Abstract
Lignocellulosic biomass, comprising of cellulose, hemicellulose, and lignin, is a difficult-to-degrade substrate when subjected to anaerobic digestion. Hydrothermal pretreatment of lignocellulosic biomass could enhance the process performance by increasing the generation of methane, hydrogen, and bioethanol. The recalcitrants (furfurals, and 5-HMF) could be formed at high temperatures during hydrothermal pretreatment of lignocellulosic biomass, which may hinder the process performance. However, the detoxification process involving the use of genetically engineered microbes may be a promising option to reduce the toxic effects of inhibitors. The key challenge lies in the scaleup of the hydrothermal process, mainly due to necessity of upholding high temperature in sizeable reactors, which may demand high capital and operational costs. Thus, more efforts should be towards the techno-economic feasibility of hydrothermal pre-treatment at full scale.
1. Lignocellulosic Biomass
Lignocellulosic biomass (LB), namely agri-wastes and energy crops, have been gaining much attention as candidate feedstocks for producing bioenergy and biobased products [1]. LB offers a promising alternative to satisfy future energy demand, since it is a widely abundant and potentially carbon-neutral source for bioenergy production [2,3]. Moreover, the agricultural activities generate large amounts of wastes, which are considered as the most important feedstock source of LB for energy production.
There are several sorts of LB. A broad classification into woody and non-woody biomass considers only the chemical composition and physical properties of biomass [4,5]. However, based on the context of the use of LB for bioenergy production, a classification according to LB origin/source seems to be more suitable [6]. Biomass is mainly generated in rural (agriculture, forestry, and livestock), urban (sewage sludge and municipal solid wastes) and industrial (cellulose and agri-food industries) areas [7,8]. Each of these biomass generation areas are comprised of different types of biomass [9]. Figure 1 gives an overview about the different LB sources used for bioenergy production [7,10,11].
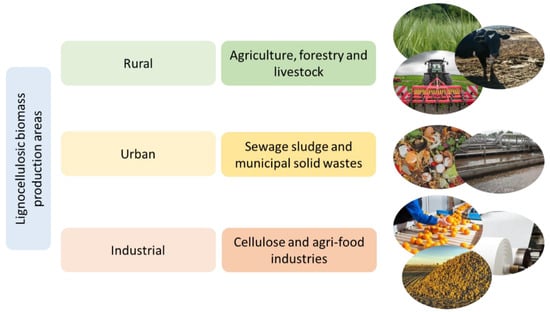
Figure 1.
Lignocellulosic biomass production areas and wastes included in each category.
Notwithstanding the LB is a promising source for bioenergy generation, not all LB types are suitable for biogas production through the Anaerobic Digestion (AD) process. Therefore, some forestry and woody residues are not suitable for biogas production due to their very high lignin composition and the poor lignin degradation, which prevent an adequate hydrolysis of macromolecules to reach high process efficiency and methane generation [12,13,14]. This is similarly applied to most of the hardwood trees, which cannot be degraded through the AD process. Poplar, willow and populous are well known short rotation tree species used as solid biomass for direct combustion.
2. Composition of Lignocellulosic Biomass
The chemical composition of biomass depends strongly on its source [6,15]. The main components of LB are polysaccharides, which are cellulose (40–50%), hemicellulose (25–35%), and lignin (15–20%) [16,17]. The ratios between these fractions vary with the plant age, stage of growth and other conditions [3,16,18,19]. These polymers are organized in complex non-uniform three-dimensional structure with different degrees and varying relative composition [20]. Table 1 resumes the average content of these three components in some LB types.

Table 1.
Average composition of the main lignocellulosic biomass (LB) categories (expressed as percentage in dry basis).
Cellulose is the major mass constituent of most natural biomass and it is found in the walls of the cells of plants. It is a structural polysaccharide in plants and it is a part of supporting tissues.
The wall of a young plant cell contains approximately 40–45% cellulose; wood 50%, while the purest example of cellulose is cotton with a percentage greater than 90% [21,22]. It is made up of interlinked glucose units which are β-1,4-O-glucosidic bonds. These bonds result in significant hydrogen bonding, both intramolecular and intermolecular cellulose molecules, which makes cellulosic material relatively hard to hydrolyze [23]. Thus, cellulose has crystalline and amorphous parts [19].
Hemicelluloses are the second most abundant polymers on the world after cellulose in lignocellulosic materials [4,24]. Hemicelluloses are heteropolysaccharides (i.e., containing more than one type of sugar unit) with branches attached to the main backbone. Nevertheless, hemicellulose is formed by a single type of monosaccharides linked by β-bonds (1–4), mostly containing pentoses (d-xylose and l-arabinose), hexoses (d-glucose, d-mannose and d-galactose) and smaller amounts of l-rhamnose, in addition to uronic acids such as glucuronic acid, 4-O-methyl-d-glucuronic acid, and galacturonic acid. The proportions of these substituents may vary according to biomass kinds [3]. Hemicelluloses connect cellulose and lignin fibers to give consistency and flexibility to the structure of the cell wall [3,19].
Lignin is a structurally important polymer in biomass and is one of the most abundant organic substances on earth, next to cellulose [25]. Lignins are major structural components of higher plants and the primary cell wall provides structural support, impermeability confers to biomass its resistance to hydrolysis and microbial degradation [26,27]. Structurally, lignin is a complex three-dimensional polymer of phenylpropane units (approx. 25). The phenylpropane units are derivatives of carbohydrates, coming from the dehydration and cyclisation of sugars. They are mostly either 4-hydroxycinnamyl alcohol (para-coumaryl alcohol, H) or its 3- and/or 3,5-methoxylated derivatives—coniferyl (guaiacyl, G) alcohol, and sinapyl alcohol (or syringal, S), respectively. The ratio of these units varies according to the plant and biomass type. According to previous studies, lignin content may vary both between species and among different tissues of an individual plant [3]. It has been found that a high lignin content is correlated with the recalcitrance of polysaccharides to enzymatic hydrolysis, which makes pretreatments necessary previously to any biological process aiming to produce bioenergy [13,15]. The lignin content and its partial degradation is related with some problems in the AD process.
The elemental analysis of biomass provides the mass concentrations of the major elements (carbon, oxygen, hydrogen, nitrogen). These concentrations depend on the chemical composition of the LB and allow the estimation of the theoretical biomethane yield and the subsequent bioenergy yield using the Buswell equation [29]. The average composition of the main wastes used in the AD process is shown in Table 2.

Table 2.
Average elemental analysis of some organic wastes classified as LB (expressed as percentage in dry basis).
3. Anaerobic Digestion of Lignocellulosic Biomass
The AD process consists in the transformation of the organic matter contained in the waste into a gaseous effluent (biogas) together with a semisolid stabilized effluent named ‘digestate’. The process is developed by a very complex microbial population operating in absence of molecular oxygen in the medium. Different stages can be distinguished in the overall AD process: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. The microorganisms responsible of those stages should synchronize their metabolic rates and work synergistically to break down the complex structures (i.e., lignocellulose) in the organic matter and hence, to obtain a stable process with a high generation of biogas [35].
