The Effect of Grinding Techniques on the Microstructural Properties of Purslane (Portulaca oleracea L.) Powder, Its Total Phenolics Before and After In Vitro Simulated Gastrointestinal Digestion, and Its Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Freeze-Drying of Collected Plant Material
2.3. Grinding of Freeze-Dried Plant Material
2.4. Characterization of Purslane Powder
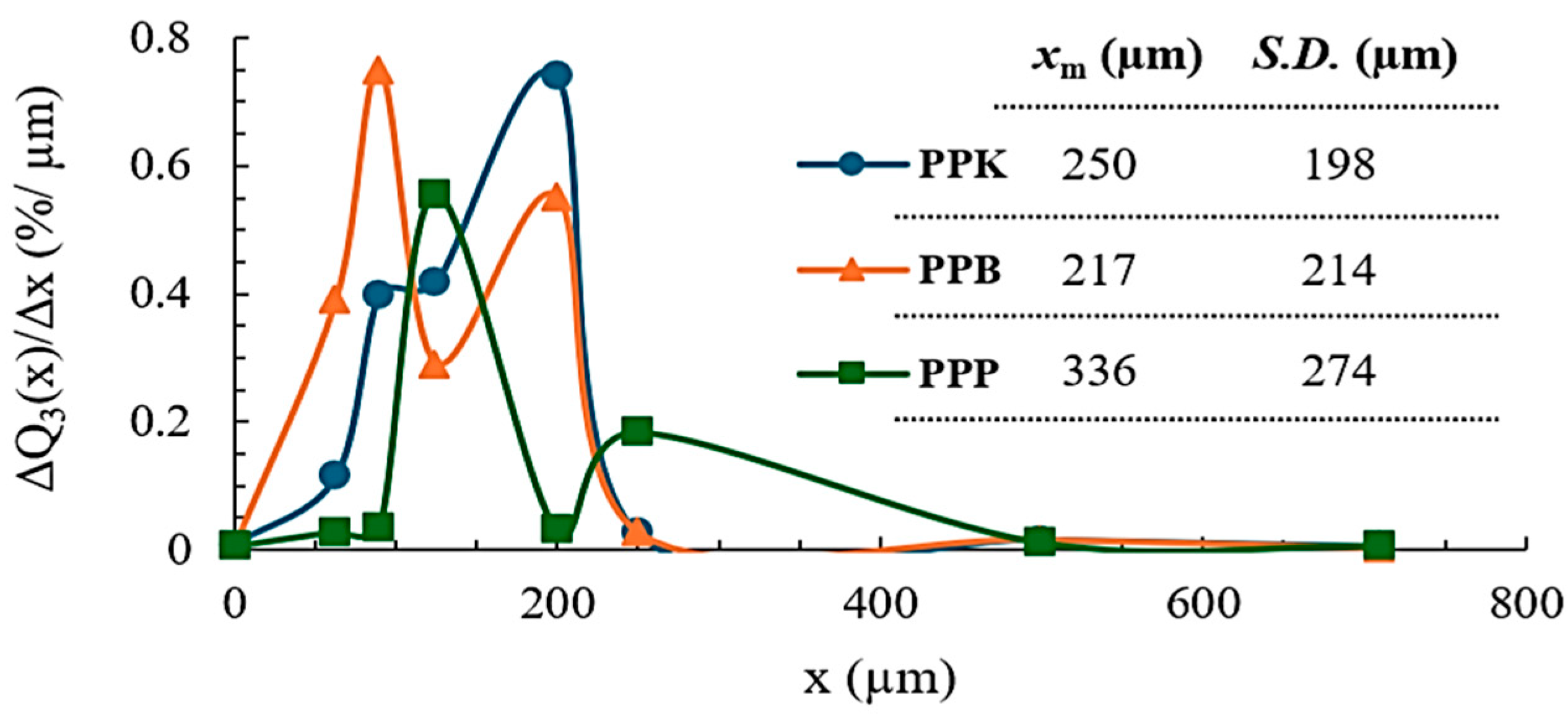
2.4.1. Determination of Particle Size Distribution (PSD)
2.4.2. Color
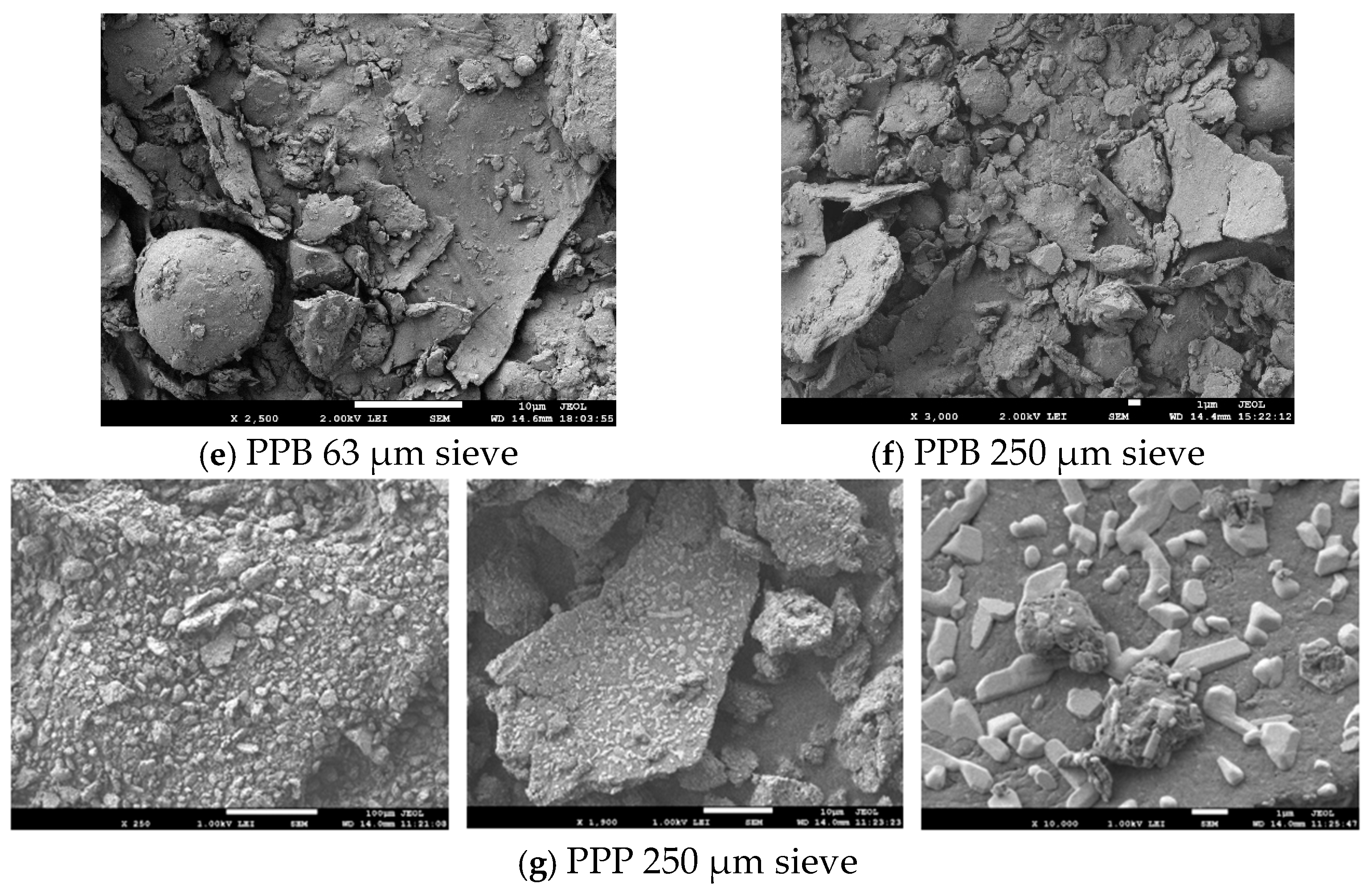
2.4.3. Powder Surface Microstructure via Scanning Electron Microscropy (SEM)
2.5. Extraction of Ground and Unground Plant Material via Hot Maceration
2.6. In Vitro Gastrointestinal Digestion
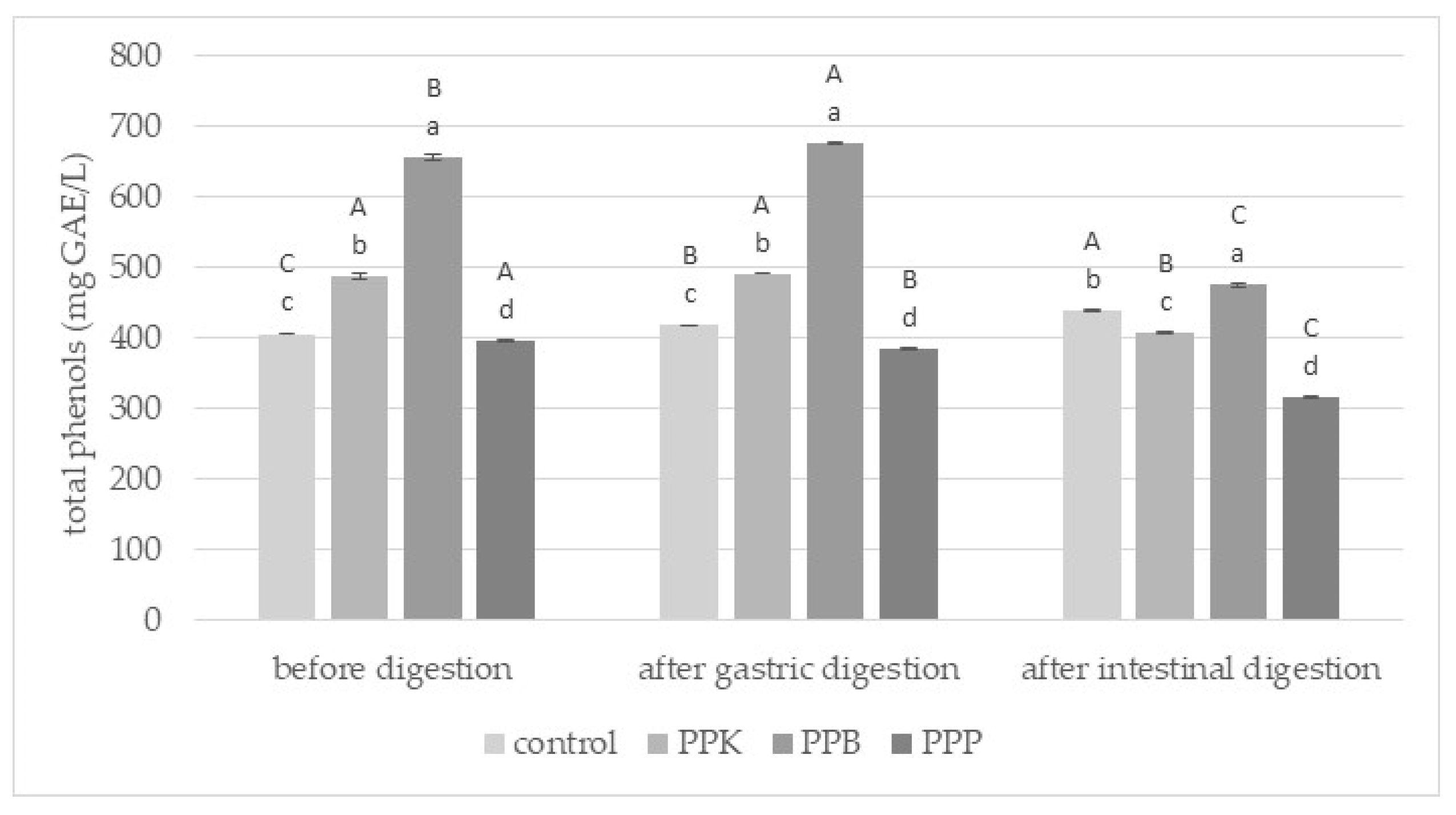
2.7. Determination of Total Phenolic (TP)
2.8. HPLC-DAD Analysis of Phenolic Compounds
2.9. In Vitro Antioxidant Activity
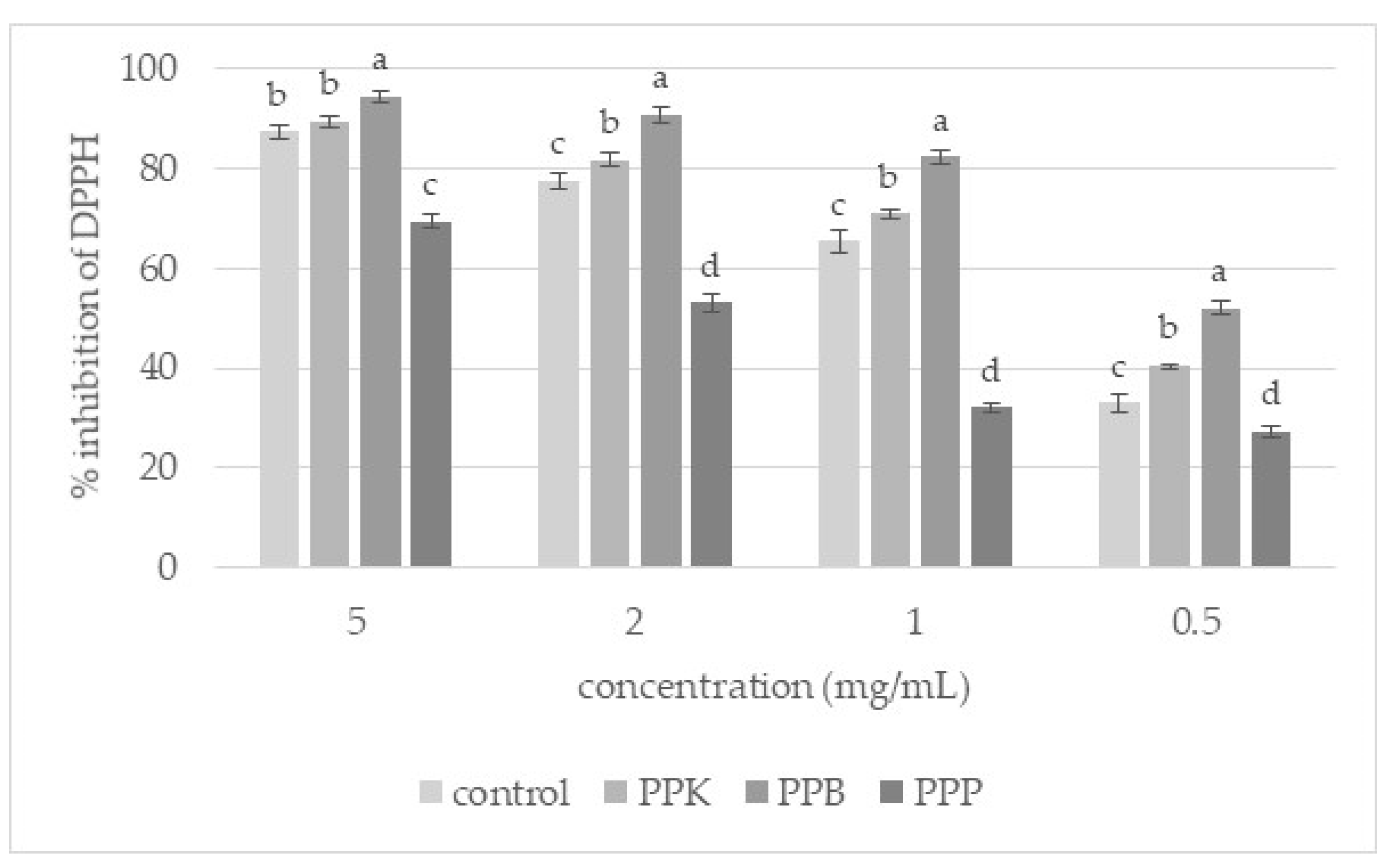
2.9.1. DPPH• (2,2-Diphenyl-1-picrylhydrazyl) Scavenging Activity
2.9.2. ABTS 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulphonic Acid) Radical Scavenging Activity
2.9.3. FRAP (Ferric Reducing/Antioxidant Power)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Purslane Powder Characterization
3.1.1. Surface Microstructure
3.1.2. Particle Size Distribution (PSD)
3.1.3. Color of Purslane Powders
3.2. Gastrointestinal Stability of Phenolic Compounds from Purslane
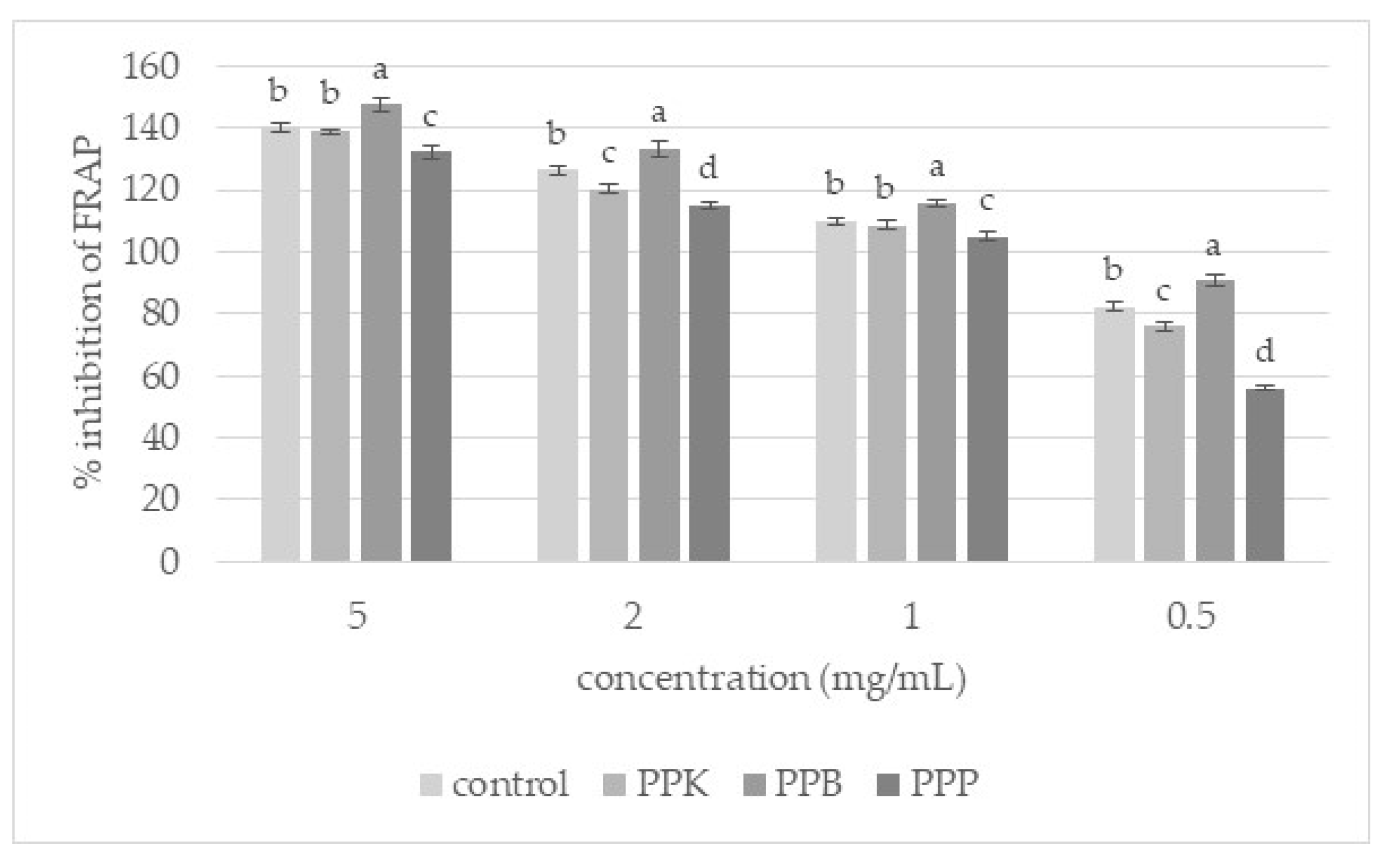
3.3. Antioxidant Activity of Extracts from Unground and Ground Purslane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thalassinos, G.; Petropoulos, S.A.; Antoniadis, V. The Response of Purslane (Portulaca olearacea) to Soil-Added Pb: Is it Suitable as a Potential Phytoremediation Species? Toxics 2023, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sreedharan, S.; Singh, P.; Achigan-Dako, E.; Ramchiary, N. Improvement of a Traditional Orpha Food Crop, Portulaca oleracea L. (Purslane) using Genomics for Sustainable Food Security and Climate-Resilient Agriculture. Front. Sustain. Food Syst. 2021, 5, 711820. [Google Scholar] [CrossRef]
