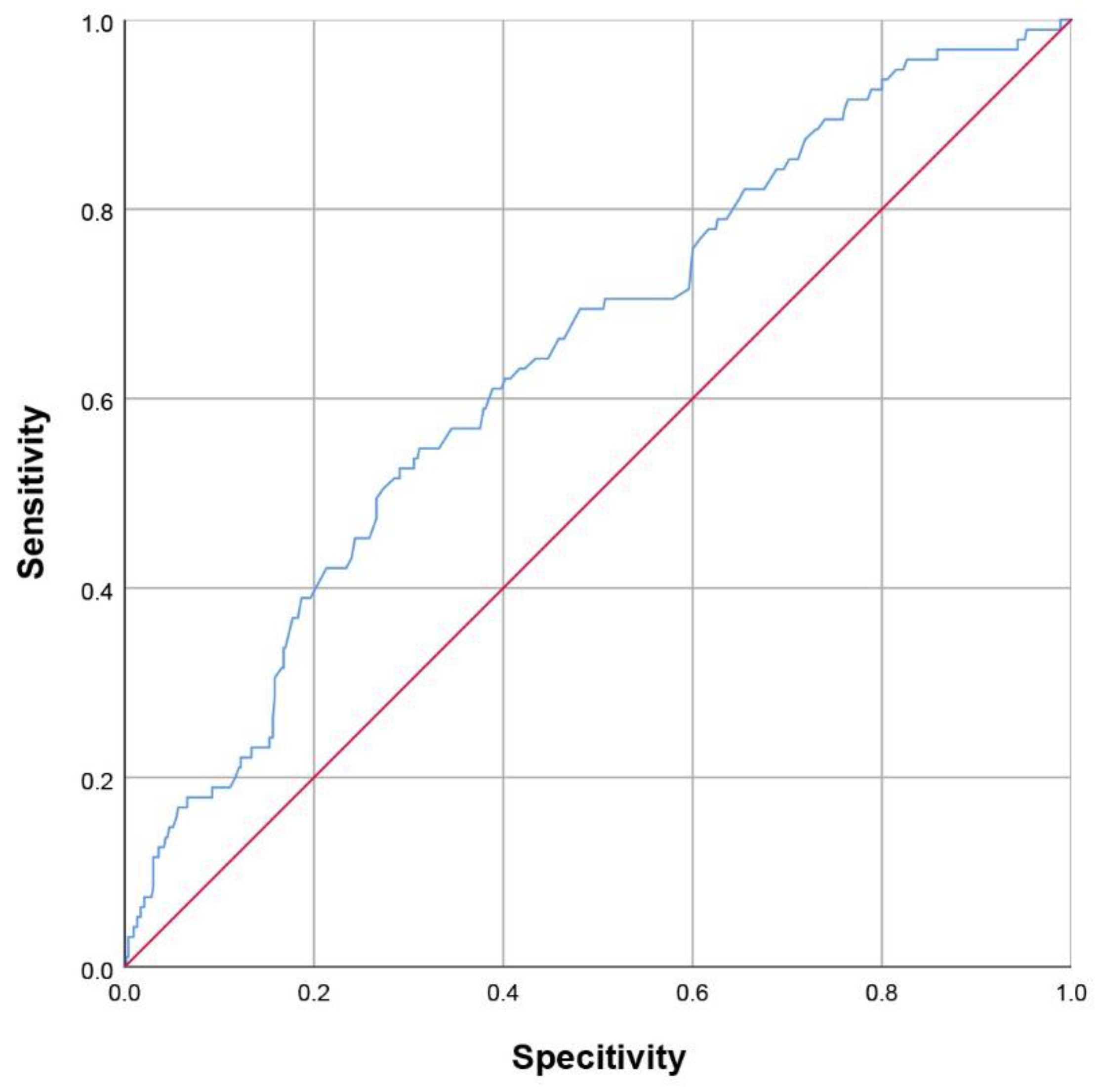
3.3. Establishment of a Nomogram for PSD at Discharge
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Ethics Statements
Abbreviations
| PSD | Post-stroke depression |
| NIHSS | National Institutes of Health Stroke Scale |
| CI | confidence interval |
| TOAST | Trial of Org 10,172 in acute stroke treatment |
| LAA | large artery atherosclerosis |
| CE | cardioembolic |
| SAO | small artery occlusion |
| OC | other causes |
| SUD | stroke of undetermined causes |
| NIHSS | National Institutes of Health Stroke Scale |
| CRP | C-reactive protein |
| WBC | white blood cell |
| PLT | platelet |
| NRL | neutrophil-to-lymphocyte ratio |
| dNRL | derived neutrophil-to-lymphocyte ratio |
| MLR | monocyte-to-lymphocyte ratio |
| SII | systemic immune–inflammation index |
| PRL | platelet-to-lymphocyte ratio |
References
- Ayerbe, L.; Ayis, S.; Crichton, S.; Wolfe, C.D.A.; Rudd, A.G. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J. Neurol. Neurosurg. Psychiatry 2013, 85, 514–521. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Wolfe, C.; Rudd, A.G. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 14–21. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of Depression after Stroke: An Updated Systematic Review and Meta-Analysis of Observational Studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Villa, R.F.; Ferrari, F.; Moretti, A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol. Ther. 2017, 184, 131–144. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Ferrari, F.; Villa, R.F. The Neurobiology of Depression: An Integrated Overview from Biological Theories to Clinical Evidence. Mol. Neurobiol. 2016, 54, 4847–4865. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Kim, J.-T.; Park, M.-S.; Cho, K.-H.; Kim, J.-M. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology 2016, 72, 156–160. [Google Scholar] [CrossRef]
- Pietzner, M.; Kaul, A.; Henning, A.-K.; Kastenmüller, G.; Artati, A.; Lerch, M.M.; Adamski, J.; Nauck, M.; Friedrich, N. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Med. 2017, 15, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, P.; Kaushal, A.; Poole, L.; Lawes, S.; Chalder, T.; Cadar, D. Systemic low-grade inflammation and subsequent depressive symptoms: Is there a mediating role of physical activity? Brain Behav. Immun. 2019, 80, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Bossù, P.; Ciaramella, A.; Bria, P.; Caltagirone, C.; Robinson, R.G. The etiology of poststroke depression: A review of the literature and a new hypothesis involving inflammatory cytokines. Mol. Psychiatry 2006, 11, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Miao, J.; Song, Y.; Wang, Y.; Pan, C.; Li, G.; Zhao, X.; Lan, Y.; Qiu, X.; Zhu, S.; et al. Systemic low-grade inflammation and depressive symptomology at chronic phase of ischemic stroke: The chain mediating role of fibrinogen and neutrophil counts. Brain Behav. Immun. 