1. Introduction
Multilayer packaging, commonly referred to as composite materials, is extensively utilized in food storage, distribution, and consumption. This type of packaging consists of multiple materials, including paper, plastic, and metal, combined to form a sandwich-like structure [
1]. The package’s polymer is laminated with other polymers, aluminum foil, and/or cardboard to provide various functions, such as tensile strength, satisfactory sealing, and protection from moisture, light, other flavors and odors, and oxygen [
2]. Each layer of packaging serves a distinct purpose in achieving the desired technological functionality.
Typical polymers employed in food packaging include polypropylene (PP), polyethylene terephthalate (PET), low-density polyethylene (LDPE), polyamide (PA), polyvinylidene chloride (PVdC), and ethylene vinyl alcohol (EVOH) [
3]. The thickness of the polymer layers varies based on their intended use. For instance, PP and PET generally range between 15–55 μm and 12–50 μm, respectively. LDPE is a thicker polymer utilized in packaging with thicknesses ranging from 25–100 μm. Thinner polymers, such as PA, PVdC, and EVOH, can range from 12–30 μm, 32 μm, and 2–10 μm, respectively. The remaining thickness of the packages results from the aluminum foil, adhesive layers, ink, and paper (if used). The thickness of the aluminum foil can be up to 12 μm [
4].
However, the use of plastic packaging composed of multiple layers of polymers, ink, paper, and metal has sparked concerns about its adverse environmental impact. Separating the layers for recycling purposes is technically challenging and financially burdensome due to the thermodynamic incompatibility of the materials. According to Eurostat Statistics Explained, the generation of packaging waste in the European Union amounted to 79 ± 1.25 million tons annually between 2007 and 2016, constituting approximately 17% of all co-collected waste, including multilayer composites [
5].
In light of this, substantial research and resources should be dedicated to developing innovative recycling techniques capable of enhancing the recycling rate of multilayer packaging while focusing on achieving high recovery rates. Delamination is considered an effective solution for multilayer plastic waste (MLPW) recycling. Several methods can be employed to achieve delamination, such as selectively dissolving polymers [
6]. However, this method requires an in-depth understanding of the chemical properties of polymers, making it difficult to apply to a mixture of packaging waste from a recycling center. Another option involves delamination through micro-perforation, which expedites the process by creating small holes in the polymers [
7]. It is advised to remove the aluminum layer before initiating the delamination process, for instance, by dissolving it in an alkali solution.
In addition to delamination, compatibilization represents another feasible recycling approach that involves the incorporation of additives to combine and stabilize different polymers [
8]. Nevertheless, this method is currently inefficient due to differences in material composition.
Recent studies have concentrated on developing novel, sustainable packaging materials to replace multilayer composite packaging. For example, researchers have developed biodegradable packaging materials from cellulose nanocrystals, chitosan, and starch [
9]. These materials exhibit exceptional oxygen and moisture barrier properties, and can be employed in food product packaging.
This study aims to determine the efficiency of MLPW in separating polymer layers by analyzing the resulting surface morphology and chemical composition of the separated layers. The study also seeks to optimize the conditions for the separation process, such as the concentration of nitric acid, exposure time, and temperature. The results of this study could have significant implications for the recycling of polymer materials, as they can provide a more efficient and environmentally friendly method for separating polymer layers compared to traditional methods, such as pyrolysis.
2. Materials and Methods
In this study, various delamination experiments were conducted using potato chip packages. Packaging materials were obtained from post-consumer waste, including a mixture of different types of packaging waste. However, due to the absence of a comparative analysis of results from the waste mixture, multilayer potato chip packaging featuring polymer, aluminum, and visually appealing print, was selected as the sample for subsequent experiments. Packaging from a single company was employed for all experiments to ensure the accuracy of the results.
It is imperative to guarantee that the acid fully covers the packing strips during the process. The procedure involves the following steps:
Place the packing strips in a flask.
Pour the desired concentration of nitric acid into the flask.
Immerse the flask in room-temperature water in an ultrasonic bath.
Insert a stirrer into the flask and activate it.
Initiate the ultrasound and gradually increase the temperature.
As the mixing speed does not significantly impact the process, set it to a minimum level.
Monitor the process as the polymer layers separate and the aluminum melts.
The process is deemed complete once the aluminum melts entirely.