- Erkoç, H.; Colak Esetlili, B. Potential of Purslane (Portulaca oleracea L.) in Phytoremediation: A Study on the Bioaccumulation and Bio-transfer of Cadmium, Nickel, and Copper in Contaminated Soils. J. Agric. Sci. 2024, 30, 284–292. [Google Scholar] [CrossRef]
- Yazdani-Biouki, R.; Karimi, M. Purslane as a Super-high K Accumulator Halophyte. In Proceedings of the Global Symposium on Salt-Affected Soils, Online, 20–22 October 2021; Available online: https://www.fao.org/global-soil-partnership/resources/events/detail/en/c/1264612/ (accessed on 29 June 2025).
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manag. 2021, 72, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; McAndrews, J. Pre-Columbian Purslane (Portulaca oleracea L.) in the New World. Nature 1975, 253, 726–727. [Google Scholar] [CrossRef]
- Uddin, K.; Quan, L.; Hasan, M.; Motmainna, M.; Madom, M. Purslane: A Perspective Plant Source of Nutrition and Antioxidant. Plant Arch. 2020, 20, 1624–1630. [Google Scholar]
- Li, Y.; Xiao, L.; Yan, H.; Wu, M.; Hao, X.; Liu, H. Nutritional Values, Bioactive Compounds and Health Benefits of Purslane (Portulaca oleracea L.): A Comprehensive Review. Food Sci. Hum. Well 2024, 13, 2480–2501. [Google Scholar] [CrossRef]
- Mishra, V.; Chugh, V.; Dwivedi, S.V.; Sharma, K.D. Food and Nutraceuticals Value of Purslane (Portulaca oleracea L.): An Overview. Pharma Inov. J. 2020, 9, 419–424. [Google Scholar]
- Younis, M.; Afzal, K.; Sultan, M.; Rabail, R.; Akhtar, S.; Ismail, T.; Khalid, M.; Shabbir, M. Quality Characteristics of Purslane (Portulaca oleracea L.) Leaf and Stem Powder-Supplemented Cupcakes. J. Food Qual. 2023, 2023, 1329249. [Google Scholar] [CrossRef]
- Obied, W.A.; Mohamoud, E.; Mohamed, O. Portulaca oleracea (purslane): Nutritive Composition and Clinico-pathological Effects on Nubian goats. Small Rumin. Res. 2023, 48, 31–36. [Google Scholar] [CrossRef]
- Badawy, W.Z.; Arafa, S.G.; Czako, M. Optimization of Purslane Plant using Cooking and Pickling Processes for Reducing Oxalate Content. J. Adv. Agric. 2018, 8, 1384–1398. [Google Scholar] [CrossRef]
