Abstract
Objective: Bone augmentation has become a significant practice in various areas of bone regeneration dentistry. This systematic review analyzes the research focused on evaluating bone substitute materials for the presence of contaminants. Methods: In June 2024, an extensive electronic search was conducted using renowned databases such as PubMed, Web of Science, and Scopus. Specific keywords employed in the search included ((bone AND (substitute) AND (remnants OR (purity)) OR ((graft AND tooth) AND (remnants OR purity)) OR ((graft AND dentin) AND (remnants OR purity)). The search adhered to the PRISMA protocol and the PICO framework. The review concentrated on the origin of bone substitute materials, the processing methods used for these materials, techniques for assessing purity, and types of contamination identified. A total of 594 articles were identified of which 22 met the criteria and were incorporated into the review. Results: Investigations into allogeneic and xenogeneic bone substitute materials have revealed that, despite manufacturers’ assurances of purity, some materials still contain contaminants. Sample analyses demonstrated the presence of donor cellular remains, cellular debris, intertrabecular fat, connective tissue, and collagen. Similarly, synthetically produced bone substitute materials (alloplastic materials) contained various impurities, such as polyvinyl alcohol (PVA), CaO phases, calcium-deficient HAp phases, oily substances containing carbon and silicone, cellulose derivatives, alpha-tricalcium phosphate (α-TCP), and heavy metals. Conclusions: Bone-derived and bone-like graft materials can contain various organic and inorganic impurities.
1. Introduction
Bone tissue is subject to a continuous process of remodeling. The activity of cells such as osteocytes, osteoblasts, and osteoclasts, in addition to the existence of the bone-signaling pathway, bone matrix, and access to nutrients from the blood, provide the potential for the rebuilding and repair of damaged or defective bone tissue []. Nevertheless, natural regeneration processes are frequently insufficient in cases of significant bone deficit. In such cases, bone grafting is one of the most frequently employed surgical techniques for augmenting bone tissue []. Bone substitute materials are pivotal in clinical applications for bone tissue regeneration and repair. These materials must closely replicate the properties of natural bone, including chemical composition, phase composition, and microstructure []. Their clinical relevance spans a variety of orthopedic and dental procedures, such as bone grafting, fracture repair, and the reconstruction of bone defects resulting from trauma, tumors, or congenital conditions [].
The purity of bone substitute materials is crucial for their clinical efficacy and safety. Impurities in these materials can significantly compromise biocompatibility, potentially leading to adverse reactions like inflammation, infection, or material rejection by the body []. Contaminants can also impair the material’s bioactivity, undermining its ability to support new bone formation through osteoconduction or osteoinduction. For example, residual cellular debris, foreign particles, or unintended phases might trigger immune responses, disrupting the healing process and increasing the risk of implant failure. Additionally, impurities can adversely affect the mechanical properties of bone substitutes. Unwanted phases or structural irregularities may weaken the material, reducing its durability and making it susceptible to degradation under physiological stress [,,]. This is especially critical for applications requiring mechanical support, where the material’s strength and longevity are essential for the success of the implant. In clinical settings, the use of high-purity bone substitute materials is essential for ensuring effective structural and functional integration with host bone while minimizing the risk of complications. Achieving the desired biomechanical properties often necessitates precise control over the material’s chemical composition, phase purity, and microstructure. Consequently, the strategic use of combinations of structurally distinct compounds is common, allowing for the optimization of material properties to meet specific clinical requirements while maintaining high standards of purity [].
The source from which the grafting material originates can be used to distinguish between several grafting materials. (see Figure 1) Autografts (autogenous materials) are derived from the same individual and are transferred from the donor area to the recipient area. Allografts (allogeneic materials) are obtained from another representative of the same species following the appropriate preparation through freezing, freeze-drying, autoclaving, irradiation, or chemical preservation to ensure the absence of an immunological response. Xenogeneic materials (xenografts) are derived from other species []. Bone graft materials can be characterized by determining their osteoinductivity, osteoconductivity, and osteogenicity. It can be observed that only autogenous materials possess all three characteristics and are currently the gold standard in surgical procedures. Allogenic materials are the second-best option, offering various forms and large quantities []. Xenografts rich in natural hydroksyapatite [] are also frequently utilized as materials due to their favorable osteoconductivity. However, they are not osteogenic or osteoinductive to the same extent as the aforementioned materials.
