Abstract
The clinical neurophysiological tests allow us to determine the type, extent, and nature of brachial plexus damage. They are crucial in decision making regarding surgical procedures or conservative treatment. This report aimed to present an algorithm for rehabilitation procedures in patients with brachial plexus injury of various origins based on the results of neurophysiology findings for the selection of procedures supporting the process of nerve and muscle regeneration. The research group consisted of patients whose medical documentation was analysed concerning the reason, level, and localization of damage to the brachial plexus structures, surgical and or rehabilitative treatment, as well as the MRI results. Among the group of fourteen patients, the clinical studies showed the greatest incidence of brachial plexus injuries of the mixed (both pre- and postganglionic), all trunks, and cervical root injuries, respectively. Results of the motor evoked potentials (MEP) and electroneurography (ENG) recordings induced at levels of spinal roots and Erb’s point showed a decrease of more than 40% in amplitudes on the symptomatic side in comparison to the asymptomatic side. This diffeence was recorded for the axillary and radial innervation and the C5, C6, and C7 root domains, ranging from 57% to 66%; the lowest decrease was recorded following electrical stimulation at Erb’s point for the ulnar nerve (34%). The latency prolongation on the symptomatic side in CMAP and MEP tests ranged from 0.2 to 1.7 ms, with the most following magnetic stimulation of the C5 cervical root for the axillary innervation. Most of the results indicated the axonotmesis and neuropraxia type of injury in motor fibers (40%) confirmed by EMG results. The sensory conduction studies (SNCS) in distal nerve branches did not confirm the severe advancement of the brachial plexus injury (63%). The proposed algorithm of the physiotherapeutic procedures should be mainly targeted for recovery of motor dysfunction as the consequence of brachial plexus injury. Rehabilitation should incorporate the treatment supporting nerve regeneration, muscle strengthening, and maintaining functional ranges of motion of the injured extremities. The rehabilitation treatment for patients with brachial plexus injuries is an individualised process, and the selection of procedures and the effectiveness of the treatment undertaken should be confronted with results of neurophysiological tests verifying the motor neural transmission from the level of the cervical motor centre to the effector, peripheral nerve function, and muscle’s motor unit activity.
1. Introduction
Damage to the brachial plexus is a diagnostic and therapeutic problem, resulting mainly from its complex anatomical structure [1]. The nerve fibers forming the brachial plexus, starting from the level of the C5–T1 spinal roots, through numerous interconnections in the form of trunks and bundles, located distally at the level of the neck, supraclavicular fossa, and axillary fossa, create terminal branches, which are the particular peripheral nerves. The fibers of these nerves conduct nerve impulses responsible for motor and sensory control in the upper extremity, according to the distribution of myotomes and dermatomes [2,3,4,5]. The complexity of the structure of the brachial plexus in one aspect may influence the extent of the damage through the interconnections of neural structures. However, from a clinical point of view, it may provide the basis for better regeneration through mutual anastomosis using properly functioning structures of nerves and muscles. This is particularly important in the case of reconstructive surgeries aimed at restoring the function of the upper extremity, primarily in the area of elbow flexion and gripping activity of the hand. The most common surgical procedures are muscle or nerve transfers [6,7,8].
Brachial plexus injuries are usually associated with the consequences of mechanical trauma, which often accompanies multi-organ injuries, especially during traffic accidents. For this reason, the diagnostic process of plexus damage and surgical treatment may be postponed until the patient returns to a stable health status. The diagnostic process itself, and surgical and rehabilitation treatment are multidisciplinary [9,10,11].
The clinical assessment analyses the sensory perception, physiological reflexes in the upper extremity, range of movement of the extremities, muscle tension, muscle strength, and atrophy, compared to the asymptomatic side [12,13]. Imaging tests such as magnetic resonance imaging (MRI) and ultrasound (USG) also provide objectivity to the clinical examination. They indicate the morphological properties of damage to the neural structures of the plexus, its extent, and, above all, location, as well as analyse the image of the tissues surrounding the brachial plexus, such as muscles, tendons, and blood vessels [14,15,16,17]. Studies that complement the above diagnostic standard are clinical neurophysiology tests used to assess the functions of the peripheral nervous system. These include electroneurography (ENG), which assesses the activity of motor and sensory nerve fibers due to electrical stimulus that stimulates neural structures [18]. Alternatively, a magnetic stimulus is also used, which, due to its physical characteristics, allows the permeating of the bone tissue and activates the neural structures [19]. This is important, especially in brachial plexus injuries at the cervical spinal roots level or the neck described as proximal damage. Depending on the type of damage to the motor fibers of the brachial plexus, in the case of axonal changes that correspond to axonotmesis according to Seddon’s classification [20], the bioelectric activity of the muscles presents the abnormality. A test that determines the nature of muscle damage, in this case, its neurogenic characteristic and the dynamics of these changes, such as the presence of active denervation and reinnervation, is an elementary (needle) electromyography (EMG) [3,4,20]. The clinical neurophysiological tests allow us to determine the type, extent, and nature of brachial plexus damage. They are crucial in making decisions regarding further treatment, including surgical procedures and conservative treatment. They also allow for a prospective assessment of ongoing rehabilitation and modification of the therapeutic protocol to optimise nerve regeneration processes and muscle reinnervation. In the available literature, most scientific reports concern the rehabilitation process of paediatric patients with brachial plexus paralysis [21,22,23,24,25] and less information regarding the targeted rehabilitation of adults with this injury [25,26,27,28,29,30].
This report aims to present an algorithm for rehabilitation procedures in adult patients with brachial plexus damage of various origins based on the results of clinical neurophysiology tests. The algorithm refers to the functional state of the nerve structures of the brachial plexus and the selection of appropriate rehabilitation techniques supporting the process of nerve regeneration and reinnervation of the examined muscles.