- Gheflati, A.; Adelnia, E.; Nadjarzadeh, A. The Clinical Effects of Purslane (Portulaca oleracea) Seeds on Metabolic Profiles in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Phytother. Res. 2019, 33, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S. Healing properties of purslane: A Systematic Review Study. Pharm. Lett. 2016, 8, 437–441. [Google Scholar]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Shao, G.; Liu, Y.; Lu, L.; Wang, L.; Ji, G.; Xu, H. Therapeutic Potential of Traditional Chinese Medicine in the Prevention and Treatment of Digestive Inflammatory Cancer Transformation: Portulaca oleracea L. as a Promising Drug. J. Ethnopharmacol. 2024, 327, 117999. [Google Scholar] [CrossRef]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Abdul Hamid, A.; Aslani, F.; Hasan, M.M.; Mohd Zainudin, M.A.; Uddin, M.K. Evaluation of Antioxidant Compounds, Antioxidant Activities, and Mineral Composition of 13 Collected Purslane (Portulaca oleracea L.) Accessions. Biomed. Res. Int. 2014, 2014, 296063. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N. Antioxidant Activity and Phenolic Compounds of Fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Santiago Saenz, Y.; Hernández-Fuentes, A.; Monroy -Torres, R.; Cariño, R.; Jimenez, R. Physicochemical, Nutritional and Antioxidant Characterization of Three Vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as Potential Sources of Phytochemicals and Bioactive Compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Yang, S.; Feng, L.; Zhang, J.; Yan, C.; Zhang, C.; Huang, Y.; Li, M.; Luo, W.; Huang, X.; Wu, J.; et al. Effect of Purslane (Portulaca oleracea L.) o Intestinal Morphology, Digestion Activity and Microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection. Int. J. Mol. Sci. 2023, 24, 10260. [Google Scholar] [CrossRef]