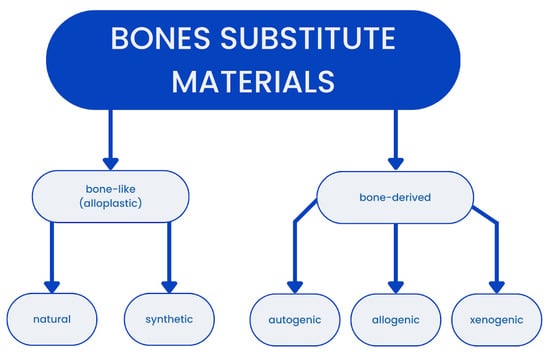
Figure 1.
Graph presenting division of grafting materials.
Despite the implementation of aseptic techniques during the harvesting and transplantation of bone grafts, the possibility of contamination remains. It is possible for microbiotas to form on the material during the interval between harvesting and transplantation []. Furthermore, environmental exposure of the donors to osteotoxic substances, such as lead, plays a significant role in graft purity. While the data in question are not routinely available, it is nevertheless the case that exemplary levels of accumulated lead in animal bone tissue have been demonstrated to impair the healing of fractures and bone metabolism []. Nevertheless, the incorporation of selected ions like strontium, iron, magnesium, zinc, or lanthanide substituted or co-substituted in hydroxyapatite has the potential to enhance the properties of the bone graft such as better biocompatibility, improved angiogenesis capability, or enhanced osteoconductivity [,,,].
It is of the utmost importance that grafting materials undergo appropriate processing, as this can significantly reduce the risk of unwanted contamination. Firstly, a comprehensive donor screening process is conducted, which includes an examination of the donor’s medical and social histories, as well as microbial and viral testing. Moreover, aseptic recovery, appropriate processing techniques, and a range of disinfection methods, including antibiotics, detergents, mechanical processes, chemical solutions, and terminal sterilization, are employed []. The demineralization of the tissue sample increases the fusion potential of the graft. A decellularization process was employed to minimize the risk of immunological response and graft rejection. However, in the case of bone grafts, the retention of some living cellular elements may be beneficial. This can be achieved through many techniques, including refrigeration in media, freeze-drying, cryopreservation, freezing, and media storage at room temperature [].
The objective of this study was to conduct a comprehensive review and synthesis of existing data in the scientific literature regarding contamination associated with the preparation and processing of bone graft materials. A systematic review of this topic is necessary as no studies addressing a similar question were identified in the databases searched. The authors, therefore, deemed it essential to address this gap systematically.
2. Materials and Methods
2.1. Focused Question
This systematic review followed the PICO framework [] as follows:
In patients needing bone substitute materials (Population), does the preparation and processing of these materials (Intervention) affect the contamination levels of alloplastic materials (Outcome) compared to bone-derived materials (Comparison)?
2.2. Protocol
The exact description of article selection for this systematic review was structured by the PRISMA protocol []. (Figure 2) The systematic review was registered on the Open Science Framework under the following link: https://osf.io/rz2hs (accessed on 3 August 2024).