Regarding the availability of feedstock for the AD process, it should be taken into account that the amount of lignocellulosic agro-industrial wastes and byproducts produced is very high and, consequently, the exploitation of LB to obtain bioenergy or high-value bioproducts constitutes a priority. However, the complexity of the LB structure greatly hinders its AD due to the high level of crystallinity of cellulose, as well as the cross-linking of carbohydrates and lignin [1,36]. Thus, lignocellulosic biomass is constituted mainly by cellulose, hemicellulose, and lignin and the above-mentioned interactions among these fractions lead to a very stable and recalcitrant structure. This complex structure of lignocellulosic material makes it resistant to enzymatic attack [37].
Solid material and macromolecules constituting organic matter should be hydrolyzed and solubilized in the medium in order to be used by the microorganisms. Therefore, this is a key step for the appropriate development of the overall AD process. Moreover, for lignocellulosic solid wastes, hydrolysis is normally the rate-limiting step of the global process [38].
This stage is performed by extracellular enzymes excreted by the acidogenic microorganisms. However, it should be taken into account that lignin is the most difficult biodegradable component in lignocellulose materials and its cross-linking with the carbohydrates reduces the surface area available for enzyme attack [39]. In addition, Yu et al. [40] reported that hemicellulose can be preferably attacked and, hence, broken down before than cellulose or lignin by the anaerobic microbiota. The structure of LB is weakened by the hemicellulose removal and, thus, the enzymatic solubilization of the cellulosic fraction is favored [41].
Therefore, considering the extraordinary relevance of this stage on the performance of the global process, multiple pretreatments of the feedstock (mechanical, physical, chemical and biological) have been tested, despite the increase in costs that may result by applying them [2,3,42,43]. The aim of these pretreatments is to promote and enhance the organic matter solubilization and the subsequent transformation into bioenergy or biobased products [2,3,4,42,43]. Consequently, the application of these pretreatments becomes relevant to enhance the methane production from LB and their effectiveness has been proved in several studies [44,45].
Among the pretreatment technologies studied, the hydrothermal pretreatment is considered an environmentally-friendly process due to not using any chemicals. It also decreases the formation of fermentation inhibitors, which are formed mainly through sugar degradation at high temperature [37].
4. Hydrothermal Pre-Treatment
The biodegradability of lignocellulosic biomass is affected by the cellulose crystallinity, exposure of the surface to enzymes and structure of lignin. The main component of cellulose is β(1→4 linked d-glucose units), which solubilises at temperature >200 °C into sugars, aldehydes, phenols, ketones, and acid groups. Similarly, hemicellulose is comprised of xyloglucans, xylans, mannans, glucomannans, and β(1→3, 1→4)-glucans, solubilising at temperature >150 °C into sugars, aldehydes, phenols, ketones, and acid groups. Lignin is made of p-coumaryl, coniferyl, and sinaphyl alchohol, solubilising at temperature at 180 °C into phenolics and oils [46].
4.1. Hydrothermal Pre-Treatment: (Principle and Mechanism)
The hydro-thermal pre-treatment of the LB can be an interesting option to achieve a high organic matter solubilization, increase in acidogenic and methanogenic biodegradability, and subsequent improvement in CH4 production. Hydrothermal pre-treatment of organic feedstocks at elevated temperatures/pressures (150–300 °C, initial pressure of 0–60 bar, 2–40 min) has garnered consideration for the production of biofuels from lignocellulosic substrate as it eliminates chemical addition and corrosion-resistant material requirements for hydrolysis reactors [47]. The recalcitrant structure of the lignocellulosic fraction gets easily broken, hemicellulose and lignin is degraded and the cellulose is hydrolysed effectively in the hydrothermal pre-treatment, thus rendering them as soluble fraction in the anaerobic digestion process (Figure 2). Thus, hydrothermal pre-treatment has been successfully applied in the anaerobic digestion of lignocellulosic biomass for the production of biogas, bioethanol, and other value-added products (VAPs) like hydrogens, hydrochar, Polyhydroxyalkanoates (PHAs) and volatile acids.
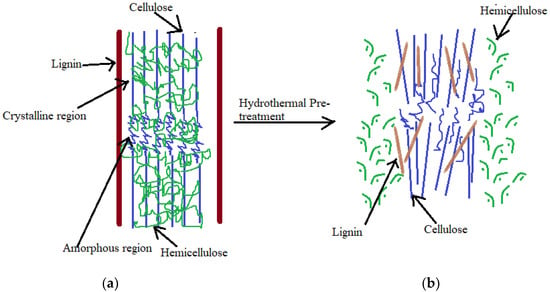
Figure 2.
Effect of hydrothermal pre-treatment on the lignocellulosic biomass: (a) biomass without pre-treatment; (b) hydrothermal pre-treatment of lignocellulose (adapted with permission from Kumar et al. [48]. Copyright (2009) American Chemical Society).
4.2. Effect of Pre-Treatment on Cellulose
Cellulose constitutes the 11–53% (dry basis) of the total lignocellulosic biomass [46]. The cellulose chains contain a number of hydroxylic groups, which leads to the formation of hydrogen bonds in the same chains or in the chains in the vicinity. Interlinking of cellulose chains by hydrogen bonds and van der Waals forces, results in high tensile strength microfibrils [49]. Cellulose molecules have different levels of crystallinity due to its different orientation throughout the structure-low or amorphous crystallinity and high or crystalline crystallinity [50]. If the crystallinity index of cellulose crystals is high, then it is difficult to degrade it and vice versa. The cellulose microfibrils are also attached to each other by pectin and hemicellulose, and they are covered by lignin. This complicated structure of cellulose makes it difficult to be degraded by chemical and biological attacks [51]. The bonds are so strong that they do not break even at high temperature boiling water [52]. The disruption of the inter and intra-hydrogen bonds by the hydrothermal pre-treatment can change the crystallinity structure of cellulose [53]. However, complete disintegration is not possible [54]. The cellulose crystal structure, amount of bound water and the degree of intermolecular regularity determines the intensity of the hydrogen bonds [52]. Sakaki et al. [55], reported that cellulose begins to decompose at temperature of 230 °C, and the reaction was completed at 295 °C. Jin et al. [56] stated that cellulose got hydrolysed to glucose in 120 s at temperature of 300 °C, and pressure of 8.9 MPa. Gao et al. [57] studied the hydrothermal pre-treatment of cellulose at temperature of 200 °C and 400 °C and reaction time of 5 min to 2 h, whereby, the solubilised products contained aldehyde, sugars, phenols, ketones, and acid groups. Girolamo et al. [58], studied the batch thermophilic anaerobic digestion of Giant reed, hydro-thermo-chemically pre-treated at temperature of 150 °C and 180 °C, time of 10 min and 20 min, and with/without sulphuric acid (2% w/w) as catalyst. The catalyst addition was done in two ways: one immediately before the hydrothermal treatment and another as 24 h prior to the steam cooking. Cellulose, owing to its crystalline and thermal-resistant structure, dissolved just modestly in the pre-soaking thermo-chemical pre-treatment. Whereas hemicellulose dissolved to a great extent at the same temperatures (150 and 180 °C).