- Li, Z.; Chu, T.; Sun, X.; Zhuang, S.; Hou, D.; Zhang, Z.; Sun, J.; Liu, Y.; Li, J.; Bian, Y. Polyphenols-rich Portulaca oleracea L. (purslane) Alleviates Ulcerative Colitis through Restiring the Intestinal Barrier, Gut Microbiota and Metabolites. Food Chem. 2025, 468, 142391. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Nisar, T.; Fang, Z.; Wang, Z.C.; Guo, Y. Effect of Superfine-grinding on the Physicochemical and Antioxidant Properties of Lycium ruthenicum Murray Powders. Powder Technol. 2020, 372, 68–75. [Google Scholar] [CrossRef]
- Duguma, H.T.; Zhang, L.; Ofoedu, C.E.; Chacha, J.S.; Agunbiade, A.O. Potentials of superfine grinding in quality modification of food powders. CyTA—J. Food 2023, 21, 530–541. [Google Scholar] [CrossRef]
- Dahran, N.; Alotaibi, B.; Abd-Elhakim, Y.; Mohamed, A.; Ibrahim, R.; Metwally, M.; Khamis, T.; Eskandrani, A.; Alosaimi, M.; Aly, M.; et al. Dietary Purslane (Portulaca oleracea L.) Leaf Powder Maintains Growth and Intestinal Health in Oreochromis niloticus under Chronic Water-borne Cadmium Exposure by Strengthening the Gut Barriers, Modulating the Intestinal Nutrient Transporters, and Relieving Oxidative Stress. Fish. Physiol. Biochem. 2025, 51, 8. [Google Scholar] [CrossRef]
- Alsubaie, N.; Abd-Elhakim, Y.M.; Mohamed, A.A.; Ibrahim, R.E.; Metwally, M.M.M.; Khamis, T.; Alhegaili, A.S.; El-Murr, A.E.; Alotaibi, B.S.; Bawahab, A.A. Purslane Leaf Powder Dietary Supplementation Rescues Cadmium-induced Disruption of Behavior, Antioxidant Status, and Expression of Tight Junction Genes, in the Brain of Nile Tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2025, 278, 111086. [Google Scholar] [CrossRef]
- Salman, K.; Ali, E.; Abd-Alla, A.E. Preparing Untraditional Kishk Formula with Purslane as Natural Source of Bioactive Compounds. J. Food Dairy. Sci. 2020, 11, 299–305. [Google Scholar] [CrossRef]