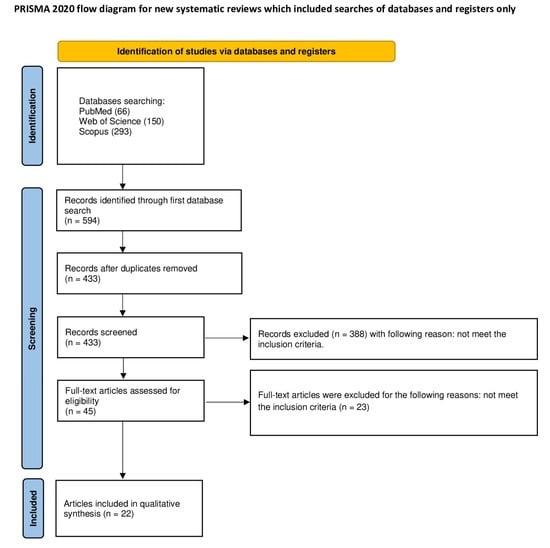
Figure 2.
The PRISMA protocol.
2.3. Eligibility Criteria
The reviewers agreed to include only the articles which met the following criteria listed below [,,,,,,]:
- Studies that focused on bone substitute materials processing;
- Examination of purity of bone substitute materials;
- In vitro studies;
- Full-text articles;
- Studies in English.
The exclusion criteria the reviewers agreed upon were as follows [,,,,,,]:
- Studies which did not concentrate on bone substitute material preparation;
- Studies examining the properties of bone substitution materials;
- Studies which did not examine the purity of processed materials;
- Non-English studies;
- Systematic review articles;
- Reviews;
- Meta-analysis.
No restrictions were imposed regarding the year of publication.
2.4. Information Sources, Search Strategy, and Study Selection
In June 2024, an extensive electronic search was conducted using renowned databases such as PubMed, Web of Science, and Scopus. Specific keywords employed in the search included ((bone AND (substitute) AND (remnants OR (purity)) OR ((graft AND tooth) AND (remnants OR purity)) OR ((graft AND dentin) AND (remnants OR purity)). The search was conducted in accordance with the PRISMA protocol and the PICO framework. The review focused on the provenance of bone substitute materials, the processing techniques employed for these materials, methods for assessing purity, and the types of contamination identified. In the Scopus database, the results were refined to titles, abstracts, and keywords, while in PubMed, they were narrowed down to titles and abstracts. In WoS, the results were refined only to abstracts. The search parameters were constrained to studies meeting the eligibility criteria. Only articles with full-text versions were included in the analysis. A total of 594 articles were identified of which 22 met the criteria and were incorporated into the review.
2.5. Data Collection and Data Items
Seven reviewers (N.S., T.H., J.K., P.P., Ł.K., J.K., and S.K.) carefully selected the articles that met the inclusion criteria. The extracted data were then introduced into a standardized Excel file.
2.6. Assessing Risk of Bias in Individual Studies
In the preliminary phase of selecting studies, the authors independently examined the titles and abstracts of each study to reduce the possibility of reviewer bias. They evaluated the level of consensus among reviewers using Cohen’s κ test [] (Watson, P.F). The authors resolved any disagreements about whether to include or exclude a study through discussions.
2.7. Quality Assessment
Two independent evaluators (J.M. and P.J.P.) assessed the procedural quality of each study included in the article. The criteria used to evaluate study design, implementation, and analysis included the physicochemical and/or microscopic characterization of evaluated alloplastic material, the quantitative assessment of contamination in alloplastic material, the presence of control group, a detailed description of material preparation/decontamination, a sample size calculation, and a biological assessment. Studies were scored on a scale of 0 to 6 points, where a higher score indicated better study quality. The risk of bias was categorized as follows: 0–1 points denoted a high risk, 2–4 points denoted a moderate risk, and 5–6 points indicated a low risk. Any discrepancies in scoring were resolved through discussion until a consensus was reached [,,,,,,].
3. Results
3.1. Study Selection
A search of the electronic databases PubMed, Scopus, and WoS yielded 594 records. Of these, 161 were duplicates and were thus removed. The remaining 433 articles were subjected to abstract screening, which resulted in the exclusion of 549 articles that did not meet the inclusion criteria. A thorough analysis of the 45 full texts yielded a further 23 exclusions. The final number of articles included in this review was 22.