4.3. Effect of Pre-Treatment on Hemicellulose
The second most important component of lignocellulosic biomass is hemicellulose, which are polysaccharides and contains xylans, mannans, xyloglucans, glucomannans and (1–3, 1–4)-glucans [46]. The hemicellulose contains hexoses and pentoses, which can be degraded into HMF, levulinic acid and formic acid [59]. On the contrary, pentose, and more specifically d-xylose and l-arabinose, is responsible for the formation of furfural. The molecular weight of hemicellulose is low and the lateral chains are short, and so they are easily hydrolysable. The hemicellulose and lignin are solubilised at temperatures greater than 150 °C and 180 °C, respectively. Under high pressure, water acts as an acid, which breaks up the biomass molecules, enhances the rate of hydrolysis of cellulose and thus leading to solubilisation of hemicellulose and lignin [46]. Costa et al. [60] studied the hydrothermal pre-treatment of sugarcane baggase at 150–200 °C for 10–30 min, and observed that soluble sugars were formed as a result from hydrolysis of hemicellulose. As per findings, when lignocellulosic biomass was pre-treated at 100 °C, the hemicellulose remained in the solid fraction, whereas at temperatures above 150 °C, hemicellulose was solubilised into the liquid part. Hydrothermal pre-treatment of hemicellulose resulting in the formation of acetic acid, which acts as a catalyst in the hydrolysis thus further degrade the biomass and leads to an increase in the sugar yield [46].
4.4. Effect of Pre-Treatment on Lignin
Lignin is an amorphous and water-insoluble heteropolymer. It is composed of phenylpropane units (coniferyl, p-coumaryl and sinapyl alcohol) held together by different linkages [61]. It binds cellulose and hemicellulose together and helps in supporting of plant structure and prevents them against microbial attack. The fermentation of lignocellulosic biomass is difficult due to the high recalcitrant lignin and inadequate accessibility of enzymes [46]. Lignin derivatives having aldehyde groups are inhibitory to methanogenesis stage [62]. The precursors in lignin, i.e., p-coumaryl, coniferyl, sinapyl alcohol, decide the solubility of lignin in acidic, neutral or alkaline environment [63]. When the hydrothermal pre-treatment of lignocellulosic biomass is performed, the dissolved lignin may inhibit the activity of cellulase, xylanase and glucosidase [64]. Recent researches conclude that irrespective of its solubilisation, the lignin content change is related to the solidification and re-deposition due to cooling after severe pre-treatment. Therefore, only re-allocation of lignin takes place, instead of lignin removal during pre-treatment at high temperature and pressure [65,66]. In the hydrothermal pre-treatment of switchgrass and paper tube residuals, such a re-allocation of lignin was observed [67,68]. Besides this, the lignin present in raw corncob has a negative yield of sugars with furans produced from the same. However, if the corncob would have undergone hydrothermal pre-treatment, the delignification can give high sugar yields, which indicates that lignin dominates in biomass conversion giving sugar yields [69].
5. Hydrothermal Pre-Treatment: Energy and Value-Added Products Recovery
5.1. Methane and Hydrogen
In order to generate a sustainable energy system, it is predicted that hydrogen will soon be in global usage. Hydrogen is environment-friendly, safe to produce, quite useful for heating purpose in residences, and fuel for non-polluting transport systems and aircraft. The energy content of hydrogen (122 kJ/g) is 2.7 times higher than the gasoline. Similarly, methane gas, produced from anaerobic reactors and digesters is also used as a fuel to generate heat and light. It is a clean fuel thus used in industries, transportation, appliances and power generation [70]. Chandra et al. [71], studied the mesophilic anaerobic digestion of rice straw for the production of methane, which involved thermo-chemical pre-treatment. Hydrothermal treatment was given for 10 min at 200 °C to the ground rice straw. For the untreated sample, the methane production was 59.8 L/kg VS. Hydrothermal pre-treatment with 5% NaOH addition (for maintaining suitable pH for biogas production) enhanced the hydrolysis step of the anaerobic digestion thereby giving a methane yield of 132.7 L/kg VS, i.e., 2.22 times higher than the untreated substrate. Girolamo et al. [58], studied the hydrothermal pre-treatment of Giant reed biomass at temperature combinations of 150 °C and 180 °C, for a time of 10 and 20 min, in the presence and absence of catalyst. The catalyst used was 2% w/w sulphuric acid, immediately before the hydrothermal pre-treatment and 24 h soaking in the catalyst. The untreated assay showed a methane yield of 273 mL/g VS, whereas the assay with no catalyst showed an increase of 10%, 7%, 23% and 4% in the 150 °C/10 min, 150 °C/20 min, 180 °C/10 min and 180 °C/20 min combination, respectively. The pre-treatments in the presence of the catalyst showed an inhibition in the methanogenic activity due to high sulphate concentration, which, led to the growth of the sulphate reducing bacteria. Li et al. [72], studied the hydrothermal pre-treatment on the methane production potential of antibiotic mycelial residue (AMR). Varying the treatment time from 0 to 60 min and temperature from 80 °C to 180 °C. The best digestion results were observed at a temperature of 120 °C for 60 min with a methane yield of 290 mL/g VS, which was almost 3 times the methane yield of raw AMR (100 mL/g VS).
Eskicioglu et al. [47] studied the hydrothermal pre-treatment followed by anaerobic digestion of five kinds of lignocellulosic biomass, namely, wheat straw, rice straw, and Douglas fir bark. Carbon dioxide was used as the catalyst in the experiment and the methane and hydrogen yield was also observed. Temperature and pressure variation of pre-treatment was from 26–175 °C and from 25–102 bars, respectively. 23–42% of hemicellulose destruction and 0–12% of delignification was observed. A 20–30% enhancement of hydrolysis rate was observed except for Douglas fir bark, which showed a 172% enhancement in digestion rate. For hydrothermally pre-treated biomass, a methane yield of 269 mL CH4/g VSadded for wheat straw, 319 mL CH4/g VSadded for rice straw, and 136 mL CH4/g VSadded for Douglas fir bark was observed. Methane yield for the pre-treated substrate was higher by 5.1% in wheat straw, 2.6% in rice straw, and 39% in Douglas fir bark in comparison with non-pre-treated biomass. Phuttaro et al. [73] studied the effect of hydrothermal pre-treatment on Napier grass and the anaerobic digestion. The highest methane yield in batch studies was observed to be 248.2 NmL/VS added at a pre-treatment temperature of 175 °C, which was 35% greater yield in comparison with untreated biomass. He et al. (2014) [74] studied the hydrothermal pretreatment of rice straw (20% TS) at 150 °C and 210 °C for 0 to 30 min operation time. The soluble carbohydrates was observed to be 80 mg/ g VS at a temperature of 210 °C and 0 min holding time. 28mL H2/VS was produced, which was 93 times higher than the control. Kongjan et al. [75], studied the hydrothermal pre-treatment of wheat straw at a temperature of 180 °C and observed a hydrogen yield of 1.59 mol/mol hexose. Jung et al. [76], also studied the hydrothermal treatment of marine algae (Laminaria japonica) at temperature 170 °C and observed a hydrogen yield of 110 L/kg COD, i.e., about 60% greater than non-pre-treated substrate.