- Ćosić, M.; Čelan, A.; Pehnes, I.; Kuzmanić, N. Investigation of Crystal Growth of Borax in Single and Dual Impeller Batch Cooling Crystallizer. Chem. Eng. Commun. 2019, 207, 847–860. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V. Evaluation of the Maillard Reaction in Potato Crisps by Acrylamide, Antioxidant Capacity and Color. J. Food Comp. Anal. 2009, 22, 589–595. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D. Physical Properties of Non-agglomerated Cocoa Drink Powder Mixtures Containing Various Types of Sugars and Sweeteners. Food Bioprocess. Techol 2013, 6, 1044–1056. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Llorent-Martínez, E.; Ruiz-Medina, A. Phytochemical Composition and Antioxidant Activity of Portulaca oleracea: Influence of the Steaming Cooking Process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Dujmović, M.; Rusak, G. Developmentally-related Changes in Phenolic and L-ascorbic Acid Content, and Antioxidant Capacity of Chinese Cabbage Sprouts. J. Food Sci. Technol. 2020, 57, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-radical and Active-oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. IJRASET 2015, 3, 636–641. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, Y.; Tamura, H.; Jono, K.; Miyamoto, M.; Tokumitsu, H.; Ichikawa, H.; Block, L.H. Dry Grinding of Chitosan Powder by a Planetary Ball Mill. Adv. Powder Technol. 1998, 9, 281–292. [Google Scholar] [CrossRef]
- Varol, T.; Ömür, F.D.; Akçay, S.B.; Güler, O.; Erdermir, F. Evolution of Morphology, Particle Size and Oxidation Resistance of Recycled Ti-6Al-4V Powders Prepared by Planetary Ball Milling. Arab. J. Sci. Eng. 2024, 50, 9123–9140. [Google Scholar] [CrossRef]
- Ozkan, G.; Sakarya, F.B.; Tas, D.; Yurt, B.; Ercisli, S.; Capanoglu, E. Effect of In Vitro Digestion on the Phenolic Content of Herbs Collected from Eastern Anatolia. ACS Omega 2023, 28, 12730–12738. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Dräger, G.; Capanoglu, E.; Esatbeyoglu, T. Bioaccessibility and Transepithelial Transportation of Cranberrybush (Viburnum opulus) Phenolics: Effects of Non-Thermal Processing and Food Matrix. Food Chem. 2022, 380, 132036. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated Gastrointestinal Digestion and In Vitro Colonic Fermentation of Spent Coffee (Coffea arabica L.): Bioaccessibility and Intestinal Permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Kashef, R.; Soliman, A.; Hassan, H.; Abd-Elhak, N. Evaluation of Total Phenolic Content and Antioxidant Activity of Different Solvent Extracts of Egyptian Purslane Leaves. Curr. Sci. Int. 2018, 7, 616–623. [Google Scholar]