2. Materials and Methods
2.1. Participants and Clinical Evaluation
Nineteen subjects were enrolled in the research group. The inclusion criteria were the diagnosis of brachial plexus damage based on clinical examination and magnetic resonance imaging. The patient’s clinical examination and analysis of the results of imaging studies qualifying for neurophysiological examination were performed by an orthopaedic surgeon. Exclusion criteria were pregnancy, stroke, oncological disorders, epilepsy, metal implants in the head and spine, cardiac pacemakers, or cochlear implants because of the use of magnetic stimulus for brachial plexus evaluation. Five patients met the exclusion criteria from the research group. Finally, the research group consisted of fourteen patients. Medical documentation was analysed concerning the surgical and rehabilitative treatment in the research group. The anthropometric data of the patients and the aetiology, type, and location of brachial plexus injury of the study group have been determined. Ethical considerations of this study were compliant with the Helsinki Declaration and granted by the Bioethical Committee of the University of Medical Sciences in Poznań, Poland (resolution no. 554/17). All patients signed a written consent form for participation in this study consisting of all information necessary to understand the purpose of the research, the scope of diagnostic procedures, and their characteristics. Preliminary neurophysiological tests were performed in a single centre at the Department of Pathophysiology of Locomotor Organs, Poznań University of Medical Sciences.
2.2. Study Design and Neurophysiological Examination
All patients were examined bilaterally once with the same neurophysiological protocol. Magnetic and electrical stimuli were used to assess the function of the brachial plexus and the magnetic one for the motor transmission from the cervical spinal roots. The three times stimulation at the Erb’s point and the selected level of the cervical roots have been performed to check the repeatability of the evoked potentials. The parameters of the compound muscle action potentials (CMAP) recording during electroneurography (ENG) and motor evoked potentials (MEP) induced with the magnetic field applied at the Erb’s point and also at the cervical roots were analysed.
The KeyPoint Diagnostic System (Medtronic A/S, Skøvlunde, Denmark) was used for the MEP and CMAP recordings. The external magnetic stimulus for MEP studies from the MagPro X100 magnetic stimulator (Medtronic A/S, Skøvlunde, Denmark) via a circular coil (C-100, 12 cm in diameter) was delivered. Table 1 shows technical data for the ENG and MEP studies.

Table 1.
Technical data for MEP and ENG recordings.
In the ENG examination, the electrical stimulation was applied at the Erb’s point over the supraclavicular region. This point also concerns the application of magnetic stimulation. To assess the MEP from the cervical roots, the magnetic coil placement was 0.5 cm laterally and slightly below the spinous process from C5 to C8. In this way, cervical roots were selectively stimulated. The same selected muscles were used for recording MEP and CMAP and represented a specific root domain and innervation from the terminal branches of the brachial plexus (Table 2).

Table 2.
Summary of data on ENG and MEP research methodology [3,4].
In the case of both CMAP and MEP recordings, their values of amplitudes in µV and latencies in ms of evoked potentials were analysed. Further details regarding the neurophysiological recordings with the above-mentioned methods are described elsewhere [3,4,18,19].
2.3. Statistical Analysis
Statistica, version 13.1 (StatSoft, Kraków, Poland) was used for the data analysis. Descriptive statistics were reported as minimal and maximal values (range), with mean and standard deviation (SD). The normality distribution and homogeneity of variances were studied with the Shapiro–Wilk test and with Levene’s test. The results from all neurophysiological tests performed on patients were also calculated from the group of healthy subjects (control group) to achieve the normative parameters used to compare the health status between the patients and the controls. Attention was paid to matching patients and healthy controls’ demographic and anthropometric properties, including sex, age, height, weight, and BMI. Results from the neurophysiological recordings have been expressed as the percentages of the changes recorded between the symptomatic and asymptomatic side for parameters recorded in patients. All statistical comparisons from neurophysiological recordings on asymptomatic and symptomatic sides as well as the results of analogical recordings in the healthy volunteers are described in other papers of our team [3,4]. The statistical software was used to determine the required minimal sample size using the primary outcome variable of MEP amplitude recorded following the stimulation at the Erb’s point with a power of 80% and a significance level of 0.05 (two tailed). The sample size software estimated that at least 9 subjects were needed for this study to differentiate between results recorded in patients and the results from previous studies on healthy volunteers using the same neurophysiological methods.
3. Results
3.1. Characteristics of Research Group
Table 3 summarises the physical examination results characterising the research group regarding both anthropometric features and the aetiology of the brachial plexus injury. The nature, level, and localization of damage to the brachial plexus of the structures that constitute it, such as spinal roots and trunks, were also considered.

Table 3.
Summary of the characteristics of research group. For the parameters of height and age of patients, the range, and mean values with standard deviation are presented. For the remaining features characterising the research group, the “+” mark confirms the presence of a given feature.
The research group was dominated by men (21% women only) aged 20–59. In terms of the aetiology of brachial plexus damage, motorcycle accidents and falls from height or shoulder girdle dislocations predominated in the research group, accounting for 43% for each. In the remaining cases, there are whiplash injuries at 7% and car accidents accounting for 7%. In 50% of the examined patients, in addition to the brachial plexus injury, also diagnosed were multi-organ injuries, bone fractures, disruption of the continuity of skin tissue, damage to the blood vessels of the upper extremity, and brain–cranial injuries. The dominant type of injury was mixed-type damage concerning the level of the cervical spinal roots and brachial plexus trunks (43%). Postganglionic damage, only at the level of the brachial plexus trunks, occurred in four patients, while preganglionic damage was present in three patients. In the case of whiplash damage, the function of the spinal roots and brachial plexus trunks did not differ from the normative.