As the separated polymer layers augment the sample’s volume, choosing an appropriately sized container and leaving sufficient empty space is vital.
Laboratory-scale reactor were used in the study.
Figure 1 shows the reactor’s main parts.
Although the packages might not have been washed before delamination, we still recommend washing them. As demonstrated throughout the process, delaminating cleaner packages enables the preservation and reuse of cleaner reagents.
The packages were cut into strips with three different widths: 0.5 cm, 1.0 cm, and 1.5 cm, to prepare the samples. The length of all samples remained consistent at 12.0 cm. The aqueous solution employed for the delamination of multilayer potato chip packaging waste consisted of nitric acid in three concentrations: 20%, 25%, and 30%. Nitric acid was diluted with distilled water. The use of non-concentrated nitric acid was deemed an intelligent solution, as it not only facilitated the delamination of the packaging layers but also dissolved the aluminum layer present in the packaging.
The temperature was a critical controlled parameter considered in the experiments. Various polymers in the packaging, each with distinct melting points, necessitated conducting these experiments at low temperatures. Ensuring that the strip edges remained unaffected by temperature was crucial for delamination. Consequently, placing the packaging strips in the solution at room temperature was vital before gradually increasing the temperature. A thermoregulated ultrasonic bath facilitated the temperature increase, continuously monitoring the acid’s temperature in the flask using a thermometer. A rate of 2–3 degrees per minute was maintained during the temperature increase. Experiment temperatures of 55 °C, 65 °C, and 75 °C were utilized. The boiling temperature of nitric acid is 83 °C [
10].
Upon completion of the process and the separation of the polymers from the acid, the hot acid was allowed to cool, and the remaining acid was washed off the polymers. The polymers were subsequently dried in a Binder drying oven at 104 °C. A portion of the used nitric acid was stored for further study, and the remainder was disposed of according to university safety regulations.
The experiment focused on studying the dissolution of aluminum in nitric acid. The primary objective was to dissolve aluminum in nitric acid using 1 g of packaging and 200 mL of nitric acid. It should be noted that the reagent proportions can be adjusted according to the experiment’s requirements.
During the process, the separation of polymer layers and the melting of aluminum became apparent. The visual monitoring of aluminum melting throughout the process facilitated a deeper understanding of aluminum dissolution in nitric acid.
The mixing speed had no significant impact on the process; thus, it was recommended to maintain a minimum speed to prevent unnecessary alterations in the procedure.
After completing the process, the separated polymer layers increased the sample’s volume. Therefore, selecting an appropriate container size and leaving sufficient space was crucial to prevent spillage or contamination.
These steps offer a comprehensive approach to dissolving aluminum in nitric acid. This method allows the experiment to be conducted with precision and accuracy.
Experiments were designed using Modde 13.0.1 (Umetrics) software, employing a factorial design (
Table 1). Each experiment was performed in triplicate to ensure the validity and reliability of the findings. The reported result (time) represents the average of all repetitions.
3. Results
3.1. Packaging Structure and Products after Delamination
Recovering valuable materials from polymer and aluminum composites presents a challenge due to the need for a delicate balance between preserving the integrity of the materials and maximizing value extraction. The delamination principle has been identified as an effective means of achieving this goal. Utilizing this principle enables the recovery of all polymer layers without causing any damage.
Multilayer plastic composites require adhesive layers for their formation. Two types of adhesives are commonly employed in polymer and aluminum composites: acrylics and polyurethanes (PUs). Acrylics are suitable for bonding polyethylene to aluminum, while PU is more versatile and widely used, even functioning as plasticizers in polymer packaging.
PU is composed of a hard segment and a soft segment. The hard segment consists of isocyanates, and the soft segment comprises polyols. During the delamination process, hydrolysis breaks the isocyanate bonds, facilitated by the addition of water. This process results in the polyols’ recovery and the polymer layers’ separation. Additionally, PUs are vulnerable to oxidative degradation in acidic or alkaline media [
11].
The kinetics of this process depend on several variables, such as moisture, heat, and UV light. Aqueous solutions and temperatures are carefully controlled during recovery to mitigate these risks.