Therefore, on average, a methane yield of 250–350 mL/g VS and hydrogen yield of 100–150 mL/g VS is produced from the hydrothermal pre-treatment of lignocellulosic biomass. It can be concluded that a methane yield has been reported to increase by 2% to 300% as compared to the non-pre-treated lignocellulosic biomass and hydrogen yield increased from 0.6 times to 93 times as compared to the non-pre-treated substrate. These numbers are an indicator that regulating the temperature and time in hydrothermal treatment, substantial methane and hydrogen yield could be achieved.
5.2. Hydrochar
Hydrochar is a high value-added carbonaceous matter obtained from hydrothermal pre-treatment of biomass at temperature ranges between 150–350 °C [77]. It is used for soil amendments, as a catalyst and in energy storage application. In the field of energy storage, hydrochar is processed to be used as electrodes in Li-ion batteries [78]. Kambo et al. [79], studied the hydrothermal carbonisation (HTC) of Miscanthus for the production of hydrochar. The treatment temperature of 190, 225, and 260 °C, reaction time of 5, 15, and 30 min and substrate to water ratio of 1:6 and 1:12 was varied. It was observed that the reaction temperature was the most important factor controlling the biomass properties. The hydrochar produced at a hydrothermal carbonisation of 260 °C gave the maximum energy density of 26–30 MJ/kg. Kim et al. [80] studied the HTC of cellulose, and observed that the pre-treatment contributed in high carbon content and high calorific value. HTC at 220 °C led to an increase in fixed carbon content of cellulose increased from 6.1% to 35.0%, which implies that decomposition of cellulose began at this temperature. Along with the fixed carbon, the calorific value of cellulose also was enhanced at temperatures of 180, 200, 220, 280 °C showing an increase from 16.5 to 18.9, 23.1, 26.5 and 27.7 MJ/kg, respectively. The effect of HTC on the quality of biochar obtained from the lignocellulosic biomass can be calculated by increasing the calorific value and this effect can be due to the pyrolysis of cellulose during HTC reactions leading to hydrolysis, chemical dehydration, and decarboxylation reactions. Thus, HTC which is a combination of high pressure and temperature uses biomass as an energy resource for the production of hydrochar, having energy density in the range of 25–30 MJ/kg. Through HTC, biomass with a low calorific value and high water content can be upgraded to a valuable carbon-rich-solid, lignite-like fuel [80].
5.3. Bioethanol
Bioethanol is a non-reactive and environment-friendly reactant to produce biodiesel. Bioethanol production through biomass fermentation makes it a sustainable energy resource, which can be a substitute to fossil fuels [81]. Use of bioethanol as a fuel in the transportation sector contributes to reduce GHG emissions thus helps in climate change mitigation. Being a liquid biofuel produced from the fermentation of corn, wheat, cane, beet and wood, bioethanol is mixed with gasoline with a gasoline: bioethanol ratio ranging from 90:10 to 15:8 [82]. Nitsos et al. [83] optimised the hydrothermal pre-treatment conditions for bioethanol production at various temperature (130–220 °C), reaction times (15–180 min) and water-to-solid ratio of 15. Hemicellulose was totally solubilised and the lignin was only partly delignified. The solubilisation of the ligno-cellulosic biomass led to an increase in surface area and pore volume to nearly 2.5 times. Mabee et al. [84] studied the hydrothermal pre-treatment, where heating of biomass under pressurised steam of 20–50 bar and temperature of 160–270 °C for few minutes followed by pressure release to the atmosphere was carried out, which leads to the desegregation of lignocellulosic biomass. The results were: hydrolysis of hemicellulose, transformation of lignin owing to high temperatures, and increase in crystallinity of cellulose. Reczey and Zacchi [85] studied the anaerobic digestion of hydrothermally pre-treated corn stover at 200 °C for 5 min with 2% sulphuric acid, which led to the four times enhanced enzymatic conversion of cellulose to glucose, and 90% higher yield of ethanol as compared to untreated raw materials. In another study, hydrothermal pre-treatment of wheat straw at 180 °C for 10 min with 0.9% sulphuric acid, gave a sugar yield of 85% of the total sugar present in the raw material.
Petersen et al. [86] studied the hydrothermal pre-treatment of wheat straw for production of bioethanol and concluded that the optimum temperature and time for pre-treatment was 195 °C and 6–12 min. Under optimized conditions, 70% of hemicellulose and 93% cellulose were solubilized, where 89% of cellulose was converted to bioethanol. Kumar et al. [68] conducted the experiments on hydrothermal pre-treatment of switchgrass and corn-stover for the production of ethanol and carbon microspores. Upon pre-treatment at 190 °C for 20 min, more than 80% of glucan was digested. Adding 0.4–0.9% of potassium carbonate could allow the digestion to occur at lower temperature whereby pre-treatment efficiency was also enhanced. On increasing the temperature from 150 °C to 190 °C, the hydrolysis of hemicellulose to water-soluble products showed an increase from 30% to 77%. 40% lignin was solubilized, however, it remained constant at temperature ranges from 150 to 190 °C. Hence, bioethanol generated from the digestion of glucan, hydrolysis of hemicellulose and delignification of lignin, fulfils the aim of hydrothermal treatment of the lignocellulosic biomass.
6. Intermediators-Inhibitory By-Products
6.1. Furfural and 5-HydroxyMethyl Furfural Production
Furfurals are produced during thermo-chemical processing of lignocellulosic biomass. 5-HydroxyMethyl Furfural (HMF) and furfurals are the most important members of furfurals. HMF is formed from hexoses and their polymers, including cellulose. Furfurals are produced from pentoses, which are obtained from hemicelluloses [87]. Acids inhibit cell growth, specifically weak non-dissociated acids cross the cell wall and energy is required to be exported out of the cell [88]. Furfural decreases specific growth rate, and HMF has a mechanism similar to furfural but produces a longer delay phase during growth. Phenolics interact with the cell membrane causing a loss of the integrity of the membrane and decreasing its permeability. However, methanogenic bacteria are able to adapt to such compounds in a certain period of time, up to a certain concentration [89].