Kinesiotherapy in the physical examination was the most frequently reported form of rehabilitation for patients at every stage of the treatment. Active–passive and isometric muscle exercises (strengthening exercises) were performed to increase muscle strength and improve the range of motion in the shoulder, elbow, and wrist joints (100% of patients). The second dominant form of rehabilitation was the functional electrical stimulation of damaged nerves or muscles (in 86% of patients). In addition, the patients also underwent exercises stimulating proprioception and neuromuscular facilitation, such as the PNF method (mainly flexion, abduction, and the external rotation pattern), massage, and manual therapy of the upper extremities. A crucial aspect of the rehabilitation process was the therapy of scars. In addition to electrostimulation, magnetotherapy, laser therapy, and phototherapy treatments, using the SOLLUX lamp were also performed. The presented types of physiotherapeutic procedures used in the research group served as an overview analysis of the scope of standardly used techniques in brachial plexus injuries. Our study did not analyse their effectiveness because the therapeutic procedures applied and the facilities in which they were performed differed.
3.2. Results of Neurophysiological Tests
Comparison of results of percentage decrease amplitude parameter concerning asymptomatic side and values of latency parameter (ms) for both symptomatic and asymptomatic side and the percentage difference between them, using electric and magnetic stimulation at Erb’s point and magnetic stimulation of cervical root C5–C8 is presented in data of Table 4. Based on the results of our previous studies [3,4], they differed from those recorded in the group of healthy volunteers.

Table 4.
Summary of CMAP and MEP amplitude values (% of change) and latency results for the symptomatic and asymptomatic side using electrical and magnetic stimulation at Erb’s point and magnetic stimulation at the cervical roots. Mean, ranges, and standard deviations are presented.
The average values of the percentage decrease in the CMAP and MEP amplitude parameters on the symptomatic side in comparison to the asymptomatic side, both in the study of electrical and magnetic stimulation at the Erb’s point and the level of the spinal roots, show differences exceeding 40%, for all nerves examined except the ulnar nerve. The difference between the symptomatic and asymptomatic side was recorded for the axillary nerves and the C5 and C6 root domains, ranging from 57% to 66%. The lowest amplitude decrease was recorded in the magnetic stimulation at the C8 root domain in the ulnar nerve (34%). The above test results correlate with the data provided in Table 3, where the most common injuries to the brachial plexus were the C5–C7 cervical roots and damage at the upper and middle trunk.
In the research group, the average values of CMAP and MEP latency prolongation on the symptomatic side in comparison with the asymptomatic side in all nerves examined ranged from 0.2 to 1.7 ms. The crucial prolongation of latency occurred in the MEP examination at the level of the C5 cervical root for the axillary nerve. The above results correlate with data on changes in the amplitude value, where the utmost percentage loss in amplitude occurred in the MEP examination at the level of the C5 cervical root for the axillary nerve. A decrease in the amplitude of evoked potentials accompanied by a prolonged latency indicates mixed nerve damage (axonal–demyelinating damage and may correspond to the axonotmesis and neuropraxia type of injury in the Seddon classification [20].
We utilised the normative parameters of the neurophysiological studies of brachial plexus function recorded in healthy volunteers assumed in the previous study [4] in Table 5 and compared them with the data of latency presented in the current study.

Table 5.
Normative values of the latency parameter (mean value and standard deviation) CMAP and MEP of the examined nerves of the brachial plexus in the control group of healthy volunteers. The data of the reference values have been utilised from previous studies [4].
In the CMAP and MEP examination of the axillary nerve, the mean latency values on the symptomatic and asymptomatic sides were prolonged compared with the reference values from the control group. Prolonged CMAP and MEP latency recorded following the stimulation of the axillary nerve, also on the asymptomatic side, indicates a coexisting demyelinating injury (neuropraxia), probably resulting from high overloads and forces in the mechanical properties of the injuries. The mean latency values for the musculocutaneous nerve on the symptomatic side were also longer than the normative values. This confirmed the above observations (Table 3 and Table 4) that the most common damage concerned the superior trunk of the brachial plexus and cervical roots C5–C7.
Figure 1 presents the application of the magnetic stimulus via the magnetic coil (A—at the Erb’s point; B—at the cervical root level). The recordings of MEP after stimulation from the level of Erb’s point and cervical roots are also presented.
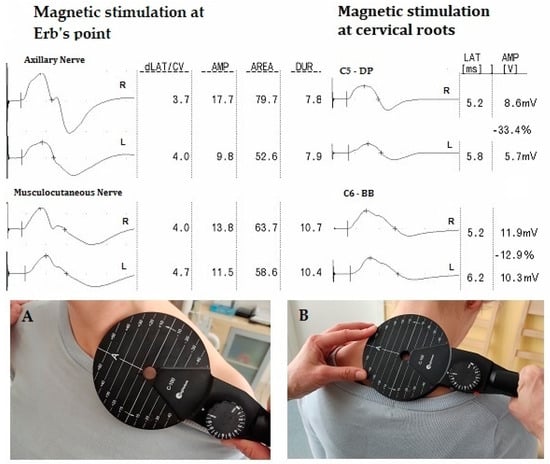
Figure 1.
Example of CMAP and MEP recordings on the right (R) and left (L) sides and the location of the magnetic coil during neurophysiological studies. Abbreviation: level of the MEP stimulation (A)—at the Erb’s point; (B)—at the cervical root level; dLAT/CV—distance latency; AMP—amplitude; AREA—surface area; DUR—duration; LAT—latency; DP—deltoid posterior muscle; BB—biceps brachii muscle.
Table 6 summarises the results of needle EMG recordings from muscles and SNCS results of the examined nerves on the symptomatic side.

Table 6.
Results of EMG and SNCS studies.