3.2. General Characteristics of the Included Studies
The studies included in the systematic review present research focused on evaluating bone substitute materials for the presence of contaminants. The general characteristics of the studies is presented in Table 1. A total of 10 studies aimed to synthesize new materials [,,,,,,,,,]. Seven of the studies compared materials [,,,,,,]. Five studies analyzed materials [,,,,]. The authors of the studies analyzed materials of various origins. Thirteen of the studies involved synthetic materials [,,,,,,,,,,,,]. Four studies dealt with animal materials [,,,], and two with human materials [,]. Also, two studies analyzed both human and animal materials [,]. In contrast, one study compared human, animal, and synthetic materials [].

Table 1.
General characteristics of the selected studies.
3.3. Main Study Outcomes
The detailed characterization of selected studies is presented in Table 2. Studies selected in this systematic review varied in their comparison research focused on evaluating bone substitute materials for the presence of contaminants.

Table 2.
Detailed characteristics of the included studies.
Six groups of researchers [,,,,,] confirmed that bone substitute materials synthesized using a higher temperature show greater purity. Ismail R. et al. [] found that pure hydroxyapatite was produced after synthesizing for 18 hours in an autoclave tube using a hydrothermal reactor. Seven studies [,,,,,,] identified the presence of organic/cellular remnants. High levels of collagen were discovered to be connected to the allogeneic bone matrix []. Additionally, six studies [,,,,,] identified the presence of nonorganic remnants. Le et al. [] confirmed the formation of calcium sulphate hemihydrate (CSH) with traces of calcium sulphate dihydrate (CSD) remnants. Furthermore, Ismail R. et al. [] verified that the hydroxyapatite powders were contaminated with various CaCO3 phases or Ca(OH)2 at each stage of production.
3.4. Quality Assessment
Among 22 articles included in the review, no entries were assigned with a high risk of bias, 16 were assigned a moderate risk of bias [,,,,,,,,,,,,,,,], and the remaining 6 with a low risk of bias [,,,,,] (see Table 3).

Table 3.
Quality assessment.
4. Discussion
During bone augmentation procedures, due to significant bone loss, the lack of contamination of the surgical field is crucial to the success of the procedure [,]. Aseptic and antiseptic techniques help reduce the number of contaminants transferred to the surgical field by the operator’s tools and hands. However, all these efforts to increase the success of treatment may be in vain when using bone substitute material containing contaminants. This systematic review included 22 papers that evaluated various contaminants in bone substitute materials. These contaminants can be of organic origin, chemical compounds, or crystalline phases that hinder or prevent new bone formation. The findings indicate significant gaps in research on the contamination of bone substitute materials. Among the studies included in the review, thirteen focused on synthetic materials [,,,,,,,,,,,,], four studies dealt with animal materials [,,,], and two investigated human materials [,]. Additionally, two studies analyzed both human and animal materials [,], and one study compared human, animal, and synthetic materials [].
Tests of allogeneic and xenogeneic bone substitute materials have shown that some materials contain contaminants, although manufacturers ensure the purity of their materials. Research by S. Ghanaati et al. [] based on the analysis of allogenic bone blocks available on the market showed that three out of five materials available on the market were contaminated. Manufacturers ensured the purity of the materials, but contaminants such as organic and cellular residues were found. Histological analysis of the Maxgraft material by J. Lorenz et al. [] showed cellular debris, intertrabecular fat, connective tissue, and collagen. The work of T. Fretwurst et al. [] involved the evaluation of allografts from different donors, showing that bone block cleaning techniques did not ensure perfect cleanliness of the samples. All samples were subjected to histological and biochemical analysis. In each case, contaminants and remains of organic tissues, including adipocytes and osteocytes, were found. Additionally, chondroblasts and fibroblasts were detected in Puro’s samples. Biochemical analysis allowed for successful isolation and purification of DNA. Lorenz J et al. [] subjected histological examination to allogeneic bone blocks implanted in patients for the purpose of regenerating the alveolar process. The collected samples contained donor cellular remains. Pollution did not negatively affect the regeneration of the alveolar process. Moreover, research by Eva Johanna Kubosch et al. [] found that only allograft blocks contained cellular remnants such as fibrocytes and adipocytes, while xenogeneic materials were free from such impurities, indicating variability in biological contamination depending on the material source. In contrast, research by Amouriq et al. [] revealed that Benecel and E4M materials were primarily contaminated with cellulose derivatives, with additional contaminants like carbon and silicone identified in Benecel products. These findings emphasize the importance of material origin and processing methods in determining contamination levels.