In cellulose, the d-glucose is dehydrated to 5-Hydroxymethylfurfural (HMF) by hydrothermal pre-treatment, and then decomposed to formic acid and levulinic acid. In supercritical water, the 99% conversion of glucose to HMF and then to formic and levulinic acids occurred at 0.01 s without the use of catalyst [52]. According to Lopez-Gonzalez et al. [90], when switchgrass was pre-treated at 200 °C for 10 min, production of furfural was observed at a rate of 0.72 g/100 g switchgrass. It was observed in several studies that when temperature was varied between 200–220 °C for 5–15 min, the furfural concentration was between 0.2 and 3.1 g/100 g switchgrass [83,91,92,93]. The hydrothermal pre-treatment of sugar cane press mud for the production of methane was studied. The methane concentration decreased at temperature >200 °C, accounting to inhibition to methanogenesis due to recalcitrant compounds (furfural) formation at high temperatures. The furfural production was observed at the rate of 0.73 g/100 g of press mud. Bougrier et al. [94], reported that Maillard reaction was responsible for the formation of recalcitrant compounds at high temperature. This was due to the polymerisation between the carbohydrates and amino acids. These recalcitrant compounds inhibited the methanogenesis and reduced the overall process efficiency.
Some soluble sugars like xylose, glucose, cell-oligomers, and xylo-oligomers, weak acids like formic, levulinic and acetic acid, furan derivatives like furfurals and HMF and phenol compounds like ferulic acid and vanillin are formed as a result of hydrothermal pre-treatment of lignocellulosic biomass. These compounds are inhibitory for the metabolic activities of substrate degrading microbes and enzymes [95].
The concentration of inhibitory compounds vary as per the type of pre-treatment applied on the lignocellulosic biomass, and the solid loading of lignocellulose on the substrate. of pre-treatment [96,97,98]. Kim et al. [99] studied that hydrothermal pre-treatment of maple wood. Pre-treatment of maple wood at 230 g/L with hot water at 200 °C for 20 min led to the formation of xylooligomers and xylose with a concentration greater than 11.2 and 9.2 g/L, respectively. The concentration of HMF and furfurals were 4.1 g/L, while as the phenol compounds were present in 1.3 g/L concentration. Garcia-Aparicio et al. [100], stated that hydrothermal pre-treatment of barley star at a temperature of 210 °C for 5 min, generated inhibitors like furfural (0.7 g/L), acetic acid (2.1 g/L), phenolic compounds (0.2 g/L) and HMF (0.2 g/L), which affected the enzymatic hydrolysis of the substrate.
Therefore, hydrothermal pre-treatment of ligno-cellulosic biomass is sometimes accompanied by the formation of inhibitors like furfurals and 5-HMF, which act as recalcitrant leading to inhibition of the biological process.
6.2. Inhibitors to Enzymatic Hydrolysis and Fermentation Derived from Hydrothermal Pre-Treatment
6.2.1. Lignin
Lignin inhibited the hydrolysis by forming physical barriers and non-productive adsorption of cellulase enzymes [36]. Thus, lignin restricts the enzymes from reaching to cellulose and thereby the active enzymes for cellulose hydrolysis also reduces [101]. Ko et al. [102,103] observed that 80–90% of lignin was recovered from solid fraction of hardwood, upon hydrothermal pre-treatment at 180–220 °C. Therefore, as the severity of hydrothermal pre-treatment increases, the lignin content in the pre-treated solids also increases due to the simultaneous de- and re-polymerization reactions of lignin. Ko et al. [102] stated that hydrothermal pre-treatment changes the structure of lignin to a more heterogeneous and condensed form, and at the same time lignin is more inhibitory to cellulase than it is before pre-treatment. At low enzyme loading, the non-productive adsorption of enzymes to lignin act as an obstacle to the efficient enzymatic hydrolysis of cellulose. Ko et al. [102,103], investigated that the hydrolysis of Avicel (a microcrystalline cellulose from wood pulp) in the presence of lignin isolated from hardwood, showed a decrease in glucose yield from 62% to 51%. Nakagame et al. [104] also observed that hydrolysis of Avicel decreased to 40% in the presence of lignin isolated from softwood. In a study, where incubation of 10 mg cellulase with lignin isolated from hydro-thermally pre-treated hardwood, a loss of 50–60% total cellulase was seen due to adsoption of lignin. Apart from this, the commercial cellulase Cellic CTec2 having the enzymatic components, >90% of β-glucosidase activity was lost due to lignin adsorption [103].
6.2.2. Lignin-Derived Phenolics
A considerable inhibitory effect on enzymes during cellulose conversion was observed due to the presence of phenolic compounds derived from lignocellulosic biomass. Ximenes et al. [105] reported that soluble phenolics may be formed at a high solid (up to 20%) hydro-thermal pre-treatment of lignocellulosic slurry, which inhibits the enzymatic hydrolysis. The phenolics, which can be extracted from pre-treated slurries can also cause inhibition to enzymatic hydrolysis. The phenolics are considered as the strongest inhibitors among all the toxic compounds derived from the hydrothermal pre-treatment. Kim et al. [99] studied that when the enzyme loading is 1 and 25 mg/g glucan, then the phenolic compounds at 1.3 g/L reduced the rate and strength of cellulose loading by half.
6.2.3. Furan Aldehydes and Weak Acids
During hydrothermal pre-treatment, degradation of sugars to furan aldehydes has been observed. The furan aldehydes include furfural and 5 HMF formed from pentose and hexose, respectively [106,107]. Furan aldehydes degradation generates weak acids like levulinic acid and formic acids. [107,108]. Although the furan aldehyde and acetic acid are inhibitors of fermenting microorganisms, they have little effect on the cellulase activity. Kim et al. [99] observed that acetic acid (13 g/L) and furfural (4 g/L) had no effect on enzymatic hydrolysis. Ask et al. [109] stated that furfurals and 5-HMF inhibit the cell growth and ethanol production of xylose-utilising Sachharomyces cerevisiae (yeast). Inhibition of yeast fermentation is caused by furan aldehydes i.e., by reduction of enzymatic and biological activities. Larsson et al. [110] demonstrated that HMF and furfurals decreased the volume of ethanol production by yeast, during the dilute acid hydrolysis of softwood, whereby furfural inhibition was greater on the cell growth than on ethanol production.