2021, 100, 332–341. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Fan, K.; Ren, W.; Wang, Q.; Ruan, Y.; Yuan, C.; Huang, G.; He, J. The Association between Systemic Inflammatory Markers and Post-Stroke Depression: A Prospective Stroke Cohort. Clin. Interv. Aging 2021, 16, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, W.; Zhou, Z.; Han, J.; Dong, W. Elevated neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict post-stroke depression with acute ischemic stroke. Exp. Ther. Med. 2020, 19, 2497–2504. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhu, L.; Zhang, B.; Gao, J.; Zhao, T.; Fang, S. Higher levels of C-reactive protein in the acute phase of stroke indicate an increased risk for post-stroke depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 134, 104309. [Google Scholar] [CrossRef]
- Arsava, E.M.; Helenius, J.; Avery, R.; Sorgun, M.H.; Kim, G.-M.; Pontes-Neto, O.M.; Park, K.Y.; Rosand, J.; Vangel, M.; Ay, H. Assessment of the Predictive Validity of Etiologic Stroke Classification. JAMA Neurol. 2017, 74, 419–426. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Alba, A.C.; Agoritsas, T.; Walsh, M.; Hanna, S.; Iorio, A.; Devereaux, P.J.; McGinn, T.; Guyatt, G. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA J. Am. Med. Assoc. 2017, 318, 1377–1384. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Crichton, S.; Wolfe, C.D.; Rudd, A.G. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013, 44, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Miao, J.; Lan, Y.; Sun, W.; Chen, Y.; Cao, Z.; Li, G.; Zhao, X.; Zhu, Z.; Zhu, S. Association of Cerebral Artery Stenosis with Post-stroke Depression at Discharge and 3 Months after Ischemic Stroke Onset. Front. Psychiatry 2020, 11, 585201. [Google Scholar] [CrossRef]
- Storor, D.L.; Byrne, G.J.A. Pre-morbid personality and depression following stroke. Int. Psychogeriatr. 2006, 18, 457–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labonté, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.-H.E.; Cahill, M.; et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017, 23, 1102–1111. [Google Scholar] [CrossRef]
- Brivio, E.; Lopez, J.P.; Chen, A. Sex differences: Transcriptional signatures of stress exposure in male and female brains. Genes Brain Behav. 2019, 19, e12643. [Google Scholar] [CrossRef]
- Mayman, N.A.; Tuhrim, S.; Jette, N.; Dhamoon, M.S.; Stein, L.K. Sex Differences in Post-Stroke Depression in the Elderly. J. Stroke Cerebrovasc. Dis. 2021, 30, 105948. [Google Scholar] [CrossRef]