Synthetically produced bone substitute materials are also not free from contaminants. Research by A. Vojevodova et al. [], aimed at assessing the purity of the synthetically produced hydroxyapatite/polyvinyl alcohol (n-HAp/PVA) composite material, showed the presence of β-TCP, free CaO and calcium-deficient HAp phases, and PVA, which has a negative impact on osteogenesis. Some additives used during the synthesis of bone substitute materials based on hydroxyapatite significantly improve the properties of the resulting product. L. Chandran et al. [] assessed the synthesis of hydroxyapatite (HAp) with La3+ and Pr3+ ions’ co-doping. The results indicate that the incorporation of Pr3+ and La3+ ions into hydroxyapatite (HAp) causes slight lattice distortion without secondary phase formation, with increased bioactivity, cell viability, and moderate antibacterial efficacy, suggesting promising biomedical applications. Processing the shells by grinding and thermal treatment at 1100 °C results in the formation of smooth spherical hydroxyapatite particles that can be used as a bone substitute, as proven by Hamidi AA et al. []. In the research of Ismat Ullah et al. [], synthesized hydroxyapatite bioceramics were co-substituted with strontium and iron. They exhibited good properties because they inhibit the activity of osteoclasts, which reduces bone resorption and increases the activity of preosteoblastic cell division and osteoblast differentiation, thus promoting bone formation functions. However, their structure showed remnants of the secondary β-TCP phase. Nhi Thao Ngoc Le et al. [] confirmed the formation of calcium sulphate hemihydrate (CSH) with traces of dihydrate residue (CSD). Furthermore, Ismail R. et al. [] verified that hydroxyapatite powders were contaminated with various phases of CaCO3 or Ca(OH)2 at each production stage.
The results of the included studies highlighted the critical issue of contamination in bone substitute materials and its implications for phase purity and biological performance. Sandeep G. [] identified contaminants like calcium silicate and tricalcium phosphate in BGHA materials. However, other studies, such as that by M.H. Fathi et al. [], showed that while hydroxyapatite can be obtained with high purity, certain processes like sintering at 700 °C can introduce a CaO phase, suggesting that processing conditions are crucial in maintaining material purity. In terms of specific bone substitute materials, Marzio Piccinini et al. [] found that OsproLife (bTCP) contains trace amounts of hydroxyapatite (HA), tetracalcium phosphate (TTCP), and alpha-tricalcium phosphate (α-TCP) within acceptable limits, indicating controlled impurity levels. Additionally, nanometric β-tricalcium phosphate synthesized by David S.H. Lee et al. [] exhibited high purity and desirable physical properties, such as increased surface area and compressive strength. Ionic doping in hydroxyapatite, as explored by Ullah I et al. [] and others, demonstrated that dopants like Sr2+, Fe3+, La3+, and Pr3+ can influence phase purity, though often without introducing new phases []. However, some doped materials, such as Sr/Fe co-doped HAp, were not entirely pure, containing secondary β-TCP phases [].
During the research, difficulties were encountered due to the limited number of available articles containing information on the purity of bone substitute materials. There is a need for more extensive research on the presence of contaminants in materials already introduced to the market. It is necessary to establish new methods and standards for graft cleansing in order to improve the quality of bone substitute materials. In the future, searches should focus on a broader analysis of synthetic materials that can be successfully used as bone substitutes. The research methodology on bone substitute materials should be unified for further meta-analysis. The small number of studies and their significant heterogeneity limit the possibility of conducting a meta-analysis.