- Ahmed, M.; AlJuhaimi, F.; Özcan, M.; Uslu, N.; Karrar, E. Determination of the Distribution of Bioactive Compounds, Antioxidant Activities, Polyphenols and Macro and Microelement Contents in Different Parts of Wild and Cultivated Purslane (Portulaca oleracea L.) Plants. J. Food Meas. Charact. 2025, 19, 3714–3724. [Google Scholar] [CrossRef]
- Liu, F.; He, C.; Wang, L.; Wang, M. Effect of Milling Method on the Chemical Composition and Antioxidant Capacity of Tartary Buckwheat Flour. Int. J. Food Sci. Technol. 2018, 53, 2457–2464. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Suttajit, M. Microchemical Components and Antioxidant Activity of Different Morphological Parts of Thai Wild Purslane (Portulaca oleracea). Weed Sci. 2010, 58, 182–188. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.; Tundis, R.; Mincione, A. Portulaca oleracea L. (Purslane) Extracts Display Antioxidant and Hypoglycaemic Effects. J. Appl. Bot. Food Qual. 2018, 91, 39–46. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Shaker, S.E.; Shawky, H. Solvent Polarity Dictates the Anti-inflammatory Potency and Mechanism of Two Purslane (Portulaca oleracea) Seed Extracts. J. Food Biochem. 2022, 46, e14281. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic Acid Is Absorbed in Its Intact Form in the Stomach of Rats1. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef]
- Shu, Y.; Li, J.; Yang, X.; Dong, X.; Wang, X. Effect of Particle Size on the Bioaccessibility of Polyphenols and Polysaccharides in Green Tea Powder and its Antioxidant Activity after Simulated Human Digestion. J. Food Sci. Technol. 2019, 56, 1127–1133. [Google Scholar] [CrossRef]