As the analyzed packages contain an aluminum layer, an oxidizing agent like nitric acid can be employed to separate the layers. Although aluminum does not dissolve in oxidizing acids, such as concentrated nitric and sulfuric acids, due to their passivating effect, it does dissolve in dilute HNO
3, being reduced to N
2O and partially to NH
4+ [
12].
The boiling temperature of nitric acid is 83 °C. At a temperature of 75 °C, the release of nitrous dioxide gas has been observed [
10].
Likewise, ammonium nitrate can split into dinitro oxide and water, but this necessitates a high temperature [
13].
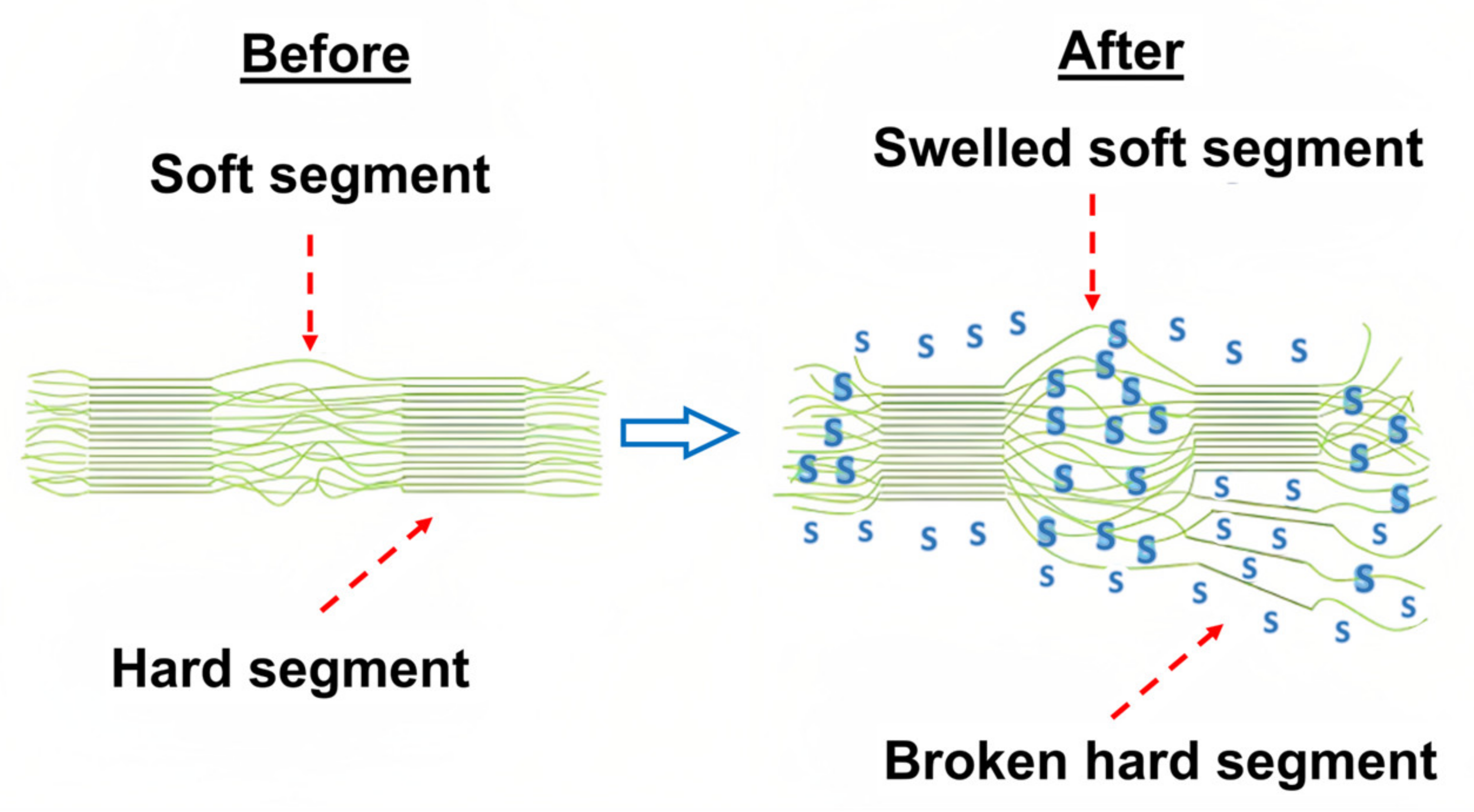
In the context of our material recovery process, we assume that the adhesive layers in the packaging consist of PU, as these are the most commonly used adhesive materials. As a strong oxidizing agent, nitric acid is also suitable for breaking down PU structures. PU is a segmented co-polymer, composed of soft and hard segments. The soft segments provide elasticity to the co-polymer while the hard segments contribute to the co-polymer’s strength [
14]. When the PU enters a solvent or solution, the hard segments swell first. During swelling, the solvent penetrates deeply into the polymer and disrupts its structure (
Figure 2) [
15,
16].