In one patient with whiplash syndrome, the EMG examination of all examined muscles and SNCS results did not differ from the normative parameters. In the remaining patients from the research group, the EMG examination was dominated by the symptoms of the neurogenic injury with signs of active denervation (39%). This is related to the results of ENG and MEP tests, where the percentage of loss of active axons on the symptomatic side exceeded 40%, thus demonstrating axonal damage to the nerve (axonotmesis), which results in neurogenic changes in the muscles supplied by the injured nerves. In the SNCS, 21% of patients had abnormal conduction values recorded in the sensory fibers of the examined nerves, which originate mainly from the level of the upper trunk of the brachial plexus, or no sensory evoked potentials were recorded (16%). In 63% of patients, the conduction values were within the normal range in all tested sensory nerves. This may result from a dominant lesion at the level of the C5-C7 spinal roots, where the excitability of the peripheral sensory nerves may be preserved about normative values.
4. Discussion
This preliminary research results provide evidence that MEP and ENG recordings induced at levels of spinal roots and Erb’s point in patients following the brachial plexus injury may precisely reveal the advancement of the abnormalities in the motor neural conduction on the symptomatic side mainly from the C5–C7 root domains. It appears that the parameter of the amplitude, rather than the latency, has greater significance in an evaluation of the pathology advancement on the symptomatic side when MEP studies are utilised. Taking into account its non-invasive and less painful properties and high diagnostic precision it seems to be superior to the ENG method. Moreover, this study’s results confirmed the axonotmesis and neuropraxia type of injury in motor fibers in correlation with EMG results.
The results of neurophysiological tests ENG, EMG, MEP, and SNCS presented in this article are consistent with our previous observations [3,4], allowing us to determine the functional state of muscles and nerves damaged at the level of the brachial plexus. The main goal of rehabilitation in patients after the brachial plexus injury is the inhibition of the degeneration and activation of the regeneration process in the nerves and reinnervation in the muscle. Nerve regeneration refers to the proliferation of axons towards the muscle and is further associated with the process of remyelination of previously damaged axons. However, muscle reinnervation is crucial for maintaining the function of myocytes and preventing their atrophy. Reinnervating motor units as well as those units that have undergone axonal damage strive to reinnervate myocytes. Due to recording in neurophysiological tests of mainly neurogenic changes with active denervation, this corresponds to the simultaneous coexistence of both the degenerative process, resulting in axonal damage, and reinnervation processes that take place in the muscle. The first step of physiotherapeutic procedures is to stop this “vicious circle”. Significant atrophy of myocytes may stop the process of returning the function of the motor unit as a functional complex [8,31,32,33,34]. Various physiotherapy techniques (electrotherapy and kinesiotherapy) may have a positive impact on the regeneration processes, both at the physiological and morphological phenomena in the nerves. Rehabilitation should incorporate the treatment supporting nerve regeneration, muscle strengthening, and maintaining functional ranges of motion of the injured extremities. It comprises the neuromuscular electrical stimulation (NMES) [12,34,35]. Contemporary researches show that NMES increases the strength of stimulated muscles, thereby preventing atrophy while waiting for the results of axonal regeneration. A type of electrical muscle stimulation is FES, i.e., functional electrical stimulation, which, using electrodes placed on the damaged muscle, stimulates the sensory and motor fibers of the supplying nerve, thereby supporting the axon’s proliferation and reinnervation of the muscle [36,37]. The proprioceptive neuromuscular facilitation method (PNF), using the patient’s exercises against the resistance of the physiotherapist stimulates muscles to contract, and also improves muscle strength and the range of motion of the injured extremity. Additionally, through the phenomenon of the spread of the impulses in the spinal cord neural connections, one can notice an improvement in the parameters of muscle activity on the opposite side [38,39,40]. Kinesiotherapy exercises can be used in patients with brachial plexus injuries both as conservative treatment and after surgical reconstructive intervention. In the work of de Santana Chagas et al. [28] concerning the rehabilitation techniques in the case of brachial plexus injury, they recommend the use of the following: passive, assisted, and active exercises to increase the range of movement, stretching exercises to improve neuromuscular control and improve the elasticity of soft tissues, resistance, and anti-gravity exercises to increase muscle strength and the use of learned exercises in the functional training. To maintain the correct range of motion, it is important to additionally use manual therapy to relax hyperactive muscles, mechanically mobilise the stiff joints, stimulate sensory perception, and reduce pain [41,42,43].
The improvement of functional state in the nerves of patients following the brachial plexus paralysis can be considered in the context of Seddon’s classification [20]. Neuropraxia, defined as a conduction block resulting from damage to the myelin sheath, is a type of demyelinating injury. Rehabilitation procedures in this area are limited to mobilisation of the structures surrounding the blocked nerve. For this purpose, the manual therapy described above can be used, focusing on lymphatic drainage and classic massage of the upper extremity [43,44]. However, Willand et al. [45] proved that the use of electrical stimulation of the nerve immediately after surgical decompression accelerates the process of axon proliferation and, consequently, the reconstruction of myelin sheath. An axonal type of brachial plexus injury, according to Seddon, can be classified as axonotmesis and neurotmesis. Axonotmesis refers to the disruption of axonal continuity while preserving connective tissue, which allows for potential axonal regrowth and functional recovery. However, in the case of neurotmesis, axonal proliferation is unlikely due to the complete disruption of the nerve structures. Taking the above into account, in the case of axonal damage, rehabilitation procedures should concern the post-operative stage, necessary for the nerve regeneration process [46]. For this purpose, the previously mentioned kinesiotherapy exercises, PNF method, or manual therapy can be used. In the literature, many researchers examine the scope of action of electrical nerve stimulation. Zuo et al. [47] reported that during electrical stimulation of damaged nerve fibers, through reverse conduction of action potentials to the cell body, intraneuronal cyclic AMP increases, which affects the regulation of genes related to nerve regenerative processes. Additional rehabilitation methods may include the sensory re-education method based on the Homunculus theory, i.e., the representation of the hand in the motor and sensory cortex of the brain. It involves the use of alternative types of stimuli that excite the motor and sensory cortex to rebuild the neuronal network, restoring the proper function of the areas supplied by the damaged peripheral nerve. An example of such therapy is mirror therapy, based on visual–tactile stimulations, or audio–tactile interaction therapy [23,48].