5. Conclusions
This systematic review focused on analyzing bone replacement materials to assess their purity and contamination levels. The results indicate that both organic and synthetic bone replacement materials contain contaminants. Despite manufacturers’ assurances of purity, contaminants such as organic and cellular residues were detected. The evaluation of allografts from different donors revealed that current bone block cleaning techniques are insufficient for achieving complete cleanliness. Future research should prioritize evaluating contamination levels in bone substitutes across various manufacturers and processing techniques. Additionally, it is essential to develop and standardize new methods and protocols for cleaning and processing bone substitute materials to ensure their purity. Furthermore, bone substitute materials come in a range of sizes and shapes, making them suitable for various applications. As the development of biodegradable materials broadens their potential uses, further studies are needed to explore these materials comprehensively.
Author Contributions
Conceptualization, J.M. and M.D.; methodology, N.S. and J.M.; software, P.J.P.; validation, N.S. and J.M.; formal analysis, N.S.; investigation, Ł.K., J.K. (Jan Kiryk), J.K. (Julia Kensy), N.S., K.W., T.H., P.J.P. and S.K.; resources, J.M., N.S. and J.K. (Jan Kiryk); data curation, J.M. and M.D.; writing—original draft preparation, Ł.K., J.K. (Jan Kiryk), J.K. (Julia Kensy), N.S., K.W., T.H., P.J.P. and S.K.; resources, J.M., N.S. and J.K. (Jan Kiryk); writing—review and editing, M.D., J.M., R.J.W. and P.S.; visualization, J.K. (Jan Kiryk); supervision, R.J.W., M.D. and J.M.; project administration, J.M. and M.D.; funding acquisition, J.M. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by a subsidy from Wroclaw Medical University, number SUBZ.B180.24.058.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available within the article.
Conflicts of Interest
Author Ł.K. was employed by the company Kor4dent Łukasz Korjat. Author T.H. was employed by the company Ortho.pl Centrum Zdrowego Uśmiechu. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fillingham, Y.; Jacobs, J. Bone Grafts and Their Substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, K.; Dudziak-Milkowska, K.; Rybak, Z.; Szymonowicz, M.; Kosior, P.; Dobrzyński, M. Zastosowanie Materiałów Hydroksyapatytowych w Regeneracji Tkanki Kostnej. Gerontol. Współczesna 2016, 4, 81–84. [Google Scholar]
- Martinez, S.A.; Walker, T. Bone Grafts. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1207–1219. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Conz, M.B.; Barros, T.C.; de Sena, L.Á.; Soares, G.d.A.; Granjeiro, J.M. Physicochemical Characterization of Two Deproteinized Bovine Xenografts. Braz. Oral Res. 2008, 22, 5–10. [Google Scholar] [CrossRef]
- Baseri, N.; Meysamie, A.; Campanile, F.; Hamidieh, A.A.; Jafarian, A. Bacterial Contamination of Bone Allografts in the Tissue Banks: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2022, 123, 156–173. [Google Scholar] [CrossRef]
- Behrend, C.; Carmouche, J.; Millhouse, P.W.; Ritter, L.; Moskal, J.; Rubery, P.; Puzas, E. Allogeneic and Autogenous Bone Grafts Are Affected by Historical Donor Environmental Exposure. Clin. Orthop. Relat. Res. 2016, 474, 1405–1409. [Google Scholar] [CrossRef][Green Version]