7. Challenges and Opportunities
The hydrothermal pre-treatment of lignocellulosic biomass comes with some challenges, for example: production of inhibitory compounds, toxification of the biomass, and production of 5-HMF and furfurals. Various studies show that xylo-oligomers and oligomer sugars can be removed by hydrolysis and fermentation of pentose [111], but other inhibitory compounds like weak acids, furan aldehydes and phenolic compounds are still inhibitory to enzymatic process and fermentation [112]. Over liming, i.e., pH adjustment by using an alkali, vacuum evaporation, sulphite addition, and adsorbent treatment are some of the methods stated by previous studies in order to remove volatile inhibitors [111]. Removal of phenolic compounds and giving higher yield of ethanol can be achieved by various chemical detoxification processes like addition of activated charcoal adsorbents and polymeric resin [113]. At the same time, these detoxification processes may also lead to high manufacturing cost, generation of waste, and fermentable sugar wastage [114,115]. Bio-abatement methods, using laccase and peroxide as microbial enzymes are also used as detoxication against furan aldehydes, phenolic compounds, and weak acids. C. ligniaria NRRL30616, an ascomyecete, was used as a mitigation bio-abatement against the inhibitors, mainly phenolics, formed during hydrothermal pre-treatment of corn stover slurries. More than 95% acetic acid and >50% HMF, phenolics, and furfurals were removed from the slurries using this technique and on the contrary, 16% higher yield of cellulose conversion was observed [114].
One of the methods for detoxification is the evolutionary engineering of fermenting microorganisms to fight the inhibitors. This method is necessary as it requires no extra detoxification treatment technique, and is based on successive cultivation [116]. S. cerevisiae, an ethanol-producing microorganism, possesses an inborn tolerance to inhibitors like furans and phenolics, and it also convert them to less harmful compounds [117,118]. HMF is reduced to 2,5-bis HMF and the furfurals are reduced to furfural alcohols [119]. Several other studies show that adaptation of yeast to hydrolysates increases the ethanol production and microbial growth [119,120,121]. Also, in order to improve microbial performance in the presence of inhibitors, genetically or metabolically engineered of yeast strains can be used. For example, for the detoxification of phenolic compounds from hydrolysates, S. cerevisiae mutants have been developed [122]. Along with this, strains of S. cerevisiae, which shows resistance to furan aldehydes, have been constructed by many oxidoreductases, such as alcohol dehydrogenases [119].
The sources and component of enzyme determine the loss of cellulase activity due to non-productive adsorption of cellulases to lignin. Upon incubation of Cellic CTec2 with lignin, the major part of β-glucosidase was adsorbed to the lignin, while 50–60% of the initial cellobiohydrolase and endoglucanase remained in the enzyme supernatant [95]. The enzymes need to be engineered to become more resistant to adsorption to insoluble lignin. This is one of the solutions for overcoming enzymatic inhibition [64]. Ko et al. [95] stated that adsorption of β-glucosidase to lignin is dependent on the pH and the salt ions concentration of the medium, thus indicating the presence of electrostatic interactions. So, by changing the surface charge, the adsorption of enzyme to lignin can be reduced.
8. Conclusions
(1) Lignocellulosic biomass is a difficult-to-degrade substrate when subjected to anaerobic digestion.
(2) A considerable increase in methane, hydrogen and bioethanol production could be achieved, when opting for hydrothermal pretreatment of lignocellulosic biomass.
(3) The production of recalcitrant such as furfurals, and 5-HMF takes place during hydrothermal pretreatment of lignocellulosic biomass at very high temperature, which leads to process inhibition.
(4) In order to convert the inhibitors into less toxic compounds, evolutionary engineering is being applied using genetically engineered microbes (e.g., S. cerevisiae, a yeast) as a promising detoxification process.
(5) Hydrothermal pre-treatment comes out to be a beneficial option for production of bio-energy from lingo-cellulosic biomass, which otherwise is difficult to degrade in anaerobic digestion alone.
Author Contributions
B.A., K.A., V.K.T. and L.A.F.-G. reviewed the literature and wrote the different sections of the paper. C.J.Á.-G., L.I.R.-G. and A.A.K. reviewed and edited the paper to enhance the scientific standard.
Funding
This research was supported by the project CTM2016-79071-R funded by the Spanish “Agencia Estatal de Investigación—AEI” and by the European Regional Development Fund (ERDF).
Acknowledgments
Authors are thankful to Department of Biotechnology-GoI (Grant No. BT/RLF/Re-entry/12/2016) for financial support to this research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | anaerobic digestion |
| LB | lignocellulosic biomass |
| PHAs | polyhydroxyalkanoates |
| TS | total solids |
| VAPs | value added products |
| HMF | hydroxy methyl furfurals |
| AMR | antibiotic mycelial residue |
| VS | volatile solids |
| HTC | hydrothermal carbonisation |
References
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.M.; Han, J.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A.; Maravelias, C.T. Synthesis of catalytic biomass-to-fuels strategies. Comput. Aided Chem. Eng. 2014, 34, 615–620. [Google Scholar]
- De Jong, E.; Gosselink, R.J.A. Lignocellulose-Based Chemical Products. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 277–313. [Google Scholar]
- Zhu, J.Y.; Pan, X.J. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef] [PubMed]
- Tye, Y.Y.; Lee, K.T.; Wan Abdullah, W.N.; Leh, C.P. The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew. Sustain. Energy Rev. 2016, 60, 155–172. [Google Scholar] [CrossRef]
- Popa, V.I. Biomass for Fuels and Biomaterials. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–37. [Google Scholar]
- International Renewable Energy Agency. Global Bioenergy Supply and Demand Projections; A Working Paper for REmap 2030; International Renewable Energy Agency: Abu Dhabi, UAE, 2014. [Google Scholar]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Rettenmaier, N.; Schorb, A.; Koppen, S. Status of Biomass Resource Assessments; Biomass Energy Europe, Institute for Energy and Environmental Research: Heidelberg, Germany, 2010. [Google Scholar]
- Key Findings: Statistical Report; Bioenergy Europe: Brussels, Belgium, 2018.