In the analysis of packaging materials, the process entails the dissolution of adhesive layers, ink, and aluminum, which result in the recovery of three distinct polymers. Upon inspecting the polymer strips, ink residues are observable, and their presence assists in visually distinguishing the individual polymer layers. Nonetheless, more advanced methods of polymer recognition, such as optical methods, are necessary to eliminate all color residues.
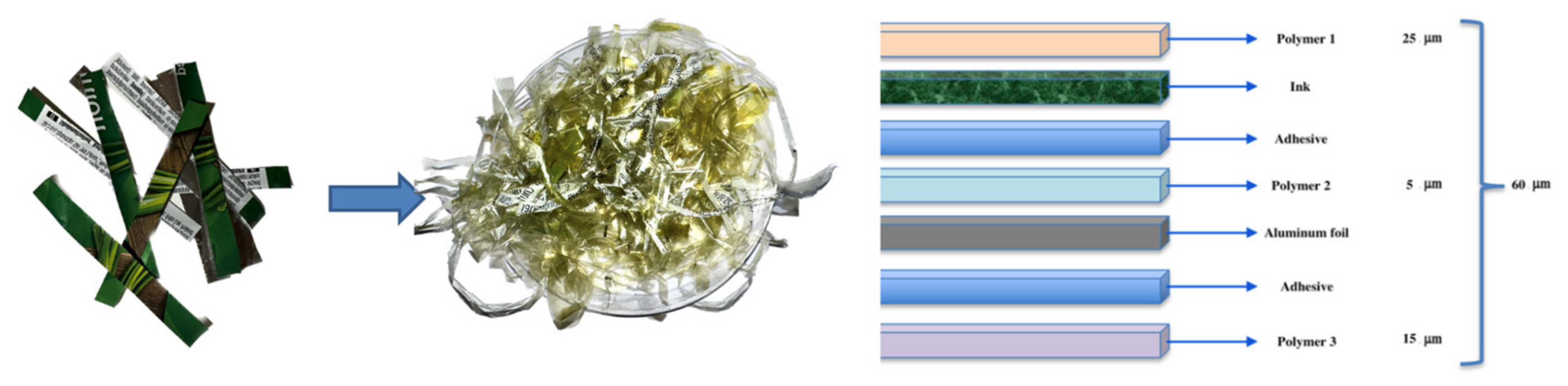
The study of packaging composition and its impact on food safety has garnered significant attention in recent years. Comprehending the composition of packaging materials is vital, as it can offer insights into material properties and their interaction with food. In this context, our research team examined the packaging material of a food product, and based on the available data, we devised a preliminary structure for the packaging composition.
Our analysis disclosed that the packaging material comprises three polymers. Polymer 1, the outermost layer of the packaging, has a thickness of 25 μm. Subsequent layers following Polymer 1 consist of adhesive and colored ink resin. Polymer 2, situated in the coil, possesses a thickness of merely 5 μm. It exhibits remarkable elasticity and can be effortlessly electrified, stretching to filament thickness. The layers succeeding Polymer 2 are composed of aluminum and adhesives. Ultimately, Polymer 3, with a thickness of 15 μm, is the innermost polymer layer of the packaging material that directly comes into contact with food.
The total thickness of the packaging material before delamination measures 60 μm, and after subtracting the thickness of the polymers, the remaining layer, consisting of residue, adhesive, and aluminum, has a thickness of 15 μm. A thickness gauge was employed to measure the thickness of each recovered polymer, enabling us to quantify the thickness of each polymer present in the packaging material.
Figure 3 shows a visual representation of the analysed packaging structure.