- Boyd, A.R.; Rutledge, L.; Randolph, L.D.; Mutreja, I.; Meenan, B.J. The Deposition of Strontium-Substituted Hydroxyapatite Coatings. J. Mater. Sci. Mater. Med. 2015, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Domingos, M.; Zhang, X. Synthesis and Characterization of Sintered Sr/Fe-Modified Hydroxyapatite Bioceramics for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 375–388. [Google Scholar] [CrossRef]
- Paramasivan, M.; Sampath Kumar, T.S.; Kanniyappan, H.; Muthuvijayan, V.; Chandra, T.S. Biomimetic Ion Substituted and Co-Substituted Hydroxyapatite Nanoparticle Synthesis Using Serratia marcescens. Sci. Rep. 2023, 13, 4513. [Google Scholar] [CrossRef]
- Chandran, L.; Ballamurugan, A.M. Apatite Matrix Substituted with Biologically Essential Rare Earth Elements as an Artificial Hard Tissue Substitute: Systematic Physicochemical and Biological Evaluation. J. Biomed. Mater. Res. A 2021, 109, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.A.; Samsell, B.; McLean, J. Allograft Tissue Safety and Technology. In Biologics in Orthopaedic Surgery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–62. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Struzik, N.; Wiśniewska, K.; Piszko, P.J.; Piszko, A.; Kiryk, J.; Matys, J.; Dobrzyński, M. SEM Studies Assessing the Efficacy of Laser Treatment for Primary Teeth: A Systematic Review. Appl. Sci. 2024, 14, 1107. [Google Scholar] [CrossRef]
- Matys, J.; Kensy, J.; Gedrange, T.; Zawiślak, I.; Grzech-Leśniak, K.; Dobrzyński, M. A Molecular Approach for Detecting Bacteria and Fungi in Healthcare Environment Aerosols: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4154. [Google Scholar] [CrossRef] [PubMed]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces—A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida Spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Hamidi, A.A.; Salimi, M.N.; Yusoff, A.H.M. Synthesis and Characterization of Eggshell-Derived Hydroxyapatite via Mechanochemical Method: A Comparative Study. AIP Conf. Proc. 2017, 1835, 20045. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous Co-Substitution of Sr2+/Fe3+ in Hydroxyapatite Nanoparticles for Potential Biomedical Applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Le, N.T.N.; Le, N.T.T.; Nguyen, Q.L.; Pham, T.L.B.; Nguyen-Le, M.T.; Nguyen, D.H. A Facile Synthesis Process and Evaluations of α-Calcium Sulphate Hemihydrate for Bone Substitute. Materials 2020, 13, 3099. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Laroybafih, M.B.; Fitriyana, D.F.; Nugroho, S.; Santoso, Y.I.; Hakim, A.J.; Mulqi, M.S.A.; Bayuseno, A.P. The Effect of Hydrothermal Holding Time on the Characterization of Hydroxyapatite Synthesized from Green Mussel Shells. J. Adv. Res. Fluid. Mech. Therm. Sci. 2021, 80, 84–93. [Google Scholar] [CrossRef]
- Vojevodova, A.; Loca, D. Hydroxyapatite/Polyvinyl Alcohol Composite In Situ Synthesis for Hydrogel Formation. Key Eng. Mater. 2017, 721, 202–207. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and Bioactivity Evaluation of Bone-like Hydroxyapatite Nanopowder. J. Mater. Process Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Sampath Kumar, T.S. Heat-Deproteinated Xenogeneic Bone from Slaughterhouse Waste: Physico-Chemical Properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef]
- Shepherd, D.V.; Kauppinen, K.; Brooks, R.A.; Best, S.M. An in Vitro Study into the Effect of Zinc Substituted Hydroxyapatite on Osteoclast Number and Activity. J. Biomed. Mater. Res. A 2014, 102, 4136–4141. [Google Scholar] [CrossRef]