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Čater, M.; Zorec, M.; Marinšek Logar, R. Methods for Improving Anaerobic Lignocellulosic Substrates Degradation for Enhanced Biogas Production. Springer Sci. Rev. 2014, 2, 51–61. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Berlin/Heidelberg, Germany, 2016; p. 8. ISBN 978-981-10-0686-9. [Google Scholar]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C. Current Approaches and Future Trends in Compost Quality Criteria for Agronomic, Environmental, and Human Health Benefits. Adv. Agron. 2017, 144, 143–233. [Google Scholar]
- Smith, A.D.; Landoll, M.; Falls, M.; Holtzapple, M.T. Chemical production from lignocellulosic biomass: Thermochemical, sugar and carboxylate platforms. In Bioalcohol Production; Woodhead Publishing: Swaston/Cambridge, UK, 2010; pp. 391–414. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Monlau, F.; Solhy, A.; Carrere, H. Mechanical dissociation and fragmentation of lignocellulosic biomass: Effect of initial moisture, biochemical and structural proprieties on energy requirement. Appl. Energy 2015, 142, 240–246. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T. Bioprocessing from Biotechnology to Biorefinery. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–24. [Google Scholar]
- Faruk, O.; Sain, M.; Patil, N.D.; Tanguy, N.R.; Yan, N. Lignin Interunit Linkages and Model Compounds. In Lignin in Polymer Composites; William Farino, Andrew Mitchell & Company: Boston, MA, USA, 2016; pp. 27–47. [Google Scholar]
- Jung, S.-J.; Kim, S.-H.; Chung, I.-M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Improvement of Exhausted Sugar Beet Cossettes Anaerobic Digestion Process by Co-Digestion with Pig Manure. Energy Fuels 2015, 29, 754–762. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, W.; Chen, S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour. Technol. 2004, 91, 31–39. [Google Scholar] [CrossRef]
- Tarvin, D.; Buswell, A.M. The Methane Fermentation of Organic Acids and Carbohydrates1,2. J. Am. Chem. Soc. 1934, 56, 1751–1755. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, C.; Liu, Y.; Zhang, R.; Liu, G.; Chen, C. Biogas production from anaerobic co-digestion of durian shell with chicken, dairy, and pig manures. Energy Convers. Manag. 2018. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Heaven, S.; Venetsaneas, N.; Banks, C.J.; Bridgwater, A.V. Slow pyrolysis of organic fraction of municipal solid waste (OFMSW): Characterisation of products and screening of the aqueous liquid product for anaerobic digestion. Appl. Energy 2018, 213, 158–168. [Google Scholar] [CrossRef]
- Angelo Basile, F.D. (Ed.) Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-815162-4. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.S.; Holtzapple, M.T. Fundamental factors affecting biomass enzymatic reactivity. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2000; Volume 84, pp. 5–37. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780813823461. [Google Scholar]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Teater, C.; Liu, Y.; MacLellan, J.; Liao, W. A sustainable pathway of cellulosic ethanol production integrating anaerobic digestion with biorefining. Biotechnol. Bioeng. 2010, 105, 1031–1039. [Google Scholar] [CrossRef]
- Yue, Z.; Teater, C.; MacLellan, J.; Liu, Y.; Liao, W. Development of a new bioethanol feedstock Anaerobically digested fiber from confined dairy operations using different digestion configurations. Biomass Bioenergy 2011, 35, 1946–1953. [Google Scholar] [CrossRef]
- Europe Observer Barometer 18th Annual Overview Barometer. Available online: https://www.eurobserv-er.org/category/2018/ (accessed on 28 May 2019).
- Renewable Energy Policy Network for the 21st Century. Renewables 2016 Global Status Report; REN21: Paris, France, 2016.
- Holm-Nielsen, J.B. Introduction to biomass supply chains. In Biomass Supply Chains for Bioenergy and Biorefining; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–13. [Google Scholar]
- World Bioenergy Association. Global Bioenergy Statistics 2018; World Bioenergy Association: Stockholm, Sweden, 2018. [Google Scholar]
- He, L.; Huang, H.; Zhang, Z.; Lei, Z. A Review of Hydrothermal Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Curr. Org. Chem. 2015, 19, 437–446. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Monlau, F.; Barakat, A.; Ferrer, I.; Kaparaju, P.; Trably, E.; Carrere, H. Assessment of hydrothermal pre-treatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res. 2017, 120, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Ha, M.A.; Apperley, D.C.; Evans, B.W.; Huxham, I.M.; Jardine, W.G.; Viëtor, R.J.; Reis, D.; Vian, B.; Jarvis, M.C. Fine structure in cellulose microfibrils: NMR evidence from onion and quince. Plant J. 1998, 16, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Atalla, R.H.; Vanderhart, D.L. Native cellulose. A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, P.; Li, F.; Jin, S.; Wang, S.; Zhou, S. A Review of Lignocellulose Change During Hydrothermal Pretreatment for Bioenergy Production. Curr. Org. Chem. 2016, 20, 2799–2809. [Google Scholar] [CrossRef]
- Xiang, H.; Sun, R.C. Unraveling the structural characteristics of lignin in hydrothermal pretreated fibers and manufactured binderless boards from Eucalyptus grandis. Sustain. Chem. Process. 2014, 9, 1–12. [Google Scholar]
- Kumar, R.; Wyman, C.E. Access of cellulase to cellulose and lignin for poplar solids produced by leading pretreatment technologies. Biotechnol. Prog. 2009, 3, 807–813. [Google Scholar] [CrossRef]
- Sakaki, T.; Shibata, M.; Sumi, T.; Yasuda, S. Saccharification of cellulose using a hot-compressed water-flow reactor. Ind. Eng. Chem. Res. 2002, 41, 661–665. [Google Scholar] [CrossRef]
- Jin, F.M.; Zhou, Z.Y.; Enomoto, H.; Moriya, T.; Higashijima, H. Conversion mechanism of cellulosic biomass to lactic acid in subcritical water and acidbase catalytic effect of subcritical water. Chem. Lett. 2004, 33, 126–127. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.H.; Yang, H.P.; Chen, H.P. Characterization of products from hydrothermal treatments of cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Grigatti, M.; Barbanti, L.; Angelidaki, I. Effects of hydrothermal pre-treatments on Giant reed (Arundo donax) methane yield. Bioresour. Technol. 2013, 147, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.G.; Pinheiro, G.C.; Pinheiro, F.G.C.; Dos Santos, A.B.; Santaella, S.T.; Leitão, R.C. The use of thermochemical pretreatments to improve the anaerobic biodegradability and biochemical methane potential of the sugarcane bagasse. Chem. Eng. J. 2014, 248, 363–372. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Den Camp, H.J.M.O.; Verhagen, F.J.M.; Kivaisi, A.K.; de Windt, F.E.; Lubberding, H.J.; Gijzen, H.J.; Vogels, G.D. Effects of lignin on theanaerobic degradation of (ligno) cellulosic wastes by rumen microorganisms. Appl. Microbiol. Biotechnol. 1988, 29, 408–412. [Google Scholar] [CrossRef]
- Grabber, J.H. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S.; Saddler, J. Inhibition of cellulase, xylanase and beta-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Ballesteros, I.; Oliva, J.; Caba_as, A.; Ballesteros, M. Hydrothermal pretreatment conditions to enhance ethanol production from popular biomass. Biotechnol. Appl. Biochem. 2003, 105, 87–100. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jørgensen, H.; Elder, T. Cellwall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 1–9. [Google Scholar] [CrossRef]
- Teghammar, A.; Yngvesson, J.; Lundin, M.; Taherzadeh, M.J.; Horváth, I.S. Pretreatment of paper tube residuals for improved biogas production. Bioresour. Technol. 2010, 101, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kothari, U.; Kong, L.; Lee, Y.Y.; Gupta, R.B. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass Bioenergy 2011, 35, 956–968. [Google Scholar] [CrossRef]