Our findings offer valuable insights into the composition and structure of packaging material, underscoring the significance of understanding packaging materials concerning food safety. Further research on the interaction between packaging materials and food is imperative to ensure food products are safe for consumption and adhere to regulatory requirements.
3.2. Design of Experiments
Table 1 presents the comprehensive experimental design which incorporates the following factors: temperature (T), concentration (C), width (a) (type: quantitative), and ultrasound (Ult) (type: qualitative). The response variable is the duration (t) (type: quantitative).
This study aimed to examine the influence of temperature, concentration, width, and ultrasound on the separation of layers in multilayer packaging. A total of 24 trials were conducted, each with varying parameters.
The most prolonged separation process in multilayer packaging occurred at the lowest temperature and concentration values of 55 °C and 20%, respectively. This trial, designated as N12, utilized a width of 1.5 cm and did not employ ultrasound, which resulted in a completion time of 1350 min. Conversely, trial N16, which employed the highest temperature and concentration along with the smallest width, took only 140 min.
The distinction between trials N1 and N23 lies solely in temperature with TN1 being 75 °C and TN23 being 65 °C. Nonetheless, the final process durations exhibit a 30 min discrepancy with tN1 requiring 240 min and tN23 necessitating 210 min. This variation in duration is similarly observed in trials N2 and N19.
A wider strip in multilayer packaging prolongs the separation time by 20 min, as evidenced by the experimental results. Notably, the lengthiest ultrasound experiment, N19, lasted 120 min. By adding 20 min to this duration, the shortest experiment without ultrasound can be determined.
3.3. Factors Influencing the Process
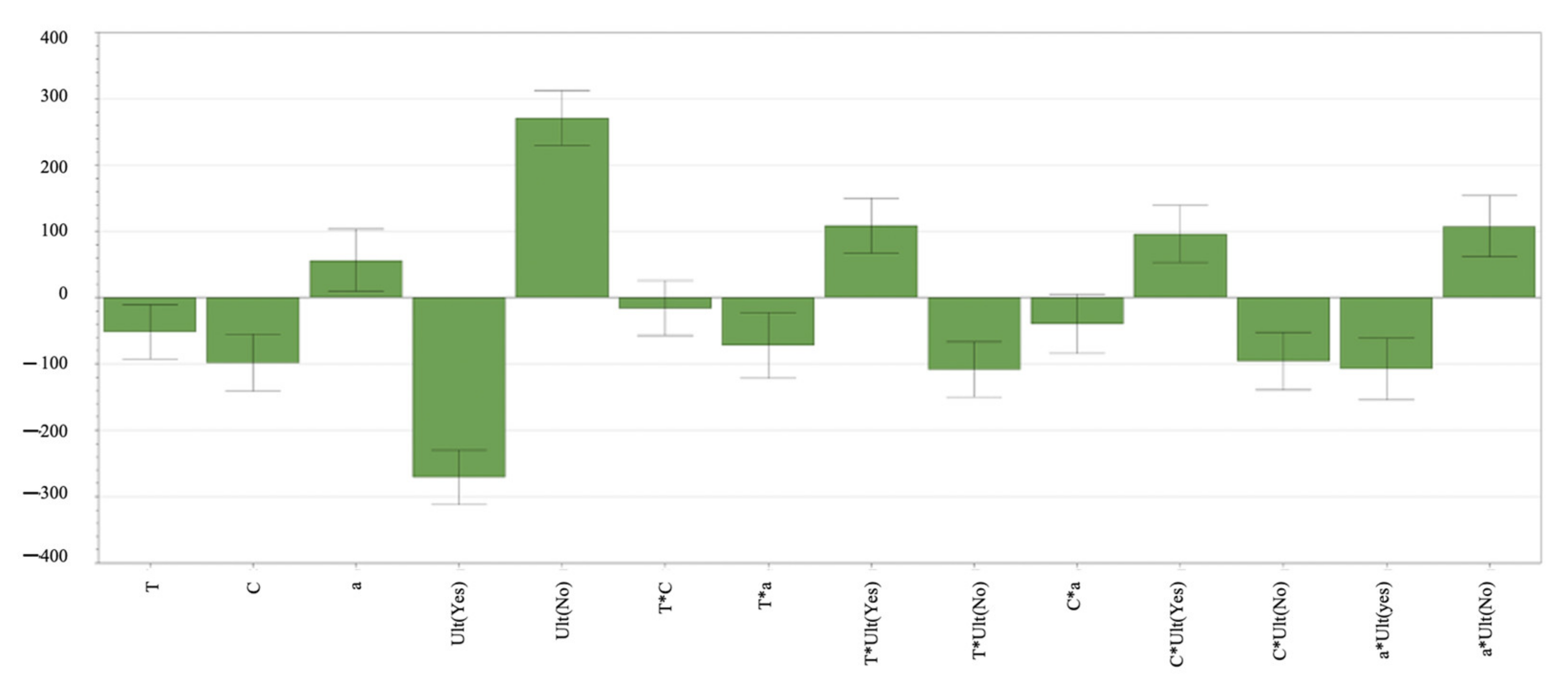
The delamination process is a complex chemical procedure influenced by numerous factors. The efficacy and efficiency of the process depends on various factors, with ultrasound identified as the most influential, followed by acid concentration. Intriguingly, the relationships between temperature and concentration or width and concentration are not statistically significant (
Figure 4). These observations are corroborated by the
p-values of T ×
C (0.420079) and
C × a (0.0741109), as demonstrated in
Appendix A.