One of this study’s limitations can be a low number of observations, presented only in fourteen patients. However, it should be pointed out that from all examined patients with brachial plexus injury, only some met the inclusion criteria for performing the MEP studies. A homogeneous rehabilitation program was not used in the research group. Therefore, there is no detailed list of kinesiotherapy and physical therapy treatments, including the characteristics of the parameters of physical stimuli used during the patient’s rehabilitation. This limitation resulted from the lack of detailed medical documentation related to rehabilitation procedures and the fact that each patient had an individually selected rehabilitation program in various medical centres dealing with the patient’s therapy. Complementing the algorithm of rehabilitation procedures would require an increase in the size of the research group and a thorough analysis of rehabilitation procedures. Such a comparison would allow for the creation of detailed rehabilitation guidelines for patients with brachial plexus injuries in correlation with the results of neurophysiological tests. Performing follow-up neurophysiological tests over time to assess rehabilitation progress in patients with brachial plexus damage by the algorithm of rehabilitation procedures proposed in this study will be the future next step in our investigation.
The algorithm was developed based on the results of neurophysiological tests determining the scope, type, and level of damage to the neural structures of the brachial plexus and based on the assessment of muscle bioelectric activity. The research results were used to select therapeutic procedures whose targeted application would lead to an effective process of nerve regeneration and muscle reinnervation. The impact of appropriate techniques on the structure and biochemical activity of both nerves and muscles was discussed at the beginning of the Discussion.
Summarising the data in Figure 2, the proposed clinical neurophysiology tests (ENG, MEP, SNCS) were the basis for assessing the functional state of nervous structures, both motor and sensory fibers of the brachial plexus. In demyelinating lesions (neuropraxia) associated with nerve compression, caused by possible conduction block, further treatment is the selection of physiotherapeutic methods influencing the elimination of the conduction block and possible spontaneous regeneration or making a decision regarding surgical intervention. The axonal nature of the damage resulting from severe damage to the brachial plexus (axonotmesis, neurotmesis) affects the change in the bioelectric activity of the muscles. Analysis of the current status of muscle function determines the acute nature (neurogenic damage with denervation potential) or chronic nature (neurogenic damage without denervation potential) of the brachial plexus injury. The current status of nerves and muscle function establishes the time, scope, and type of surgical intervention. Subsequent physiotherapeutic treatment, implementation time, and type are aimed at accelerating the processes of nerve regeneration and muscle reinnervation. At each stage of treatment, neurophysiological control tests are necessary, to show the possible progress or absence of regeneration and reinnervation. The absence of positive results from neurophysiological studies allows for the revision of therapeutic procedures such as surgical and physiotherapeutic. The effectiveness of the treatment algorithm will be measured through clinical and neurophysiological control tests. The first neurophysiological study will be the basis for a comparative analysis of nerve regeneration and reinnervation processes in muscles. Additionally, the patient’s subjective assessment, particularly towards sensory perception and pain, is significant. The main benefits resulting from algorithm application are an objective evaluation of muscle and nerve function, the possibility of the individualised diagnostic and therapeutic process in each patient, correlation of the current status of neural structures function with imaging studies, and planning and controlling the surgical treatment.
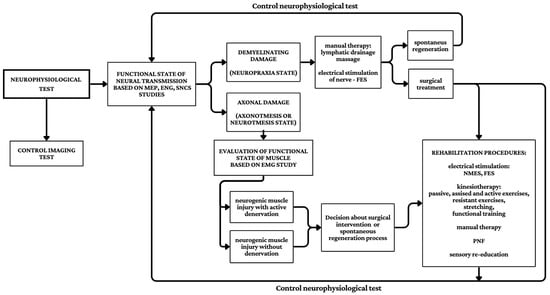
Figure 2.
Proposal of the rehabilitation algorithm in patients after the brachial plexus injury based on the neurophysiological test results presented in this study.
The available literature databases do not provide clear and comprehensive data on the algorithms of rehabilitation procedures in patients with brachial plexus injuries, similar to those presented in our study, developed especially using the results of neurophysiological tests. Few scientific reports correlated the results of neurophysiological tests with imaging tests or selected rehabilitation procedures, but they did not concern the starting base for further therapeutic procedures [49,50,51]. An extensive review by Li et al. [52] shows that a clear rehabilitation process has not been developed yet. The rehabilitation focuses mainly on exercise therapy, sensory training, neuroelectromagnetic stimulation, and the strategy of neurotrophic factors utilising acupuncture and massage therapy. In contrast, interventions like hydrotherapy, photo-therapy, and neural stem cell therapy are described as secondary significance. Exercise therapy concerns mostly passive range of motion, active-assisted range of motion, active range of motion, strength-increasing procedures, and other elements of PNF kinesiotherapy [53]. Objective functional assessment of the brachial plexus based on clinical neurophysiology studies also refers to the classification of peripheral nerve damage according to Seddon (neuropraxia, axonotmesis, neurotmesis) and allows for the adaptation of therapeutic procedures, both surgical and rehabilitation, following the current functional state of the brachial plexus neural structures and bioelectrical activity of upper extremity muscles. Each rehabilitation procedure has the potential to physically affect tissues in a specific way, influencing their functions, including molecular and biochemical ones [45]. The selection of an appropriate rehabilitation technique should therefore refer directly to the registered functional changes in nerves and muscles, which would influence the optimisation of rehabilitation progress. Moreover, neurophysiological tests performed before rehabilitation become the basis for prospective assessment of the health patient’s statement.