- Baldelli, A.; Aguilera, J.M. Size Reduction and Particle Size Influence the Quantification of Phenolic-type Compounds and Antioxidant Activity in Plant Food Matrices. Trends Food Sci. Technol. 2025, 162, 105081. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Karam, M.C.; Dicko, A. Effect of Particle Size on Antioxidant Activity and Catechin Content of Green Tea Powders. J. Food Sci. Technol. 2016, 53, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, Q.-X.; Qiao, M.; Ma, F.; Thakur, K.; Wei, Z.-J. Effect of Superfine Grinding on Properties of Vaccinium bracteatum Thunb Leaves Powder. Food Sci. Biotechnol. 2017, 26, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Chen, D.; Shuzhi, C. Antioxidant Activity and Mechanism of Protocatechuic Acid in vitro. Funct. Food Health Dis. 2011, 7, 232–244. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurozzi; Junassilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of Particle Size on Phytochemical Composition and Antioxidant Properties of Sargassum cristaefolium Ethanol Extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef]
- Habibian, M.; Sadeghi, A.; Karimi, A. Phytochemicals and Antioxidant Properties of Solvent Extracts from Purslane (Portulaca oleracea L.): A Preliminary Study. Food Sci. Eng. 2019, 1, 12. [Google Scholar] [CrossRef]



| Color Parameters | |||||
|---|---|---|---|---|---|
| L* | a* | b* | Chroma | Hue | |
| PPK | 53.64 ± 0.09 ab | 1.4 ± 0.01 b | 25.87 ± 0.04 a | 25.91 ± 0.04 a | 86.9 ± 0.03 b |
| PPB | 61.68 ± 2.22 a | 1.32 ± 0.13 b | 26.73 ± 0.77 a | 26.76 ± 0.78 a | 87.18 ± 0.19 a |
| PPP | 39.50 ± 17.34 b | 1.67 ± 0.02 a | 22.77 ± 0.03 b | 22.83 ± 0.03 b | 85.80 ± 0.05 c |
| Phenolic Compounds | Control | PPK | PPB | PPP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UN (µg/g) | GA (%) | IN (%) | UN (µg/g) | GA (%) | IN (%) | UN (µg/g) | GA (%) | IN (%) | UN (µg/g) | GA (%) | IN (%) | |
| Benzoic | 20.90 | 100 | 34.54 | 16.03 | 74.85 | 100 | 14.06 | 99.43 | 100 | 10.12 | 72.13 | 76.18 |
| Protocatehuic | 2010.03 | 88.60 | 41.29 | 2318.08 | 74.50 | 61.10 | 2059.76 | 69.47 | 29.08 | 804.40 | 65.67 | 57.34 |