The duration of the delamination process is also affected by the relationships between temperature and ultrasound, and between nitric acid concentration and ultrasound. These relationships may impact the reaction rate and the extent of delamination. Consequently, meticulous control of these factors is imperative for achieving optimal results in the delamination process.
In the absence of ultrasound, the dissolution rates of the aluminum and adhesive layers are similar. However, when ultrasound is employed, the aluminum layer dissolves substantially faster than the adhesive layer. This phenomenon can be attributed to the breakdown of the aluminum foil in the packaging layers facilitated by ultrasound. Additionally, a mixing process aids in removing the aluminum foil from the composite. The larger reaction surface area of the metal also results in accelerated dissolution in acid. It is crucial to note that prematurely halting the reactor before the adhesive layers are fully oxidized may yield suboptimal outcomes in the delamination process.
3.4. Contour Plots and Duration
The findings presented in the contour plot in
Figure 5 are noteworthy. The straight lines depicted on the plot indicate that the surface is planar and adheres to a linear model. Furthermore, these findings reveal significant differences among the time zones. Specifically, when the strip widths are 1 cm or 1.5 cm, and ultrasound is not employed, the curves traverse diagonally across the graph area, which imply that the process duration decreases with an increase of temperature and concentration. Conversely, when the strip width is 0.5 cm, the curves run more horizontally, signifying that the temperature effect is less pronounced. In this instance, the process completion rate depends more on the nitric acid concentration. A similar trend is observed when the strips are 1.5 cm wide, and ultrasound is utilized, with the curves appearing nearly horizontal.
Intriguingly, the model exhibits a starkly different scenario when the width is 0.5 cm and 1 cm, and ultrasound is applied. Primarily, it is important to note that the curves are nearly vertical, indicating that nitric acid concentration exerts minimal influence. In this situation, the temperature factor assumes a critical role with the reaction time decreasing as the temperature diminishes. Consequently, to achieve optimal layer separation via ultrasound with widths of 0.5 cm and 1 cm, selecting a lower acid concentration and a reduced temperature is recommended.
This study’s findings hold considerable implications for future research in this field. The observed differences among the time zones and the varying effects of factors, such as temperature and concentration, establish a basis for the further investigation of this process. Moreover, these findings can be employed to optimize the layer separation process using ultrasound, which boasts numerous applications across diverse fields, including medicine, chemistry, and engineering. The results suggest carefully considering strip width and ultrasound usage is crucial for ensuring optimal outcomes. Additionally, this study underscores the significance of temperature and nitric acid concentration in this process, which can inform the design of future experiments and the development of more efficient layer separation methods.
4. Discussion and Conclusions
Multilayer food packaging is prevalent in global industries due to its adaptability in packaging diverse products. Nonetheless, the non-recyclable, multi-material structure of such packaging has raised concerns regarding its environmental impact [
17]. In response, delamination of multilayer packaging can be achieved using methods such as selective melting, micro-perforation, and ultrasound. In this study, we have chosen the latter approach. Additionally, making polymers in compatible packaging can help reduce waste.