In the current research, the progression of functional deficits concerned the scope of innervation and function of the deltoid and biceps brachii muscles. This corresponds to damage at the level of the upper trunk of the brachial plexus and the axillary and musculocutaneous nerves. Depending on the deficit range in bioelectrical muscle activity, isometric muscle exercises that demonstrate at least minimal voluntary activity are recommended. For other muscles that show moderate functional deficits in EMG studies, active–passive and active exercises with loading are recommended. Due to the primarily post-ganglionic nature of the nerve damage (at the level of Erb’s point), attention should be turned to the need for electrostimulation of the motor fibers of the brachial plexus as well as muscle stimulation. Electrical stimulation of muscles allows for maintaining the activity of myocytes and prevents their atrophy until regenerative processes in the nerve appear. The orientation of the stimulation electrodes from the anode to the cathode is consistent with the orthodromic (physiological) conduction of nerve impulses towards the effector. The sensory deficits demonstrated in the SNCS examination should be an indication to improve proprioception in those areas of the upper limb that showed the extent of deficits in terms of peripheral and dermatomal innervation (in the case of damage also preganglionic at the level of the spinal roots). Activation of the upper limb muscles also creates correct patterns of arm abduction movement, but mainly flexion in the elbow joint and the wrist, which determines the return of motor function in the upper extremities.
Based on the results of the neurophysiological examination, the above-mentioned targeted activities make it possible to personalize rehabilitation treatment according to the patient’s capabilities, who, seeing the effectiveness of the therapy, also shows greater involvement in the further treatment process.
5. Conclusions
The proposed algorithm of physiotherapeutic procedures should be focused mainly on recovery from motor dysfunctions resulting from brachial plexus damage, according to the results of the complex neurophysiological testing. Rehabilitation should include treatment that supports nerve regeneration, muscle strengthening, and maintaining functional ranges of motion on the symptomatic side. Rehabilitation treatment in patients with brachial plexus injuries is an individualised process, and the selection of procedures and the effectiveness of the treatment undertaken should be compared with the results of neurophysiological tests checking the motor neuronal transmission from the level of the cervical motor centre to the effector, the activity of peripheral nerves and the activity of muscle motor units. This is guaranteed by comparative MEP, ENG, and EMG tests.
Author Contributions
Conceptualisation, K.L. and A.W.-K.; methodology, A.W.-K.; software, K.L. and A.W.-K.; validation, K.L., A.W.-K. and J.H.; formal analysis, K.L., A.W.-K. and J.H.; investigation, K.L. and A.W.-K.; resources, J.H.; data curation, K.L. and A.W.-K.; writing—original draft preparation, K.L.; writing—review and editing, A.W.-K. and J.H.; visualisation, K.L and A.W.-K.; supervision, A.W.-K.; project administration, A.W.-K. and J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of Poznan University of Medical Science, decision no. 554/17, dated 22 June 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Enoka, R.M.; Amiridis, I.G.; Duchateau, J. Electrical Stimulation of Muscle: Electrophysiology and Rehabilitation. Physiology 2020, 35, 40–56. [Google Scholar] [CrossRef]
- Neto, J.H.S.; Neto, B.C.; Eiras, A.B.D.; Botelho, R.H.S.; Carmo, J.M.d.M.; Passos, M.A.R.F. The 2-Dimensional and 3-Dimensional Anatomy of the Adult Brachial Plexus Divisions and Cords. Hand 2022, 17, 50–54. [Google Scholar] [CrossRef]
- Wiertel-Krawczuk, A.; Huber, J. Standard Neurophysiological Studies and Motor Evoked Potentials in Evaluation of Traumatic Brachial Plexus Injuries—A Brief Review of the Literature. Neurol. Neurochir. Pol. 2018, 52, 549–554. [Google Scholar] [CrossRef]
- Wiertel-Krawczuk, A.; Huber, J.; Szymankiewicz-Szukała, A.; Wincek, A. Neurophysiological Evaluation of Neural Transmission in Brachial Plexus Motor Fibers with the Use of Magnetic versus Electrical Stimuli. Sensors 2023, 23, 4175. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Cowey, A.; Jones, M.; Pickard, S.; Ford, D. The Anatomy, Investigations and Management of Adult Brachial Plexus Injuries. Orthop. Trauma 2009, 23, 420–432. [Google Scholar] [CrossRef]
- Estrella, E.P.; Montales, T.D. Functioning Free Muscle Transfer for the Restoration of Elbow Flexion in Brachial Plexus Injury Patients. Injury 2016, 47, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, P.; Huber, J.; Szymankiewicz-Szukała, A.; Górecki, M.; Romanowski, L. End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats. Int. J. Mol. Sci. 2023, 24, 10428. [Google Scholar] [CrossRef]
- Lyberis, P.; Balsamo, L.; Fontana, E.C.; Ruffini, E.; Nicosia, S.; Roffinella, M. VATS Phrenic Nerve Harvesting for Brachial Plexus Neurotization: Literature Review and Our Experience. Minerva Surg. 2023, 78, 558–561. [Google Scholar] [CrossRef]
- Jiménez-Del-Barrio, S.; Cadellans-Arróniz, A.; Ceballos-Laita, L.; Estébanez-de-Miguel, E.; López-de-Celis, C.; Bueno-Gracia, E.; Pérez-Bellmunt, A. The Effectiveness of Manual Therapsaliy on Pain, Physical Function, and Nerve Conduction Studies in Carpal Tunnel Syndrome Patients: A Systematic Review and Meta-Analysis. Int. Orthop. 2022, 46, 301–312. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Kim, D.D.; Burkett, C.; Kline, D.G. Nerve Transfer Surgery for Adult Brachial Plexus Injury: A 10-Year Experience at Louisiana State University. Neurosurgery 2009, 65, A55-62. [Google Scholar] [CrossRef]
- Thomeer, R.T.; Malessy, M.J. Surgical Repair of Brachial Plexus Injury. Clin. Neurol. Neurosurg. 1993, 95, S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Kuncoro, J.; Deapsari, F.; Suroto, H. Clinical and Functional Outcome after Different Surgical Approaches for Brachial Plexus Injuries: Cohort Study. Ann. Med. Surg. 2022, 78, 103714. [Google Scholar] [CrossRef]
- Rich, J.A.; Newell, A.; Williams, T. Traumatic Brachial Plexus Injury Rehabilitation Using Neuromuscular Electrical Muscle Stimulation in a Polytrauma Patient. BMJ Case Rep. 2019, 12, e232107. [Google Scholar] [CrossRef] [PubMed]
- MRI of the Brachial Plexus: A Practical Review, Applied Radiology. Available online: https://appliedradiology.com/Articles/mri-of-the-brachial-plexus-a-practical-review (accessed on 2 February 2024).