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Zeng, Y.; Wu, W. Risk Factors for Post-stroke Depression: A Meta-analysis. Front. Aging Neurosci. 2017, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Panova-Noeva, M.; Schulz, A.; Arnold, N.; Hermanns, M.I.; Prochaska, J.H.; Laubert-Reh, D.; Spronk, H.M.; Blettner, M.; Beutel, M.; Pfeiffer, N.; et al. Coagulation and inflammation in long-term cancer survivors: Results from the adult population. J. Thromb. Haemost. 2018, 16, 699–708. [Google Scholar] [CrossRef]
- Shenhar-Tsarfaty, S.; Assayag, E.B.; Bova, I.; Shopin, L.; Cohen, M.; Berliner, S.; Shapira, I.; Bornstein, N.M. Persistent hyperfibrinogenemia in acute ischemic stroke/transient ischemic attack (TIA). Thromb. Haemost. 2008, 99, 169–173. [Google Scholar]
- Peycheva, M.; Deneva, T.; Zahariev, Z. The role of fibrinogen in acute ischaemic stroke. Neurol. Neurochir. Polska 2021, 55, 74–80. [Google Scholar] [CrossRef]
- Ruan, Y.; Yuan, C.; Liu, Y.; Zeng, Y.; Cheng, H.; Cheng, Q.; Chen, Y.; Huang, G.; He, W.; He, J. High fibrinogen-to-albumin ratio is associated with hemorrhagic transformation in acute ischemic stroke patients. Brain Behav. 2021, 11, e01855. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, J.M.; Lee, S.E.; Kang, D.R.; Ovbiagele, B.; Demchuk, A.M.; Lee, J.S. Association of fibrinogen level with early neurological deterioration among acute ischemic stroke patients with diabetes. BMC Neurol. 2017, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xing, C.; Li, Y.; Zhu, X. Elevated plasma fibrinogen indicates short-term poor outcome in patients with acute ischemic stroke after intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 104991. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Zhao, K.; He, W.; Lin, S.; He, J. High levels of plasma fibrinogen are related to post-stroke cognitive impairment. Brain Behav. 2019, 9, e01391. [Google Scholar] [CrossRef]
- Luan, X.; Cheng, H.; Chen, Y.; Cheng, L.; Zhou, S.; Song, J.; Lin, G.; Qiu, H.; He, J. High levels of plasma fibrinogen and prothrombin time are related to post-stroke emotional impairment. Brain Res. 2020, 1748, 147017. [Google Scholar] [CrossRef] [PubMed]
- Liegey, J.; Sagnier, S.; Debruxelles, S.; Poli, M.; Olindo, S.; Renou, P.; Rouanet, F.; Moal, B.; Tourdias, T.; Sibon, I. Influence of inflammatory status in the acute phase of stroke on post-stroke depression. Rev. Neurol. 2021, 177, 941–946. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Lan, Y.; Miao, J.; Pan, C.; Sun, W.; Li, G.; Wang, Y.; Zhao, X.; Zhu, Z.; et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry 2022, 22, 162. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Kowalska, K.; Pasinska, P.; Klimiec-Moskal, E.; Pera, J.; Slowik, A.; Klimkowicz-Mrowiec, A.; Dziedzic, T. C-reactive protein and post-stroke depressive symptoms. Sci. Rep. 2020, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.; Rand, K.L.; Muldoon, M.F.; Kamarck, T.W. A prospective evaluation of the directionality of the depression–inflammation relationship. Brain Behav. Immun. 2009, 23, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Rajanikant, G.K. Post stroke depression: The sequelae of cerebral stroke. Neurosci. Biobehav. Rev. 2018, 90, 104–114. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Sun, W.; Liu, X. The advances of post-stroke depression: 2021 update. J. Neurol. 2021, 269, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, T.; Sales, C. Understanding Why Post-Stroke Depression May Be the Norm Rather than the Exception: The Anatomical and Neuroinflammatory Correlates of Post-Stroke Depression. J. Clin. Med. 2021, 10, 1674. [Google Scholar] [CrossRef]
- Wen, H.; Weymann, K.B.; Wood, L.; Wang, Q.M. Inflammatory Signaling in Post-Stroke Fatigue and Depression. Eur. Neurol. 2018, 80, 138–148. [Google Scholar] [CrossRef] [PubMed]



| Total (n = 625) | Without PSD (n = 530) | With PSD (n = 95) | p Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, year, median (IQR) | 65 (57–72) | 65 (56–72) | 65 (61–70) | 0.647 |
| Female sex, n (%) | 219 (35.04%) | 172 (32.45%) | 47 (49.47%) | 0.002 |
| Marriage, n (%) | 614 (98.55%) | 523 (98.68%) | 91 (95.79%) | 0.141 |
| Education level | 0.632 | |||
| No education or primary school, n (%) | 280 (44.80%) | 234 (44.15%) | 46 (48.42%) | |
| Middle school, n (%) | 276 (44.16%) | 237 (44.72%) | 39 (41.04%) | |
| College and above, n (%) | 69 (11.04%) | 59 (11.13%) | 10 (10.53%) | |
| Vascular risk factors | ||||
| Smoking, n (%) | 175 (28.00%) | 156 (29.44%) | 19 (20.00%) | 0.063 |
| Drinking, n (%) | 134 (21.44%) | 119 (22.45%) | 15 (15.79%) | 0.174 |
| Hypertension, n (%) | 452 (72.32%) | 382 72.07%) | 70 (73.68%) | 0.804 |
| Diabetes mellitus, n (%) | 186 (29.76%) | 157 (29.63%) | 29 (30.53%) | 0.903 |
| Dyslipidemia, n (%) | 20 (3.20%) | 18 (3.40%) | 2 (2.10%) | 0.753 |
| Atrial fibrillation, n (%) | 49 (7.84%) | 44 (8.30%) | 5 (5.26%) | 0.408 |
| Previous stroke, n (%) | 129 (20.64%) | 105 (19.81%) | 24 (25.26%) | 0.270 |
| Coronary heart diseases, n (%) | 18 (2.88%) | 15 (2.83%) | 3 (3.15%) | 0.745 |
| Neurological and neuropsychological evaluation | ||||
| NIHSS on admission, median (IQR) | 2 (1–5) | 2 (1–4) | 4 (2–9) | <0.001 |
| TOAST subtype | 0.494 | |||
| LAA | 339 (54.24%) | 280 (52.83%) | 59 (62.10%) | |
| CE | 47 (7.52%) | 40 (7.55%) | 7 (7.37%) | |
| SAO | 197 (31.52%) | 173 (32.64%) | 24 (25.26%) | |