Our delamination method employs nitric acid, temperature, stirring, and ultrasound to separate the packaging layers while preserving the polymer layers. One notable advantage of our method is that it enables the separation of dissolved aluminum from the liquid medium through further investigation. We successfully recovered three distinct polymers that can be recycled and used for producing new products through utilizing nitric acid, temperature, mixing, and ultrasound. Aluminum can also be reused to its full potential, considering the existing abundance of aluminum in the market.
The foundation of our method lies in the degradation of adhesive layers. For example, if PU adhesive layers are present, they can be decomposed by a strong oxidizing agent, such as nitric acid. PU comprises soft and hard segments, and monomers are formed when the polymer is oxidized by acid. These monomers are subsequently removed from the multilayer packaging structure by mixing, breaking down the packaging structure, and separating the polymers. The use of non-concentrated nitric acid further enables the dissolution of aluminum from the packaging. However, controlling gas emissions remains a significant challenge in the delamination process, warranting further research.
We conducted 24 experiments using the Modde 13.0.1 (Umetrics) program to identify the factors influencing the process. The results revealed that ultrasound has the most substantial impact on the process with temperature and acid concentration exhibiting no significant statistical correlation. Ultrasound application enhanced the process’s efficiency, and the width of the packaging strips emerged as a crucial factor in the process’s success. The contour plots demonstrated that acid concentration and temperature are equally important when the strips are 1 cm and 1.5 cm wide. In contrast, when the strips are 0.5 cm wide, the influence of concentration outweighs that of temperature. The situation alters when ultrasound is applied, rendering the influence of temperature insignificant when the width of the packaging strips is 1.5 cm.
In conclusion, based on the presented results, we successfully separated the packaging layers and recovered three polymers with the potential for aluminum deposition. All chosen process parameters, including acid concentration, temperature, the width of MLPW strips, and ultrasound, were vital for the final outcome. Although the process is effective without ultrasound, ultrasound is the most critical factor in achieving faster results.
Author Contributions
Conceptualization, A.Š. and G.D.; methodology, A.Š.; software, A.Š.; validation, A.Š., T.M. and G.D.; formal analysis, T.M.; investigation, A.Š.; resources, T.M.; data curation, A.Š.; writing—original draft preparation, A.Š.; writing—review and editing, G.D.; visualization, A.Š. and T.M.; supervision, G.D.; project administration, G.D.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
Research is carried out with EU financial support, implemented in the frame of GRMP, EU fellowships for Georgian researchers, 2023 (57655523).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Scaled and centered coefficients, standard errors, p-values, and confidence intervals for parameter relations.
Table A1.
Scaled and centered coefficients, standard errors, p-values, and confidence intervals for parameter relations.
| Factors | Coefficient | Stand. Errors | p | Conf. Int± |
|---|
| Constant | 307.12 | 19.4201 | 7.1779 × 10−10 | 41.9547 |
| Temperature | −51.7446 | 19.0135 | 0.01746 | 41.0762 |
| Concentration | −98.5458 | 19.862 | 0.00026 | 42.9092 |
| Width | 56.3552 | 21.7409 | 0.022337 | 46.9684 |
| Ult | DF = 1 | | | |
| Ult (Yes) | −271.053 | 18.9615 | 2.4971 × 10−9 | 40.9639 |
| Ult (No) | 271.053 | 18.9615 | 2.4971 × 10−9 | 40.9639 |
| T × C | −16.0422 | 19.2665 | 0.420079 | 41.6229 |
| T × a | −72.165 | 22.7591 | 0.00737012 | 49.168 |
| T × Ult | DF = 1 | | | |
| T × Ult (Yes) | 108.717 | 19.2512 | 7.9658 × 10−5 | 41.5896 |
| T × Ult (No) | −108.717 | 19.2512 | 7.9658 × 10−5 | 41.5896 |
| C × a | −40.0397 | 20.6168 | 0.0741109 | 44.5399 |
| C × Ult | DF = 1 | | | |
| C × Ult (Yes) | 95.9689 | 20.1414 | 0.000369499 | 43.513 |
| C × Ult (No) | −95.9689 | 20.1414 | 0.000369499 | 43.513 |
| a × Ult | DF = 1 | | | |
| a × Ult (Yes) | −107.645 | 21.463 | 0.000236435 | 46.368 |
| a × Ult (No) | 107.645 | 21.463 | 0.000236435 | 46.368 |
| N = 24 | Q2 = | 0.559 | Cond. No. = | 2.63 |
| DF = 13 | R2 = | 0.968 | RSD = | 80.52 |
| COMP. = 2 | R2 adj. = | 0.943 | | |
| | | | Confidence = | 0.95 |
References
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Piringer, O.G.; Baner, A.L. Plastic Packaging Materials for Food: Barrier Function, Mass Transport, Quality Assurance, and Legislation; John and Wiley Sons: Hoboken, NJ, USA, 2008; Available online: https://books.google.lt/books?id=7Uz9XW-VihgC&lpg=PP1&hl=lt&pg=PP1#v=onepage&q&f=false (accessed on 15 April 2023).