- Gasparotti, R. New Techniques in Spinal Imaging. Neuroradiology 2011, 53 (Suppl. S1), S195–S197. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, W. Application and Research Progress of Ultrasound-Guided Brachial Plexus Block Through Costoclavicular Space Approach in Upper Limb Surgery. Altern. Ther. Health Med 2023, 30, 24–30. [Google Scholar]
- Jongbloed, B.A.; Bos, J.W.; Rutgers, D.; van der Pol, W.L.; van den Berg, L.H. Brachial Plexus Magnetic Resonance Imaging Differentiates between Inflammatory Neuropathies and Does Not Predict Disease Course. Brain Behav. 2017, 7, e00632. [Google Scholar] [CrossRef]
- Rajczewski, A.; Daroszewski, P.; Fabijański, A.; Bogusławski, K.; Kaźmierczak, M.; Huber, J. Incidence of Carpal Tunnel Syndrome and Other Coexisting Brachial Plexus Neuropathies in Bullseye Shooters—A Pilot Retrospective Clinical and Neurophysiological Assessment. Appl. Sci. 2023, 13, 8020. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Huber, J.; Leszczyńska, K.; Wietrzak, P.; Kaczmarek, K. Relationships between the Clinical Test Results and Neurophysiological Findings in Patients with Thoracic Outlet Syndrome. Bioengineering 2022, 9, 598. [Google Scholar] [CrossRef]
- Seddon, H.J. A Classification of Nerve Injuries. Br. Med. J. 1942, 2, 237–239. [Google Scholar] [CrossRef]
- Ferrante, M.A. Brachial Plexopathies: Classification, Causes, and Consequences. Muscle Nerve 2004, 30, 547–568. [Google Scholar] [CrossRef]
- Grossman, J.A.I.; Price, A.; Chim, H. Complications in Surgery for Brachial Plexus Birth Injury: Avoidance and Treatment. J. Hand. Surg. Am. 2018, 43, 164–172. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, S.; Alsharif, R. Effect of Virtual Reality versus Conventional Physiotherapy on Upper Extremity Function in Children with Obstetric Brachial Plexus Injury. J. Musculoskelet. Neuronal. Interact. 2017, 17, 319–326. [Google Scholar]
- Palomo, R.; Sánchez, R. Physiotherapy applied to the upper extremity in 0 to 10-year-old children with obstetric brachial palsy: A systematic review. Rev. Neurol. 2020, 71, 1–10. [Google Scholar] [CrossRef]
- Sicari, M.; Longhi, M.; D’Angelo, G.; Boetto, V.; Lavorato, A.; Cocchini, L.; Beatrici, M.; Battiston, B.; Garbossa, D.; Massazza, G.; et al. Modified Constraint Induced Movement Therapy in Children with Obstetric Brachial Plexus Palsy: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2022, 58, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Smania, N.; Berto, G.; Marchina, E.L.; Melotti, C.; Midiri, A.; Roncari, L.; Zenorini, A.; Ianes, P.; Picelli, A.; Waldner, A.; et al. Rehabilitation of Brachial Plexus Injuries in Adults and Children. Eur. J. Phys. Rehabil. Med. 2012, 48, 483–506. [Google Scholar]
- Saliba, S.; Saliba, E.N.; Pugh, K.F.; Chhabra, A.; Diduch, D. Rehabilitation Considerations of a Brachial Plexus Injury with Complete Avulsion of C5 and C6 Nerve Roots in a College Football Player: A Case Study. Sports Health 2009, 1, 370–375. [Google Scholar] [CrossRef]
- de Santana Chagas, A.C.; Wanderley, D.; de Oliveira Ferro, J.K.; Alves de Moraes, A.; Morais de Souza, F.H.; da Silva Tenório, A.; Araújo de Oliveira, D. Physical Therapeutic Treatment for Traumatic Brachial Plexus Injury in Adults: A Scoping Review. PM&R 2002, 14, 120–150. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Y.; Xu, X.; Zhang, S.; Zhao, X. Clinical research of comprehensive rehabilitation in treating brachial plexus injury patients. Chin. Med. J. 2012, 125, 2516. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Badilas, N.K.; Stavropoulos, N.A.; Mazis, G.; Kotoulas, H.K.; Kyriakopoulos, S.; Tagkalegkas, I.; Sofianos, I.P. Treatment Options for Brachial Plexus Injuries. ISRN Orthop. 2014, 2014, 314137. [Google Scholar] [CrossRef]
- Komirishetty, P.; Zubkow, K.; Areti, A.; Ong, H.; Zochodne, D.W. Delayed Manipulation of Regeneration within Injured Peripheral Axons. Neurobiol. Dis. 2021, 155, 105383. [Google Scholar] [CrossRef]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving Peripheral Nerve Regeneration: From Molecular Mechanisms to Potential Therapeutic Targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef]
- Hunt, M.; Lu, P.; Tuszynski, M.H. Myelination of Axons Emerging from Neural Progenitor Grafts after Spinal Cord Injury. Exp. Neurol. 2017, 296, 69–73. [Google Scholar] [CrossRef]