| OC | 28 (4.48%) | 24 (4.53%) | 4 (4.21%) | |
| SUD | 14 2.24%) | 13 (2.45%) | 1 (1.05%) | |
| Laboratory tests | ||||
| WBC (/109/L), median (IQR) | 6.98 (5.69–8.48) | 6.90 (5.67–8.44) | 7.26 (5.79–8.68) | 0.143 |
| Lymphcyte (/109/L), median (IQR) | 1.64 (1.25–2.08) | 1.65 (1.29–2.09) | 1.57 (1.18–1.99) | 0.384 |
| Neutrophil (/109/L), median (IQR) | 4.47 (3.47–5.87) | 4.40 (3.43–5.87) | 4.70 (3.77–5.78) | 0.169 |
| PLT (/109/L), median (IQR) | 203 (166–240) | 200 (166–240) | 208 (164–261) | 0.168 |
| Fibrinogen (g/L), median (IQR) | 2.70 (2.27–3.37) | 2.64 (2.23–3.28) | 3.21 (2.47–3.67) | <0.001 |
| CRP (mg/L), median (IQR) | 2.36 (0.97–6.17) | 2.24 (0.96–5.66) | 3.69 (1.11–9.03) | 0.012 |
| NRL (%), median (IQR) | 2.64 (1.93–3.78) | 2.61 (1.92–3.69) | 3.02 (2.03–4.43) | 0.095 |
| dNRL (%), median (IQR) | 1.88 (1.43–2.50) | 1.87 (1.43–2.48) | 1.97 (1.42–2.77) | 0.265 |
| MLR (%), median (IQR) | 0.31 (0.22–0.44) | 0.31 (0.22–0.44) | 0.34 (0.22–0.50) | 0.315 |
| SII, median (IQR) | 549 (363–818) | 534 (359–812) | 642 (369–844) | 0.097 |
| PRL (%), median (IQR) | 124 (96–160) | 121 (96–157) | 136 (94–175) | 0.069 |
| PSD | ORunadjusted | 95% CI | p Value | ORadjusted | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Female sex, n (%) | 2.308 | 1.311–3.169 | 0.002 | 2.043 | 1.287–3.245 | 0.002 |
| NIHSS on admission, median (IQR) | 1.126 | 1.072–1.183 | <0.001 | 1.108 | 1.055–1.165 | <0.001 |
| Fibrinogen, median (IQR) | 1.462 | 1.228–1.740 | <0.001 | 1.388 | 1.129–1.706 | 0.002 |
| CRP, median (IQR) | 1.057 | 1.020–1.094 | 0.002 | 1.013 | 0.969–1.059 | 0.566 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, L.; Shao, H.; Tang, X.; Zhang, L.; Zhou, Y.; Jiang, Y.; Fang, Q.; Cai, X. Higher Plasma Fibrinogen Level at Admission Is Associated with Post-Stroke Depression at Discharge. Brain Sci. 2022, 12, 1032. https://doi.org/10.3390/brainsci12081032
Zhu J, Wang L, Shao H, Tang X, Zhang L, Zhou Y, Jiang Y, Fang Q, Cai X. Higher Plasma Fibrinogen Level at Admission Is Associated with Post-Stroke Depression at Discharge. Brain Sciences. 2022; 12(8):1032. https://doi.org/10.3390/brainsci12081032
Chicago/Turabian StyleZhu, Juehua, Li Wang, Han Shao, Xiang Tang, Lulu Zhang, Yun Zhou, Yongjun Jiang, Qi Fang, and Xiuying Cai. 2022. "Higher Plasma Fibrinogen Level at Admission Is Associated with Post-Stroke Depression at Discharge" Brain Sciences 12, no. 8: 1032. https://doi.org/10.3390/brainsci12081032
APA StyleZhu, J., Wang, L., Shao, H., Tang, X., Zhang, L., Zhou, Y., Jiang, Y., Fang, Q., & Cai, X. (2022). Higher Plasma Fibrinogen Level at Admission Is Associated with Post-Stroke Depression at Discharge. Brain Sciences, 12(8), 1032. https://doi.org/10.3390/brainsci12081032