- Mishra, A.; Mohite, A.M.; Sharma, N. Influence of Particle Size on Physical, Mechanical, Thermal, and Morphological Properties of Tamarind-Fenugreek Mucilage Biodegradable Films. Polym. Bull. 2022, 80, 3119–3133. [Google Scholar] [CrossRef]
- Dixon, J. Packaging Materials 9. Multilayer Packaging for Food and Beverages; ILSI: Washington, DC, USA, 2011; Available online: https://ilsi.eu/publication/packaging-materials-9-multilayer-packaging-for-food-and-beverages (accessed on 15 April 2023).
- Statistical Office of the European Union. Available online: https://ec.europa.eu/eurostat (accessed on 15 April 2023).
- Walker, T.W.; Frelka, N.; Shen, Z.; Chew, A.K.; Banick, J.; Grey, S.; Kim, M.S.; Dumesic, J.A.; Van Lehn, R.C.; Huber, G.W. Recycling of multilayer plastic packaging materials by solvent-targeted recovery and precipitation. Sci. Adv. 2020, 6, eaba7599. [Google Scholar] [CrossRef] [PubMed]
- Berkane, I.; Cabanes, A.; Horodytska, O.; Aracil, I.; Fullana, A. The delamination of metalized multilayer flexible packaging using a microperforation technique. Resour. Conserv. Recycl. 2023, 189, 106744. [Google Scholar] [CrossRef]
- Lahtela, V.; Silwal, S.; Kärki, T. Re-Processing of Multilayer Plastic Materials as a Part of the Recycling Process: The Features of Processed Multilayer Materials. Polymers 2020, 12, 2517. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.A.; Ahmad, I.; Thomas, S.; Dufresne, A. Recent developments in nanocellulose-based biodegradable polymers. J. Polym. Environ. 2017, 25, 1–27. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529, ISBN 0-07-049439-8. [Google Scholar]
- Aznar, M.; Vera, P.; Canellas, E.; Nerín, C.; Mercea, P.; Störmer, A. Composition of the adhesives used in food packaging multilayer materials and migration studies from packaging to food. J. Mater. Chem. 2011, 21, 4358–4370. [Google Scholar] [CrossRef]
- Janickis, V.; Kreivėnienė, N. Neorganinės Chemijos Pagrindai: Teorija, Praktika, Uždaviniai; Technologija: Kaunas, Lithuania, 2009; ISBN 978-9955-25-706-6. [Google Scholar]
- Lee, J.D. Concise Inorganic Chemistry; Oxford Publications: Oxford, UK, 2016; ISBN 0-412. [Google Scholar]
- De Mello Soares, C.T.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Z.; Zhao, W.; Liu, W.; Liu, H.; Zhu, C. Synthesis and structure/properties characterizations of four polyurethane model hard segments. R. Soc. Open Sci. 2018, 5, 180536. [Google Scholar] [CrossRef] [PubMed]
- Koltzenburg, S.; Maskos, M.; Nuyken, O. Polymer Chemistry; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-49277-2. [Google Scholar]
- Mohite, A.M.; Sharma, N.; Aggarwal, S.; Sharma, S. Effect of Tamarindus coating on post-harvest quality of apples and pears stored at different conditions. Carpathian J. Food Sci. Technol. 2018, 10, 17–25. Available online: https://www.researchgate.net/publication/328262151_Effect_Of_Tamarindus_coating_on_post-harvest_quality_of_apples_and_pears_stored_at_different_conditions (accessed on 15 April 2023).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).