- Goroszeniuk, T.; Pang, D. Peripheral Neuromodulation: A Review. Curr. Pain Headache Rep. 2014, 18, 412. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Vicente, F.; San Juan, A.F.; Larumbe-Zabala, E.; Estévez-González, A.J.; Donadio, M.V.F.; Pérez-Ruiz, M. Neuromuscular Electrical Stimulation Improves Muscle Strength, Biomechanics of Movement, and Functional Mobility in Children With Chronic Neurological Disorders: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab170. [Google Scholar] [CrossRef] [PubMed]
- Vaz, D.V.; Mancini, M.C.; da Fonseca, S.T.; Arantes, N.F.; Pinto, T.P.d.S.; de Araújo, P.A. Effects of Strength Training Aided by Electrical Stimulation on Wrist Muscle Characteristics and Hand Function of Children with Hemiplegic Cerebral Palsy. Phys Occup. Ther. Pediatr. 2008, 28, 309–325. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Zhang, Q.; Jiang, M.; He, J.; Yang, L.; Li, Y.; Zhang, Q.; Jiang, M.; He, J. The Role of Functional Electrical Stimulation in Brachial Plexus Injury Repair. In Brachial Plexus Injury—New Techniques and Ideas; IntechOpen: London, UK, 2021; ISBN 978-1-83969-687-9. [Google Scholar]
- Trofin, D.; Matei, D.-V.; Trofin, D.M.; Onu, I.; Iordan, D.A.; Stamate, T. Clinic-Electrophysiologic Correlations in Rehabilitation of Adult Patients with Traumatic Brachial Plexus Lesions. Appl. Sci. 2023, 13, 5679. [Google Scholar] [CrossRef]
- Guiu-Tula, F.X.; Cabanas-Valdés, R.; Sitjà-Rabert, M.; Urrútia, G.; Gómara-Toldrà, N. The Efficacy of the Proprioceptive Neuromuscular Facilitation (PNF) Approach in Stroke Rehabilitation to Improve Basic Activities of Daily Living and Quality of Life: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef] [PubMed]
- Deodhe, N.P.; Dhage, P.; Harjpal, P. Functional Electrical Stimulation in Conjunction With Proprioceptive Neuromuscular Facilitation (PNF) Technique to Improve Upper Limb Function in Traumatic Brachial Plexus Injury: A Case Report. Cureus 2023, 15, e46386. [Google Scholar] [CrossRef]
- Frampton, V. Problems in Managing Reconstructive Surgery for Brachial Plexus Lesions Contrasted with Peripheral Nerve Lesions. J. Hand Surg. 1986, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Frampton, V. Management of Pain in Brachial Plexus Lesions. J. Hand. Ther. 1996, 9, 339–343. [Google Scholar] [CrossRef]
- Lustosa, L.; Silva, A.E.L.; Carvalho, R.d.P.; Vargas, C.D. Upper Limb Joint Coordination Preserves Hand Kinematics after a Traumatic Brachial Plexus Injury. Front. Hum. Neurosci. 2022, 16, 944638. [Google Scholar] [CrossRef] [PubMed]
- Milicin, C.; Sîrbu, E.A. Comparative Study of Rehabilitation Therapy in Traumatic Upper Limb Peripheral Nerve Injuries. NeuroRehabilitation 2018, 42, 113–119. [Google Scholar] [CrossRef]
- Willand, M.P.; Nguyen, M.-A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2016, 30, 490–496. [Google Scholar] [CrossRef]
- Zaneteas, P.D. Brachial Plexus Injuries and the Electrodiagnostic Examination. Curr. Sports Med. Rep. 2003, 2, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical Stimulation to Enhance Peripheral Nerve Regeneration: Update in Molecular Investigations and Clinical Translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef] [PubMed]
- Osumi, M.; Inomata, K.; Inoue, Y.; Otake, Y.; Morioka, S.; Sumitani, M. Characteristics of Phantom Limb Pain Alleviated with Virtual Reality Rehabilitation. Pain Med. 2019, 20, 1038–1046. [Google Scholar] [CrossRef]
- Casaletto, E.; Lin, B.; Wolfe, S.W.; Lee, S.K.; Sneag, D.B.; Feinberg, J.H.; Nwawka, O.K. Ultrasound imaging of nerves in the neck: Correlation with MRI, EMG, and clinical findings. Neurol. Clin. Pract. 2020, 10, 415–421. [Google Scholar] [CrossRef]
- Wolny, T.; Glibov, K.; Granek, A.; Linek, P. Ultrasound Diagnostic and Physiotherapy Approach for a Patient with Parsonage-Turner Syndrome-A Case Report. Sensors 2023, 23, 501. [Google Scholar] [CrossRef]
- Andersen, L.L.; Magnusson, S.P.; Nielsen, M.; Haleem, J.; Poulsen, K.; Aagaard, P. Neuromuscular activation in conventional therapeutic exercises and heavy resistance exercises: Implications for rehabilitation. Physic. Ther. 2006, 86, 683–697. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Wang, J.; Zhang, T.; Chen, Z. Review of rehabilitation protocols for brachial plexus injury. Front. Neurol. 2023, 14, 1084223. [Google Scholar] [CrossRef]
- Kinlaw, D. Pre-/postoperative therapy for adult plexus injury. Hand Clin. 2005, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).