| Chlorogenic | 380.20 | 100 | 80.33 | 467.55 | 65.12 | 68.71 | 365.89 | 85.44 | 72.27 | 389.15 | 95.31 | 73.53 |
| Syringic | 11.57 | 71.39 | 93.17 | 40.57 | 45.00 | 14.61 | 8.77 | 57.24 | 91.67 | 7.68 | 93.88 | 32.42 |
| Ferulic | 242.17 | 86.83 | 86.83 | 287.80 | 63.66 | 76.17 | 253.05 | 89.73 | 73.34 | 666.61 | 88.36 | 64.24 |
| Synapic | 8.93 | 56.77 | 56.77 | 8.00 | 77.37 | 46.12 | 9.20 | 100 | 49.89 | 6.81 | 63.14 | 56.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilušić, T.; Runtić, D.; Šola, I.; Benković, M.; Bilušić, A.; Ćosić, M.; Đorđević, D. The Effect of Grinding Techniques on the Microstructural Properties of Purslane (Portulaca oleracea L.) Powder, Its Total Phenolics Before and After In Vitro Simulated Gastrointestinal Digestion, and Its Antioxidant Capacity. Appl. Sci. 2025, 15, 7448. https://doi.org/10.3390/app15137448
Bilušić T, Runtić D, Šola I, Benković M, Bilušić A, Ćosić M, Đorđević D. The Effect of Grinding Techniques on the Microstructural Properties of Purslane (Portulaca oleracea L.) Powder, Its Total Phenolics Before and After In Vitro Simulated Gastrointestinal Digestion, and Its Antioxidant Capacity. Applied Sciences. 2025; 15(13):7448. https://doi.org/10.3390/app15137448
Chicago/Turabian StyleBilušić, Tea, Dora Runtić, Ivana Šola, Maja Benković, Ante Bilušić, Marija Ćosić, and Dani Đorđević. 2025. "The Effect of Grinding Techniques on the Microstructural Properties of Purslane (Portulaca oleracea L.) Powder, Its Total Phenolics Before and After In Vitro Simulated Gastrointestinal Digestion, and Its Antioxidant Capacity" Applied Sciences 15, no. 13: 7448. https://doi.org/10.3390/app15137448
APA StyleBilušić, T., Runtić, D., Šola, I., Benković, M., Bilušić, A., Ćosić, M., & Đorđević, D. (2025). The Effect of Grinding Techniques on the Microstructural Properties of Purslane (Portulaca oleracea L.) Powder, Its Total Phenolics Before and After In Vitro Simulated Gastrointestinal Digestion, and Its Antioxidant Capacity. Applied Sciences, 15(13), 7448. https://doi.org/10.3390/app15137448