- Daorattanachai, P.; Viriya-empikul, N.; Laosiripojana, N.; Faungnawakij, K. Effects of kraft lignin on hydrolysis/dehydration of sugars, cellulosic and lignocellulosic biomass under hot compressed water. Bioresour. Technol. 2013, 144, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Hydrothermal pretreatment of rice straw biomass: A potential and promising method for enhanced methane production. Appl. Energy 2012, 94, 129–140. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhang, Z.; Ma, D.; Wang, L.; Xu, G. Hydrothermal pretreatment for biogas production from anaerobic digestion of antibiotic mycelial residue. Chem. Eng. J. 2015, 279, 530–537. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef]
- He, L.; Huang, H.; Lei, Z.; Liu, C.; Zhang, Z. Enhanced hydrogen production from anaerobic fermentation of rice straw pretreated by hydrothermal technology. Bioresour. Technol. 2014, 171, 145–151. [Google Scholar] [CrossRef]
- Kongjan, P.; Angelidaki, I. Extreme thermophilic biohydrogen production from wheat straw hydrolysate using mixed culture fermentation: Effect of reactor configuration. Bioresour. Technol. 2010, 101, 7789–7796. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Shin, H.S. Fermentative hydrogen production from Laminaria japonica and optimization of thermal pretreatment conditions. Bioresour. Technol. 2011, 102, 2745–2750. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zhou, H.; Shang, S.; Luo, J.; Tsang, D.C. Hydrothermal Carbonization for Hydrochar Production and Its Applications. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 15; pp. 275–294. [Google Scholar]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Kim, D.; Yoshikawa, K.; Park, K.Y. Characteristics of biochar obtained by hydrothermal carbonization of cellulose for renewable energy. Energies 2015, 8, 14040–14048. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- López-Aparicio, S.; Hak, C. Evaluation of the use of bioethanol fuelled buses based on ambient air pollution screening and on-road measurements. Sci. Total Environ. 2013, 452, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Mabee, W.E.; Gregg, D.J.; Arato, C.; Berlin, A.; Bura, R.; Gilkes, N.; Mirochnik, O.; Pan, X.; Pye, E.K.; Saddler, J.N. Updates on softwood-to-ethanol process development. Appl. Biochem. Biotechnol. 2006, 129–132, 55–70. [Google Scholar] [CrossRef]
- Reczey, V.K.; Zacchi, G. Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility. Biotechnol. Appl. Biochem. 2004, 113, 509–523. [Google Scholar]
- Petersen, M.Ø.; Larsen, J.; Thomsen, M.H. Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenergy 2009, 33, 834–840. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production-A review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y. Pretreatment of corn stover by soaking in aqueous ammonia. Biotechnol. Appl. Biochem. 2005, 124, 1119–1131. [Google Scholar] [CrossRef]
- Monlau, F.; Trably, E.; Barakat, A.; Quemeneur, M.; Steyer, J.P.; Carrère, H. Do by-products of thermochemical treatment of lignocellulosic materials inhibit anaerobic mixed cultures? Overview of recent findings. In Proceedings of the 13th World Congress on Anaerobic Digestion, Santiago de Compostela, Spain, 25–28 June 2013; p. 171. [Google Scholar]
- López González, L.M.; Pereda Reyes, I.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Suryawati, L.; Wilkins, M.R.; Bellmer, D.D.; Huhnke, R.L.; Maness, N.O.; Banat, I.M. Effect of hydrothermolysis process conditions on pretreated switchgrass composition and ethanol yield by SSF with Kluyveromyces marxianus IMB4. Process Biochem. 2009, 44, 540–545. [Google Scholar] [CrossRef]
- Yu, G.; Yano, S.; Inoue, H.; Inoue, S.; Endo, T.; Sawayama, S. Pretreatment of rice straw by a hot-compressed water process for enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2010, 160, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.A.; González, A.; Oliva, J.M.; Ballesteros, I.; Manzanares, P. Effect of process variables on liquid hot water pretreatment of wheat straw for bioconversion to fuel-ethanol in a batch reactor. J. Chem. Technol. Biotechnol. 2007, 82, 929–938. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenes, J.P.; Carrere, H. Effects of thermal treatments onfive different waste activated sludge samples solubilisation, physical propertiesand anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Ko, J.K.; Um, Y.; Park, Y.C.; Seo, J.H.; Kim, K.H. Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl. Microbiol. Biotechnol. 2015, 99, 4201–4212. [Google Scholar] [CrossRef]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol. Prog. 2004, 20, 200–206. [Google Scholar] [CrossRef]
- Dekker, R.F.H. Inhibitors of Trichoderma reesei β-glucosidase activity derived from auto-hydrolysis-exploded Eucalyptous regnans. Appl. Microbiol. Biotechnol. 1988, 29, 593–598. [Google Scholar]
- Excoffier, G.; Toussaint, B.; Vignon, M.R. Saccharification of steam exploded poplar wood. Biotechnol. Bioeng. 1991, 38, 1308–1317. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef] [PubMed]
- García-Aparicio, M.P.; Ballesteros, I.; González, A.; Oliva, J.M.; Ballesteros, M.; Negro, M.J. Effect of inhibitors released during steamexplosion pretreatment of barley straw on enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2006, 129, 278–288. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The influence of lignin on the enzymatic hydrolysis of pretreated biomass substrates. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; Zhu, J.Y., Zhang, X., Pan, X.J., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 145–167. [Google Scholar]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A. Furfural formation and behavior. Ind. Eng. Chem. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Ulbricht, R.J.; Sharon, J.; Thomas, J.A. A review of 5-hydroxymethylfurfural (HMF) in parental solutions. Fundam. Appl. Toxicol. 1984, 4, 843–853. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Ask, M.; Bettiga, M.; Mapelli, V.; Olsson, L. The influence of HMF and furfural on redox-balance and energy-state of xylose-utilizing Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Hendrickson, R.; Parenti, J.; Ladisch, M.R. Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour. Technol. 2013, 135, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Quiroga, X.; Aboudi, K.; Álvarez-Gallego, J.C.; Romero-García, I.L. Enhancement of Methane Production in Thermophilic Anaerobic Co-Digestion of Exhausted Sugar Beet Pulp and Pig Manure. Appl. Sci. 2019, 9, 1791. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Grant, T.M.; King, C.J. Mechanism of irreversible adsorption of phenolic compounds by activated carbons. Ind. Eng. Chem. Res. 1990, 29, 264–271. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Zhang, L.; Ladisch, M. Biological abatement of cellulase inhibitors. Bioresour. Technol. 2013, 146, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Reimann, A.; Nilvebrant, N.O.; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl. Biochem. Biotechnol. 2005, 121, 451–460. [Google Scholar] [CrossRef]
- Liu, Z.L.; Moon, J.; Andersh, B.J.; Slininger, P.J.; Weber, S. Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxy methylfurfural by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008, 81, 743–753. [Google Scholar] [PubMed]
- Keller, F.A.; Bates, D.; Ruiz, R.; Nguyen, Q. Yeast adaptation on softwood prehydrolysate. Appl. Biochem. Biotechnol. 1998, 70, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Cassland, P.; Jönsson, L.J. Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl. Environ. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).