- Barbeck, M.; Jung, O.; Xiong, X.; Krastev, R.; Korzinskas, T.; Najman, S.; Radenkovic, M.; Wegner, N.; Knyazeva, M.; Walther, F. Balancing Purification and Ultrastructure of Naturally Derived Bone Blocks for Bone Regeneration: Report of the Purification Effort of Two Bone Blocks. Materials 2019, 12, 3234. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential Lack of “Standardized” Processing Techniques for Production of Allogeneic and Xenogeneic Bone Blocks for Application in Humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef]
- Piccinini, M.; Prosperi, S.; Preve, E.; Rebaudi, A.; Bucciotti, F. In Vitro Biocompatibility Assessment and in Vivo Behavior of a New Osteoconductive ΒtCP Bone Substitute. Implant. Dent. 2016, 25, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kubosch, E.J.; Bernstein, A.; Wolf, L.; Fretwurst, T.; Nelson, K.; Schmal, H. Clinical Trial and In-Vitro Study Comparing the Efficacy of Treating Bony Lesions with Allografts versus Synthetic or Highly-Processed Xenogeneic Bone Grafts. BMC Musculoskelet. Disord. 2016, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Spanou, A.; Nelson, K.; Wein, M.; Steinberg, T.; Stricker, A. Comparison of Four Different Allogeneic Bone Grafts for Alveolar Ridge Reconstruction: A Preliminary Histologic and Biochemical Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 424–431. [Google Scholar] [CrossRef]
- Sandeep, G.; Varma, H.K.; Kumary, T.V.; Suresh Babu, S.; John, A. Characterization of Novel Bioactive Glass Coated Hydroxyapatite Granules in Correlation with in Vitro and in Vivo Studies. Trends Biomater. Artif. Organs 2006, 19, 99–107. [Google Scholar]
- Amouriq, Y.; Bourges, X.; Weiss, P.; Bosco, J.; Bouler, J.M.; Daculsi, G. Skin Sensitization Study of Two Hydroxypropyl Methylcellulose Components (Benecel® and E4M®) of an Injectable Bone Substitute in Guinea Pigs. J. Mater. Sci. Mater. Med. 2002, 13, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.H.; Pai, Y.; Chang, S.; Kim, D.H. Microstructure, Physical Properties, and Bone Regeneration Effect of the Nano-Sized β-Tricalcium Phosphate Granules. Mater. Sci. Eng. C 2016, 58, 971–976. [Google Scholar] [CrossRef]
- Lorenz, J.; Schlee, M.; Al-Maawi, S.; Chia, P.; Sader, R.A.; Ghanaati, S. Variant Purification of an Allogeneic Bone Block. Acta Stomatol. Croat. 2017, 51, 141. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Al-Maawi, S.; Schwarz, F.; Sader, R.A.; Schlee, M.; Ghanaati, S. Allogeneic Bone Block for Challenging Augmentation—A Clinical, Histological, and Histomorphometrical Investigation of Tissue Reaction and New Bone Formation. Clin. Oral Investig. 2018, 22, 3159–3169. [Google Scholar] [CrossRef]
- Hsu, H.J.; Waris, R.A.; Ruslin, M.; Lin, Y.H.; Chen, C.S.; Ou, K.L. An Innovative α-Calcium Sulphate Hemihydrate Bioceramic as a Potential Bone Graft Substitute. J. Am. Ceram. Soc. 2018, 101, 419–427. [Google Scholar] [CrossRef]
- Kanchana, P.; Sekar, C. Influence of Sodium Fluoride on the Synthesis of Hydroxyapatite by Gel Method. J. Cryst. Growth 2010, 312, 808–816. [Google Scholar] [CrossRef]
- Targonska, S.; Dominiak, S.; Wiglusz, R.J.; Dominiak, M. Investigation of Different Types of Micro- and Nanostructured Materials for Bone Grafting Application. Nanomaterials 2022, 12, 3752. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, M.; Hnitecka, S.; Olchowy, C.; Dominiak, S.; Gedrange, T. Possible Treatment of Severe Bone Dehiscences Based on 3D Bone Reconstruction—A Description of Treatment Methodology. Appl. Sci. 2021, 11, 10299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).